Abstract
The La/SSB autoantigen is a major target of long-term humoral autoimmunity in primary Sjögren's Syndrome (SS) and systemic lupus erythematosus. A majority of patients with linked anti-Ro60/Ro52/La responses target an NH2-terminal epitope designated LaA that is expressed on Ro/La ribonucleoprotein complexes and the surface membrane of apoptotic cells. In this study, we used high-resolution Orbitrap mass spectrometry to determine the clonality, isotype and V-region sequences of LaA-specific autoantibodies in seven patients with primary SS. Anti-LaA immunoglobulin (Ig)Gs purified from polyclonal sera by epitope-specific affinity chromatography were analysed by combined database and de-novo mass spectrometric sequencing. Autoantibody responses comprised two heavily mutated IgG1 kappa-restricted monoclonal species that were shared (public) across unrelated patients; one clonotype was specified by an IGHV3-30 heavy chain paired with IGKV3-15 light chain and the second by an IGHV3-43/IGKV3-20 pairing. Shared amino acid replacement mutations were also seen within heavy and light chain complementarity-determining regions, consistent with a common breach of B cell tolerance followed by antigen-driven clonal selection. The discovery of public clonotypic autoantibodies directed against an immunodominant epitope on La, taken together with recent findings for the linked Ro52 and Ro60 autoantigens, supports a model of systemic autoimmunity in which humoral responses against protein–RNA complexes are mediated by public sets of autoreactive B cell clonotypes.
Keywords: La/SSB, mass spectrometry, primary Sjögrens syndrome, public clonotypes
Introduction
High-titre IgG autoantibodies to the 48 kDa La/SSB protein are a serological hallmark of primary Sjögren's syndrome (SS), but are also associated with systemic lupus erythematosus (SLE) and the neonatal lupus syndrome [1–3]. La is linked physically with 60 kDa Ro/SSA (Ro60) protein in ribonucleoprotein (RNP) complexes comprising small non-coding cytoplasmic (Y) RNAs termed hYRNAs [4] that are thought to drive combined anti-Ro60/La humoral responses via T cell dependent intermolecular determinant spreading [5]. Autoantibodies to a structurally unrelated 52 kDa Ro/SSA protein (Ro52) [also termed tripartite motif (TRIM)21] are invariably part of these linked sets, but can also occur as an isolated species [6]. The physiological role of La is to serve as a termination factor for RNA polymerase III [7] and to stabilize single-stranded DNA during DNA metabolism [8]. Epitope mapping of the La autoantigen in patients with primary SS has revealed three immunodominant regions: LaA [amino acids (aa)1–107]; LaC (aa111–242); and LaL2/3 (aa346–408) [9]. Autoantibodies directed against the conserved winged-helix LaA determinant [10] are of particular significance because: they occur in ∼100% of precipitin-positive sera and appear to arise early in the anti-La response [9,11]; are present at the highest concentration (mg/ml range) of any determinant [12]; bind the analagous LaA apotope on the surface of apoptotic cells where they form immunoglobulin (Ig)G-immune complexes [13,14]; react with a conserved discontinuous epitope [15]; and are present in sera from ∼80% of mothers of children with congenital heart block [14].
While an early study reported restricted heterogeneity and kappa light chain oligoclonality of humoral responses against recombinant fragments encompassing LaC and LaL2/3[16], nothing is known at a molecular level about the variable (V) gene usage and V region mutational status of spontaneous human anti-La autoantibodies in patients with primary SS and degree of sharing of V region structures among anti-Ro52/Ro60/La responses. We have recently developed a proteomic method that allows near full-length protein sequencing of epitope-specific populations of autoantibodies purified from whole serum [17–19]. This approach utilizes high-resolution Orbitrap mass spectrometry (MS) and differs from traditional single-cell approaches by determining the molecular signature of the secreted autoantibody proteome. Our initial studies have shown that the response against an immunodominant determinant on Ro60, termed Ro60peg, is monoclonal, IgG1 kappa-restricted and specified by a characteristic HV3-23 heavy chain and KV3-20 light chain pairing that is shared (public) among unrelated patients [17]. Similarly, responses to Ro52/TRIM21 are restricted to two public IgG1 kappa clonotypes comprising a HV3-7 or HV3-23 encoded heavy chain paired with a KV3-20 light chain [18]. Direct autoantibody sequencing has also revealed common and random somatic mutations in the heavy and light chain complementarity determining regions (CDRs) of these autoantibodies, consistent with antigen-driven clonal selection. The aim of the current study was to determine whether humoral responses to the immunodominant LaA epitope comprise similar sets of public clonotypes and whether these share molecular signatures with anti-Ro52/60 autoantibody-specific proteomes. Accordingly, we have characterized serum Ig proteomes selected on LaA protein in patients with primary SS using MS and identified additional patterns of public clonotypes linking humoral anti-Ro/La autoimmunity.
