Abstract
Graves' disease (GD) is an autoimmune disease that involves aberrant B and T lymphocyte responses. Detailed knowledge about lymphocyte subpopulation composition will therefore enhance our understanding of the pathogenesis of GD and might support the development of new immunomodulatory treatment approaches. The aim of this study was to gain detailed insight into the composition of the peripheral blood lymphocyte compartment in GD before and during anti-thyroid drug therapy. Major B and T lymphocyte subpopulations were investigated by flow cytometry in peripheral blood from newly diagnosed GD patients (n = 5), GD patients treated with anti-thyroid drugs (n = 4), patients with recurrent GD (n = 7) and healthy controls (HC; n = 10). In GD patients, numbers of activated T lymphocytes [human leucocyte antigen D-related (HLA-DR)+ and CD25+] were increased. The B lymphocyte compartment in GD was characterized by significantly higher numbers of transitional (CD38highCD27−, P < 0·03) and pre-naive mature (CD38lowCD27−IgD+CD5+, P < 0·04) B lymphocytes, while memory populations were slightly decreased. The increased numbers of CD5+, transitional and pre-naive mature B lymphocytes correlated positively with fT4 plasma levels. GD is associated with increased numbers of activated T lymphocytes and transitional and pre-naive mature CD5+ B lymphocytes within the peripheral blood. The increase in CD5+ B lymphocytes was due mainly to an increase in transitional and pre-naive mature B lymphocytes. Increased fT4 plasma levels might be associated with this increase in transitional and pre-naive mature CD5+ B lymphocytes.
Keywords: B lymphocytes, Graves' disease, T lymphocytes
Introduction
Graves' disease (GD) is an autoimmune disease of the thyroid gland, characterized by hyperthyroidism, which can be accompanied by extra-thyroidal symptoms such as ophthalmopathy and dermopathy [1]. GD is an autoantibody-mediated autoimmune disease in which hyperthyroidism is caused by thyroid-stimulating hormone receptor (TSHR) specific autoantibodies (TRAb) which mimic the effects of pituitary TSH [1]. In addition, neutral and inhibiting TRAb and antibodies to other thyroid autoantigens such as thyroglobulin (Tg) and thyroid peroxidase (TPO) can also be present in the serum of GD patients. Thus, autoantibody-producing B lymphocytes are important contributors to GD pathogenesis. Moreover, lymphoid infiltrates in thyroid and retro-orbital tissue of GD patients consist of secondary lymphoid follicles containing both B and T lymphocytes [2,3]. Consequently, GD is currently considered as a B lymphocyte-mediated T lymphocyte-dependent autoimmune disease [4].
T lymphocyte involvement in GD is evident by infiltration of activated memory T lymphocytes [CD45RO+humal leucocyte antigen D-related (HLA-DR+)] into the thyroid gland [5,6]. Also, T lymphocytes in the peripheral blood exhibit signs of activation as reflected by increased expression of HLA-DR, CD25 and CD69 [7–12]. However, contradictory data exist on the composition of the peripheral blood T lymphocyte compartment. Naive and memory T lymphocyte subpopulations have been described as being normal, increased or decreased [7,8,10,13–15]. Also, regulatory T lymphocytes [Tregs; CD4+CD25+forkhead box protein 3 (FoxP3+)] have been described at normal or increased levels in GD [16,17]. Due to the central pathogenic role of TRAb in GD, T helper type 2 (Th2) lymphocytes are considered as important contributors to GD [4]. This is supported further by increased levels of typical Th2-associated cytokines such as interleukin (IL)-4, IL-5 and IL-13 in GD serum and the in-vitro production of these cytokines by stimulated T lymphocytes from GD patients. However, increased levels of the Th1-associated cytokines interferon (IFN)-γ and IL-12 have also been reported frequently, especially in serum from early GD patients with ophthalmopathy, suggesting the involvement of Th1 responses in GD as well [9,18–20].
The B lymphocyte-mediated immune response in GD is characterized by autoantibody formation and infiltration of memory, germinal centre and marginal zone B lymphocytes into the thyroid gland [21,22]. In addition, increased peripheral blood B lymphocyte numbers, in particular CD5+ B lymphocytes, have been reported in GD [23,24]. TRAb are mainly of the immunoglobulin (Ig)G1 subclass [25], an IgG subclass formed in the presence of the Th1 cytokine IFN-γ, which underscores the importance of T lymphocyte-dependent B lymphocyte responses in GD. The occurrence of IgM, IgA and IgE deposits in thyroid and extra-ocular muscle tissues indicate that B lymphocytes producing Ig subclasses other than IgG can also contribute to GD [26,27].