Materials and methods
Patients and controls
Sera were collected from seven primary SS patients with anti-Ro/La autoantibodies who were positive for anti-LaA on a glutathione-S-transferase (GST)-LaA fusion protein enzyme-linked immunosorbent assay (ELISA). The relative affinities of autoantibodies directed against the LaA, LaC and LaL2/3 epitopes determined by the KCSN elution method [12] are included in Table S1. Patient characteristics and serological findings are presented in Table 1. Controls included two anti Ro/La-positive primary SS patients who were anti-LaA-negative, one asymptomatic donor with anti-Ro/La with anti-LaA and four healthy donors. Patients with primary SS fulfilled at least four of the six American–European Consensus Group Criteria [20]. None of the patients had evidence of malignant B cell disorders nor were being treated with steroids or other immunosuppressive drugs. The study was approved by the Southern Adelaide Human Research Ethics Committee.
Table 1.
Characteristics of patients with primary Sjögren's syndrome (SS)
| Duration of symptoms (years) | Autoantibodies (OD)* | Total IgG† (mg/ml) | RF† (IU/ml) | ||||
|---|---|---|---|---|---|---|---|
| Patient | Age/sex | Ro52 | Ro60 | LaA | |||
| SS127 | 68/female | 14 | 2·4 | 2·4 | 2·1 | 15·3 | 697 |
| SS118 | 82/female | 32 | 2·5 | 2·2 | 2·3 | 30·1 | 579 |
| SS102 | 54/ female | 15 | 2·4 | 2·4 | 1·7 | 23·8 | 154 |
| SS155 | 63/female | 19 | 2·4 | 2·4 | 2·3 | 31·1 | 421 |
| SS179 | 80/female | 31 | 2·1 | 2·1 | 2·5 | 50·4 | 1110 |
| SS137 | 23/female | 5 | 1·7 | 1·4 | 2·5 | 16·1 | 540 |
| SS107 | 69/ emale | 14 | 2·5 | 2·3 | 2·5 | 35·3 | 172 |
Autoantibodies were measured by enzyme-linked immunosorbent assay, with *optical density (OD) < 0·2 considered normal; †total immunoglobulin (Ig)G and rheumatoid factor (RF) were measured by nephelometry.
Preparation of affinity-purified anti-LaA autoantibodies
CNBr-activated Sepharose 4B beads (Pharmacia, Stockholm, Sweden) were coupled with the soluble recombinant GST-LaA fragment (aa 1–107), according to the manufacturer's recommendations. Five millilitres of serum from each patient or control subject was passed over the column and washed overnight with phosphate-buffered saline (PBS; pH 7·4). The bound IgG fraction was eluted with 0·1 M glycine in 0·5 M NaCl, pH 2·3 and neutralized in 1 M Tris HCl, pH 8·0. The resulting fraction was dialyzed against PBS overnight at 4 degrees and then concentrated in an Amicon-Ultra 15 centrifugal filter (Millipore, Bedford, MA, USA) to a final volume of ∼500 μl.
Analysis of specificity of affinity-purified anti-LaA by ELISA
The activity and specificity of affinity-purified IgGs were determined by testing the starting serum (1:500 dilution), flow-through fraction (volume normalized to the starting serum) and the eluted bound fraction (1:50 dilution) on two ELISA sets. The first tested the specificity of the affinity-purified IgG on various recombinant La fragments and native La. The various La fragments were expressed as GST fusion protein constructs prepared from the pGEX vector (New England BioLabs, Ipswich, MA, USA). The plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with 5 μg/ml of native La (Arotec Diagnostics, Wellington, New Zealand), GST-LaA, GST-LaC (aa111–242) and GST control in 0·03 M carbonate buffer (pH 8·2). Non-specific sites were blocked with 3% skimmed milk powder and duplicate wells were incubated with the different fractions. Bound IgG was detected with anti-human IgG conjugated with alkaline phosphatase, as described previously [21]. The second ELISA set tested the affinity-purified anti-LaA sample against native Ro60 (Arotec Diagnostics), full-length Ro52 expressed in a maltose binding protein (MBP) fusion construct from the pMAL vector (New England Biolabs) and MBP control.
Two-dimensional gel electrophoresis (2-DE)
Affinity-purified anti-LaA samples were precipitated using a 2-DE Clean Up Kit, according to the manufacturer's recommendations (Bio-Rad, Hercules, CA, USA), and were analysed using 2-DE as described previously [17].
Mass spectrometry (MS)
Samples were prepared for MS from either in-solution digests of affinity-purified IgGs or from heavy and light chain or intact immunoglobulin plugs excised from 2-DE gels, as described previously [18]. Analysis of peptides was carried out in a Thermo Orbitrap XL linear ion trap mass spectrometer fitted with a nanospray source (Thermo Scientific, Waltham, MA, USA), as detailed previously [18].
Protein sequence data analysis
Database searches were first carried out with Proteome Discoverer version 1·2 (Thermo Scientific) using the Sequest algorithm against two combined databases; the ImMunoGeneTics (IMGT) (http://www.imgt.org) and the Uniprot 2010–06 databases. The database search parameters were as follows: a maximum of two missed trypsin cleavages; cross-correlation scores (Xcorr) matches of >1·5, 2·0, 2·25, 2·5 and 2·75 for charge states of 1, 2, 3, 4 and 5, respectively; high peptide probabilities; and two or more unique peptides sequenced for each protein. The mass tolerance for peptide identification was 10 parts per million (ppm) for precursor ions and 0·01 Da for product ions. Searches were carried out with the following modifications: the oxidation of methionine; phosphorylation of serine, threonine and tyrosine; and carboxymethylation of cysteine.
De-novo sequencing and mutational analysis of purified IgGs
De-novo sequencing was performed on raw data files with peaks Studio version 5·3 (Bioinformatics Solutions, Waterloo, ON, Canada). As there is a large degree of sequence homology between the various Ig gene families the following strict set of rules were followed to minimize erroneous assignments of families: all spectra were inspected manually for quality; peptide lists were generated from the peaks software program; a minimum of two framework (FR) peptides were matched back to the germline sequence; and sequence homology had to be >80% when the sequences were Ig blasted (http://www.ncbi.nlm.nih.gov/projects/igblast) against the IMGT database. Further data refinement parameters were set in the peaks software program; scans were merged with a retention time of 1 min, a precursor m/z error tolerance of 10 ppm and a minimum charge state of 2. Scans were filtered for a precursor mass of between 350 and 5000 Da and a quality value of >0·65. Mutational analysis and deviations from the germline IMGT sequence were carried out using the spider search tool by searching against the combined IMGT/Uniprot 2010–06 databases with the following parameters: a homology match query type; a mass error tolerance of 0·01 Da; and the previously described variable modifications [18].
Results
Isolation of monospecific anti-LaA IgG from polyclonal anti-Ro/La sera
Previously, we have developed a proteomic approach to analyse self-reactive anti-Ro52/Ro60 clonotypes present in human polyclonal sera [17,18]. The first step involves affinity selection of autoantibodies specific for either an immunodominant determinant or the intact autoantigen, in this case the clinically important LaA epitope located at the NH2-terminus of La protein (Fig. 1a). Accordingly, anti-Ro/La-positive sera from seven subjects with primary SS were passed over individual GST-LaA columns followed by extensive washing, and eluted IgGs confirmed as specific for LaA by analysing starting serum, flow-through and eluted fractions on Ro52/Ro60/La ELISAs (Fig. 1b,c). In control experiments, no eluted IgG was detected after normal human sera (n = 4) or sera from primary SS patients without anti-LaA (n = 2) were passed over the GST-LaA column. Furthermore, no IgGs were detected in column eluates after anti-LaA-positive sera (n = 3) were passed over a sham GST column.
Fig. 1.