Despite the autoimmune pathogenesis of GD, current treatment modalities focus mainly on ablation of thyroid function by anti-thyroid drug therapy with thionamides, radioactive iodine therapy or thyroidectomy [28]. These therapies, however, do not largely affect the underlying pathogenic autoimmune response, although it has been suggested that thionamides have some immunomodulatory actions [28,29]. Currently, B cell-directed therapy with anti-CD20 (Rituximab) is investigated in GD ophthalmopathy. Early clinical studies report promising results on clinical improvement of ophthalmopathy, but the effects on hyperthyroidism are less pronounced [28,30].
In-depth knowledge with regard to alterations in the composition of the peripheral blood lymphocyte compartment in GD will contribute to improved understanding of its pathogenesis and may lead to new immunomodulatory treatment strategies. To date, however, detailed phenotypic studies on peripheral blood B and T lymphocyte subpopulations are lacking. In this study, we confirm activation of the T lymphocyte compartment in GD being present in non-treated and treated GD patients. Anti-thyroid drug therapy does thus not markedly affect the activation status of T lymphocytes. In addition, we demonstrate increased numbers of transitional and pre-naive mature B lymphocytes in GD, while memory B lymphocyte numbers are slightly decreased. The numbers of transitional and pre-naive mature B lymphocytes correlated positively with plasma fT4 levels in GD, suggesting that thyroid hormones influence B lymphocyte development.
Materials and methods
Patients and controls
Sixteen patients with Graves' disease (GD) and 10 healthy controls (HC) were included in this study. The GD patients were divided into three groups: a group of recently diagnosed patients prior to anti-thyroid drug therapy, a group that received anti-thyroid drug therapy for 2–4 months and a group of patients with recurrent GD receiving anti-thyroid drug therapy for a second period of time. Characteristics of the subjects are summarized in Table 1. GD was diagnosed based on typical clinical symptoms, including diffuse enlargement of the thyroid and homogeneous increased uptake in a [I123] thyroid scan combined with the presence of TRAb, suppressed TSH and increased free thyroxine (fT4) serum levels (Fig. 1a–c). One patient had clinically active ophthalmopathy. The patients had no co-existent autoimmune diseases and had not used corticosteroids or antibiotics during the last 3 months before study inclusion. All subjects gave their written informed consent. The study was approved by the medical ethical committee of the Erasmus Medical Center, Rotterdam, the Netherlands and the Reinier de Graaf hospital, Delft, the Netherlands.
Table 1.
Characteristics of Graves' disease (GD) patients and healthy controls (HC)
| HC | GD diagnosis | GD treatment | GD recurrent | |
|---|---|---|---|---|
| Group size | 10 | 5 | 4 | 7 |
| Age (year) | 31·5 ± 6·1 | 47·2 ± 6·5 | 30·8 ± 8·5 | 32·9 ± 8·7 |
| Gender | ||||
| Female | 8 | 4 | 4 | 4 |
| Male | 2 | 1 | 0 | 3 |
| Treatment | ||||
| Thiamazole | 3 | 6 | ||
| Propylthiouracil (PTU) | 2 | 1 | ||
| Levothyroxine | 1 | 5 | ||
| β-adrenergic blocking drugs | 1 | 1 | 1 | |
| Ophthalmopathy | 1 |
Fig. 1.