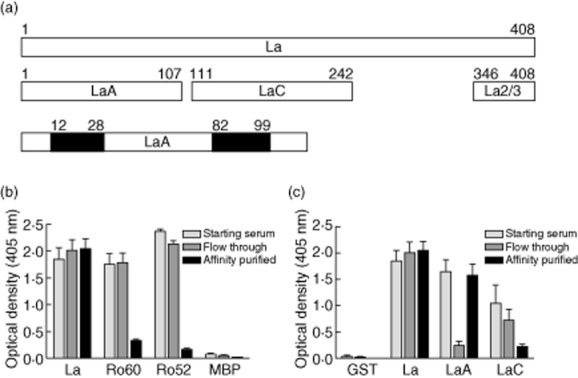
Specificity of anti-LaA immunoglobulin (Ig)Gs purified by LaA epitope-specific affinity chromatography from sera of patients with primary Sjögren's syndrome (SS) containing anti-Ro52/Ro60/La autoantibodies. (a) Immunodominant epitopes on La are mapped as LaA (aa 1–107), LaC (aa 111–242) and LaL2/3 (aa 346–408). LaA is an NH2-terminal conformation epitope requiring two regions of the protein to come together (filled areas) for immunoreactivity. (b) Purified IgGs tested by enzyme-linked immunosorbent assay (ELISA) for specificity to intact La using native La, native Ro60, maltose binding protein (MBP)-Ro52 and MBP control. The starting, flow-through and eluted (affinity-purified) fractions are compared. (c) Fine specificity of purified IgGs determined by ELISA using recombinant glutathione-S-transferase (GST)-full-length La, GST-LaA and GST-LaC proteins. Bars show the mean ± standard error of the mean of duplicate optical density values (n = 7 patients with primary SS).
The anti-LaA autoantibody population is biclonal and comprises unique heavy/light chain pairings
The clonality of the affinity-purified anti-LaA samples was then assessed using high-resolution 2-DE. Under reduced conditions, anti-LaA IgG resolved into several overlapping heavy chain species migrating at 55 kD with a range of isoelectric points (pI) from 7 to 8·5 (Fig. 2a). These spots probably represent charge variants due to post-translational modifications of oligoclonal species such as glycosylation, as has been observed previously for mouse monoclonal antibodies and a clonotypic anti-Ro60 autoantibody [17,22,23]. Light chains, evident at ∼25 kD, resolved into four equally spaced spots ranging in pI from 5·2 to 6·8, identified as IGKV3-15 (gel plugs 2 and 3) and IGKV3-20 (gel plugs 4 and 5) (Fig. 2a). The complete heavy chain area was gel excised and found to comprise two dominant V gene families, IGHV3-43 and IGHV3-30. In order to determine actual heavy and light chain pairing, non-reduced 2-DE gels of anti-LaA IgG were performed and revealed biclonal species of similar staining intensity migrating to 150 kD, one comprising a IGHV3-30/IGKV3-15 pairing and the second a IGHV3-43/IGKV3-20 pairing as identified by MS (Fig. 2b).
Fig. 2.
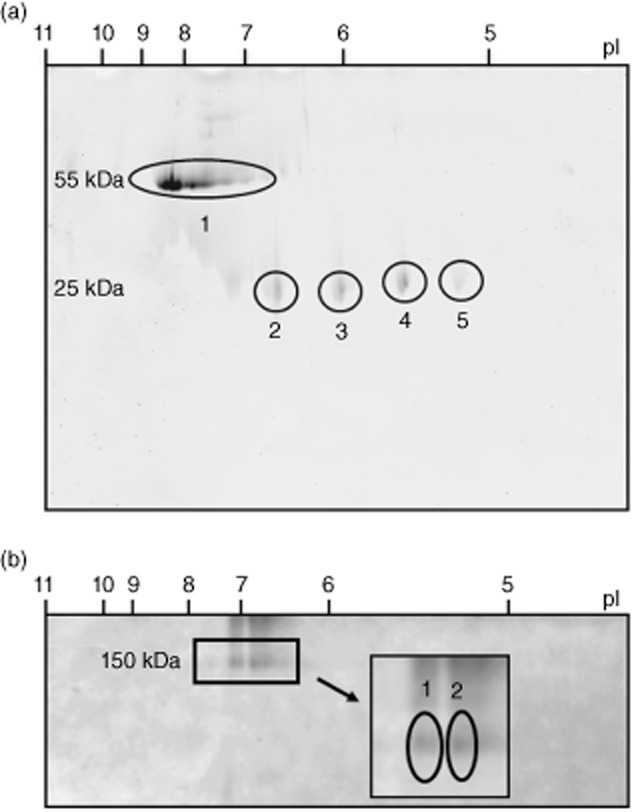
Representative two-dimensional (2-DE) gel electrophoresis gels of affinity-purified anti-LaA immunoglobulin (Ig)G from patient SS107. (a) Under reduced conditions, the heavy chain species running at ∼55 kDa (gel plug 1) identifies as IGHV3-43 and IGHV3-30, and the light chain species running at ∼25 kDa as IGKV3-15 (gel plugs 2, 3) and IGKV3-20 (gel plugs 4, 5). (b) Under non-reduced conditions the biclonal intact IgG species running at ∼150 kDa comprises IGHV3-30/IGKV3-15 (gel plug 1) and IGHV3-43/IGHV3-20 (gel plug 2) heavy and light chain pairings.
De-novo MS sequencing of LaA-specific clonotypic autoantibodies
In order to confirm both the clonality and heavy and light chain pairings of anti-LaA IgGs as detected on gel plug digests, and determine the extent of IgV-region somatic hypermutation, we performed in-solution trypsin digests of purified anti-LaA IgGs followed by combined database and de-novo Orbitrap MS sequencing, as described previously [17,18]. This confirmed the presence of the two IgG1 heavy chain species, IGHV3-30 and IGHV3-43, and two kappa light chains encoded by IGKV3-20 and IGKV3-15. JK2 and JK4 regions were identified from in-solution digests together with a single heavy chain JH2, indicating that the IGHV3-43 and IGHV3-30 clonotypes utilize a single J-region. Extensive aa replacement mutations within the V- and J- regions were identified using the spider analysis module (peaks software) in the anti-LaA samples from the seven patients with primary SS (Supporting information, Fig. S1a–e). Public (common) mutations that are shared among patients are tabulated by proteomic heat maps shown in Fig. 3. These are present in both FR and CDRs of each clonotypes; notably, a serine to asparagine substitution at aa31 in the CDR1 region of the IGKV3-20-encoded light chain was present in all seven primary SS patients, as was an arginine to lysine at aa16 in the FR1 region of the IGHV3-30-encoded heavy chain.
Fig. 3.
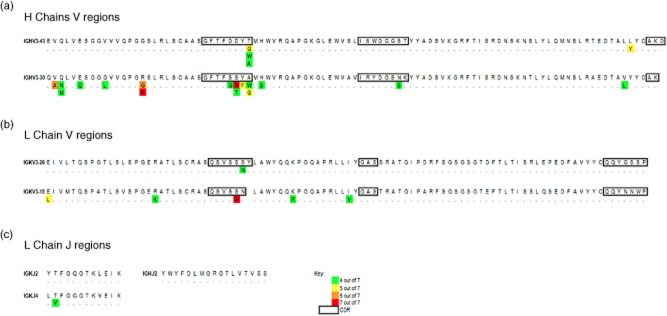
Variable (V)-region peptide heat map of compiled de-novo sequencing data from seven patients with primary Sjögren's syndrome (SS) showing public (shared) mutations. (a) Heavy chain V-region sequences align with germline HV3-43 and HV3-30 (IMGT database). (b) Light chain V-region sequences align with germline KV3-20 and KV3-15. (c) Light chain J-regions align with KJ2 and KJ4 germline sequence and heavy chain J-regions aligned with HJ2. Due to a lack of overlapping peptides from the CDR3 region into the J-regions, the exact pairing of V- and J- regions could not be determined. Common mutations divergent from the germline sequence are depicted in the text and colour-coded according to the frequency of the mutation detected in the primary SS patient cohort analysed. Dots indicate amino acids matching to the germline sequence. Complementary determining regions (CDR) regions are boxed.
Discussion
In this study, we have determined the secreted autoantibody proteome specific for an epitope on La protein that is expressed as an apotope on the surface of apoptotic cells and available for binding of maternal anti-La in congenital heart block. A combination of affinity purification, isoelectric focusing and de-novo MS sequencing identified two dominant public IgG1 kappa clonotypes in unrelated patients with primary SS: one specified by a heavy chain derived from the HV3-30 gene segment paired with a KV3-15 light chain; and the second comprising a HV3-43/KV3-20 pairing. These findings extend recent proteomic analyses of anti-Ro60 and anti-Ro52 autoantibodies that show similar properties of clonal restriction and expression of public clonotypes [17,18]. The MS approach used herein can identify fine clonotypic specificities undetectable by routine immunoassays, and has a key advantage over human hybridoma and recombinant antibody single-cell methods in that it represents the global autoantibody repertoire against a structurally defined determinant [19]. Furthermore, the proteomic approach can readily detect expressed public clonotypes in unrelated patients without selection bias and use their molecular signatures in a targeted MS diagnostic platform [18].
Combined data from the anti-Ro52/Ro60/La autoantibody proteomes tested thus far support a model of autoantibody production against linked protein-RNA complexes in systemic autoimmunity that is based on the utilization of a remarkably restricted set of autoreactive public B cell clonotypes, and challenge the general view that these responses are highly diversified, heterogeneous and polyclonal in outbred populations. The origin of these disease-specific, interlinked public clonotypes, that we consider hallmarks of long-term humoral autoimmunity, remains unknown. An implied corollary is that patients expressing public Ro/La-specific clonotypes share common pathways of autoimmunity to multiple determinants on Ro/La complexes. In brief, we propose that intrinsic defects in early B cell tolerance checkpoints in patients predisposed to Ro/La autoimmunity [24] may allow the emergence of low-affinity germline-encoded clonotypes that preferentially use certain heavy and light chain pairings; these may be exposed in secondary lymphoid organs to Ro52/Ro60/La determinants on poorly cleared apoptotic and/or necrotic remnants and undergo clonal selection, expansion and affinity maturation in dysregulated germinal centres [25]. The role of clonal selection in the generation of these clonotypes is supported by the presence of shared and random aa replacement mutations in the CDRs of the LaA-specific clonotypic IgGs, notably those from serine to arginine or asparagine that are known to produce changes in binding affinity [26]. Autoreactive memory B cells arising from primary immune responses in germinal centres may conceivably migrate to inflamed salivary glands in patients with primary SS, where they may reside for months or years before expansion to clones of plasma cells during recall responses, thereby maintaining long-lived humoral Ro/La autoimmunity. Interestingly, plasma cells specific for Ro52, Ro60 and La have been identified earlier in salivary glands from patients with seropositive primary SS, but their IgV region signatures have yet to be determined [27].
A key finding now emerging from the sequencing of Ro/La autoantibody proteomes is the shared expression of Ig light and heavy chain V and J gene segments across these responses. For example, anti-Ro52 proteomes have the same HV3-23/KV3-20 pairing signature as the anti-Ro60peg clonotype, and KV3-20 is shared by clonotypes that encompass the entire Ro52/Ro60/La response, with KV3-20 combined with KJ2 for anti-LaA/Ro60peg clonotypes [17,18]. In addition to the sharing of germline-encoded structures, which suggests a role for recombinatorial bias in the generation of these linked, autoreactive species, clonotypes are specified further by shared somatic mutations consistent with the notion that they are generated through independent antigen-driven clonal selection events. It is also conceivable that presentation of clonotypic V-region determinants on major histocompatibility complex (MHC) class II by Ro- or La-specific B cells to cognate idiotypic-specific T helper cells may, in part, drive determinant spreading in Ro/La humoral autoimmunity [28–30]. This receptor presentation model of autoantibody diversification provides an alternative to an intermolecular T–B cell help model based on MHC class II presentation of antigenic Ro or La peptide determinants to cognate T cells [5]. Further proteomic studies on other La epitope-selected autoantibodies will help to determine whether intramolecular responses beyond LaA, in particular high-affinity autoantibodies against the central LaC epitope, share public clonotypes. It will also be important to examine whether the findings for Ro/La are generalizable to other linked sets of human autoantibodies.
The affinity enrichment and MS sequencing protocol detailed herein can be used to determine IgV-region molecular signatures of secreted autoantibodies present in complex serum mixtures. In conjunction with a targeted mass spectrometry diagnostic platform that utilizes Ig proteome-derived V-region surrogate peptides, it potentially represents a major advance over routine immunoassays that have dominated autoimmune serology for decades. One limitation is that the methodology does not always obtain full sequence through the diversity (D) region [18], compounded by the marked DH segment variability of autoantibodies from patients with SLE [31]. Priority should be given to isolate autoantigen-specific cells from blood or inflamed salivary glands, using labelled Ro/La determinants or single-cell recombinant antibody techniques, potentially in combination with PCR-based methods to target specific gene families utilized by secreted Ro/La-specific clonotypes. Recently published refinements of the methodology have compared mass spectra data derived from mass spectrometry-based sequencing to reference V-region databases derived from next-generation sequencing of B cell repertoires [32–34]. Finally, it may be feasible to identify specific clonotypes in whole cell proteomes of patient memory B cells and/or plasma cells by targeted mass spectrometry (which can detect a peptide in a complex sample at pg/ml sensitivity), using mutated V-region surrogate peptides derived from serum anti-Ro/La clonotypes.
Acknowledgments
This work was supported by an Australian National Health and Medical Research Council grant 1041900 to T. P. Gordon and T. K. Chataway.
Disclosure
The sequencing method has been included in a United States Provisional Patent.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's website:
Fig. S1. De-novo sequencing data of anti-LaA immunoglobulin (Ig)Gs purified from the sera of seven patients with primary Sjögren's syndrome (SS), showing clonal restriction to (a) KV3-20; (b) KV3-15; (c) HV3-43; and (d) HV3-30 gene families. Public (shared) mutated V-region peptides collected from these sequences are tabulated by proteomic heat maps in Fig. 3. (e) Sequencing of J-regions reveals KJ2, KJ4 and HJ2. Dots indicate homology with the germline sequence while mutations divergent from the germline areindicated in the text. The sequence in red text indicates peptides that could not be assigned specifically to either light chain due to a high degree of sequence homology between KV3-20 and KV3-15. CDRs are boxed.
Table S1. Comparison of the relative affinities of anti-La immunoglobulin (Ig)G binding directed against immunodominant epitopes of La.
References
- 1.Alexander EL, Hirsch TJ, Arnett FC, Provost TT, Stevens MB. Ro(SSA) and La(SSB) antibodies in the clinical spectrum of Sjögrens syndrome. J Rheumatol. 1982;9:239–246. [PubMed] [Google Scholar]
- 2.Harley JB, Alexander EL, Bias WB, et al. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjogren's syndrome. Arthritis Rheum. 1986;29:196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- 3.Whittingham S, Naselli G, McNeilage LJ, Coppel RL, Sturgess AD. Serological diagnosis of primary Sjogren's syndrome by means of human recombinant La (SS-B) as nuclear antigen. Lancet. 1987;2:1–3. [PubMed] [Google Scholar]
- 4.Hendrick JP, Wolin SL, Rinke J, Lerner MR, Steitz JA. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981;1:1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCluskey J, Farris AD, Keech CL, et al. Determinant spreading: lessons from animal models and human disease. Immunol Rev. 1998;164:209–229. doi: 10.1111/j.1600-065x.1998.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 6.Schulte J, Fritzler M, Mahler M. Latest update on the Ro/SSA autoantibody system. Autoimmun Rev. 2009;8:632–637. doi: 10.1016/j.autrev.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Stefano JE. Purified lupus antigen La recognizes an oligouridylate strech common to the 3′ termini of RNA polymerase III transcripts. Cell. 1984;36:145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- 8.Roy R, Kozlov AG, Lohman TM, Ha T. SSB protein diffusion on single-stranded DNA stimulates RecA filament formation. Nature. 2009;461:1092–1097. doi: 10.1038/nature08442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeilage LJ, Macmillan EM, Whittingham SF. Mapping epitopes on the La (SS-B) autoantigen of primary Sjogrens syndrome: identification of a cross-reactive epitope. J Immunol. 1990;145:3829–3835. [PubMed] [Google Scholar]
- 10.Kenan DJ, Keene JD. La gets its wings. Nat Struct Mol Biol. 2004;11:303–305. doi: 10.1038/nsmb0404-303. [DOI] [PubMed] [Google Scholar]
- 11.Gordon TP, Mavrangelos C, McCluskey J. Restricted epitope recognition by precipitin-negative anti-La/SS-B-positive sera. Arthritis Rheum. 1992;35:663–666. doi: 10.1002/art.1780350609. [DOI] [PubMed] [Google Scholar]
- 12.Gordon TP, Greer M, Reynolds P, Guidolin A, McNeilage LJ. Estimation of amounts of anti-La (SS-B) antibody directed against immunodominant epitopes of the La(SS-B) autoantigen. Clin Exp Immunol. 1991;85:402–406. doi: 10.1111/j.1365-2249.1991.tb05739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed JH, Neufing PJ, Jackson MW, et al. Different temporal expression of immunodominant Ro60/60 kDa-SSA and La/SSB apotopes. Clin Exp Immunol. 2007;148:153–160. doi: 10.1111/j.1365-2249.2007.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neufing PJ, Clancy RM, Jackson MW, Tran HB, Buyon JP, Gordon TP. Exposure and binding of selected immunodominant La/SSB epitopes on human apoptotic cells. Arthritis Rheum. 2005;52:3934–3942. doi: 10.1002/art.21486. [DOI] [PubMed] [Google Scholar]
- 15.McNeilage LJ, Umapathysivam K, Macmillion E, Guidolin A, Whittingham S, Gordon T. Definition of a discontinuous immunodominant epitope at the NH2 terminus of the La/SS-B ribonucleoprotein autoantigen. J Clin Invest. 1992;89:1652–1656. doi: 10.1172/JCI115762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bini P, Chu J-L, Okolo C, Elkon K. Analysis of autoantibodies to recombinant La (SS-B) peptides in systemic lupus erythematosus and primary Sjogren's syndrome. J Clin Invest. 1990;85:325–333. doi: 10.1172/JCI114441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindop R, Arentz G, Chataway TK, et al. Molecular signature of a public clonotypic autoantibody in primary Sjogrens syndrome: a ‘forbidden’ clone in systemic autoimmunity. Arthritis Rheum. 2011;63:3477–3486. doi: 10.1002/art.30566. [DOI] [PubMed] [Google Scholar]
- 18.Arentz G, Thurgood LA, Lindop R, Chataway TK, Gordon TP. Secreted human Ro52 autoantibody proteomes express a restricted set of public clonotypes. J Autoimmun. 2012;39:466–470. doi: 10.1016/j.jaut.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Lindop R, Arentz G, Thurgood LA, Reed JH, Jackson MW, Gordon TP. Pathogenicity and proteomic signatures of autoantibodies to Ro and La. Immunol Cell Biol. 2012;90:304–309. doi: 10.1038/icb.2011.108. [DOI] [PubMed] [Google Scholar]
- 20.Vitali C, Bombardieri S, Jonsson R, et al. European Study Group on Classification Criteria for Sjögrens Syndrome. Classification criteria for Sjögrens syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed JH, Dudek NL, Osborne SE, et al. Reactivity with dichotomous determinants of Ro 60 stratifies autoantibody responses in lupus and primary Sjogrens syndrome. Arthritis Rheum. 2010;62:1448–1456. doi: 10.1002/art.27370. [DOI] [PubMed] [Google Scholar]
- 22.Layer A, Tissot JD, Schneider P, Duchosal MA. Micropurification and two-dimensional polyacrylamide gel electrophoresis of immunoglobulins for studying the clonal diversity of antigen-specific antibodies. J Immunol Methods. 1999;227:137–148. doi: 10.1016/s0022-1759(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Gaza-Bulseco G, Faldu D, Chumsae C, Sun J. Heterogeneity of monoclonal antibodies. J Pharm Sci. 2008;97:2426–2447. doi: 10.1002/jps.21180. [DOI] [PubMed] [Google Scholar]
- 24.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 26.Ho M, Kreitman RJ, Onda M, Pastan I. In vitro antibody evolution targeting germline hot spots to increase activity of an anti-CD22 immunotoxin. J Biol Chem. 2004;280:607–617. doi: 10.1074/jbc.M409783200. [DOI] [PubMed] [Google Scholar]
- 27.Tengner P, Halse A-K, Haga H-J, Jonsson R, Wahren-Herlenius M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjogren's syndrome. Arthritis Rheum. 1998;41:2238–2248. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 28.Xiang X, Smith DS, Guth A, Wysocki LJ. A receptor presentation hypothesis for T cell help that recruits autoreactive B cells. J Immunol. 2001;166:1562–1571. doi: 10.4049/jimmunol.166.3.1562. [DOI] [PubMed] [Google Scholar]
- 29.Kalsi JK, Hahn BH. The role of VH determinants in systemic lupus erythematosus. Lupus. 2002;11:878–884. doi: 10.1191/0961203302lu310rr. [DOI] [PubMed] [Google Scholar]
- 30.Munthe LA, Corthay A, Os A, Zangani M, Bogen B. Systemic autoimmune disease caused by autoreactive B cells that receive chronic help from Ig V region-specific T cells. J Immunol. 2005;175:2391–2400. doi: 10.4049/jimmunol.175.4.2391. [DOI] [PubMed] [Google Scholar]
- 31.Demaison C, Chastagner P, Theze J, et al. Somatic diversification in the heavy chain variable region genes expressed by human autoantibodies bearing a lupus-associated nephritogenic anti-DNA idiotype. Proc Natl Acad Sci USA. 1994;91:514–518. doi: 10.1073/pnas.91.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung WC, Beausoleil SA, Zhang X, et al. A proteomics approach for the identification and cloning of monoclonal antibodies from serum. Nat Biotechnol. 2012;30:447–452. doi: 10.1038/nbt.2167. [DOI] [PubMed] [Google Scholar]
- 33.Sato S, Beausoleil SA, Popova L, et al. Proteomics-directed cloning of circulating antiviral human monoclonal antibodies. Nat Biotechnol. 2012;30:1039–1043. doi: 10.1038/nbt.2406. [DOI] [PubMed] [Google Scholar]
- 34.Wine Y, Boutz DR, Lavinder JL, et al. Molecular deconvolution of the monoclonal antibodies that comprise the polyclonal serum response. Proc Natl Acad Sci USA. 2013;110:2993–2998. doi: 10.1073/pnas.1213737110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