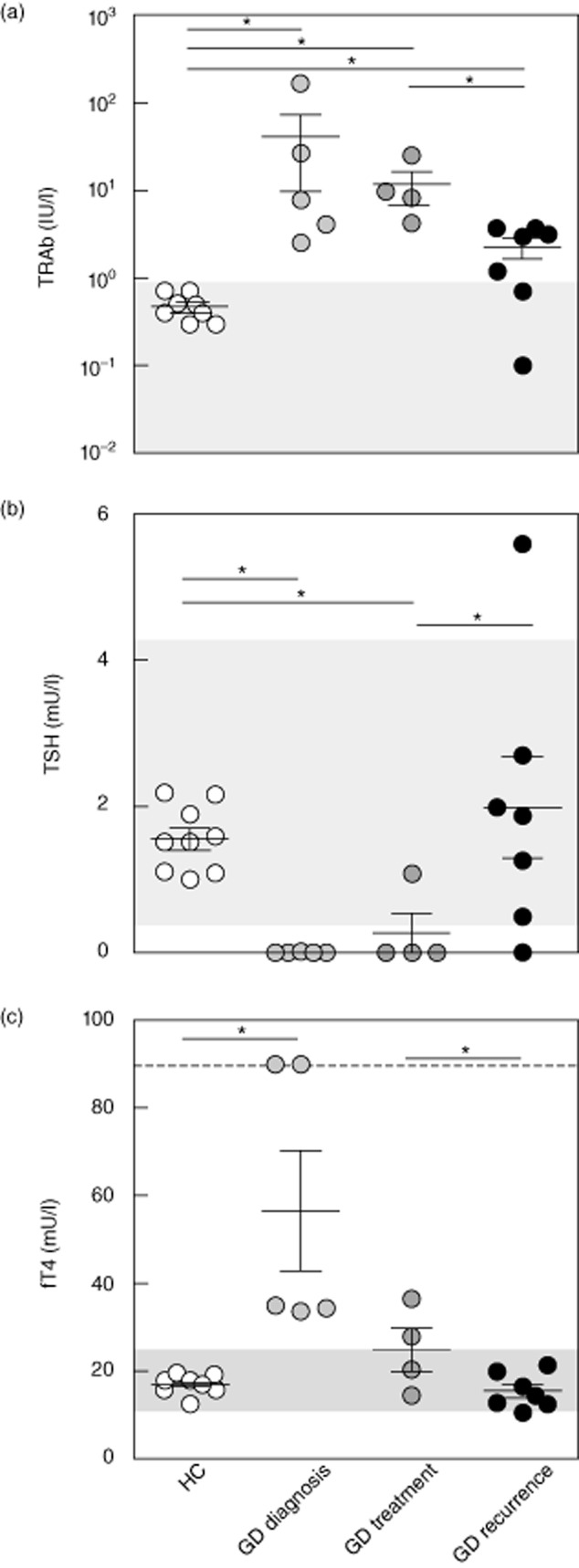
Serum levels of thyroid stimulating hormone receptor (TSHR)-specific autoantibodies (TRAb) (a), thyroid stimulating hormone (TSH) (b) and free thyroxine (fT4) (c) in healthy controls (HC) (n = 10), Graves' disease (GD) diagnosis (n = 5), GD treatment (n = 4) and GD recurrent (n = 7). Grey area = normal values; dashed line = upper limit. *P < 0·05.
Study design
Blood was collected prior to a diagnostic [I123] thyroid scan. All patients receiving anti-thyroid drug therapy stopped using thyroid hormone supplementation 3 weeks before and thyrostatics 1 week before the blood collection. Blood was collected in BD Vacutainer® SST™ II advance tubes for serum collection and in BD Vacutainer® lithium heparin tubes for collection of peripheral blood mononuclear cells (PBMCs; BD, Plymouth, UK) and processed further within 1 h after collection. Serum was isolated by centrifugation and frozen for further analyses. PBMCs were isolated using Ficoll density separation and viably frozen for further analyses.
Laboratory testing
TRAb were measured by radioimmunoassay with Dyno tests (BRAHMS, Berlin, Germany; normal range 0·0–0·9 IU/l). Serum-free T4 (fT4) and TSH were measured by chemiluminescence assays (Vitros ECi Immunodiagnostic System, Ortho-Clinical Diagnostics, Amersham, UK; TSH normal range 0·4–4·3 mU/l; fT4 normal range 11–25 pmol/l).
Flow cytometry
Total leucocyte count was determined in freshly collected blood using a CoulterCounter (Beckman Coulter B.V., Woerden, the Netherlands) and leucocyte subpopulations were identified by flow cytometry based on CD45 expression and side-scatter.
For immunophenotypic characterization of T and B lymphocyte subpopulations, viably frozen PBMCs were used. T lymphocyte subpopulations were defined as naive (TN; CD45RA+CCR7+CD27+CD28+), central memory (TCM; CD45RO+CCR7+CD27+CD28+), effector memory (TEM; CD45RO+CCR7−) and terminally differentiated (TTD; CD45RA+CCR7−). Regulatory T lymphocytes (Tregs; CD4+CD25+FoxP3+) were identified by intracellular detection of the transcription factor FoxP3 using a FoxP3 staining kit (eBioscience, San Diego, CA, USA). In order to identify Th1, Th2 and Th17 lymphocytes, PBMCs were stimulated with phorbol-12-myristate-13-acetate (PMA; 50 ng/ml; Sigma-Aldrich, Saint Louis, MO, USA) and ionomycin (500 ng/ml; Invitrogen Ltd, Paisley, UK) for 4 h in the presence of GolgiStop (BD Biosciences, San Jose, CA, USA). Thereafter, cells were first stained for extracellular markers, then fixed with 2% paraformaldehyde, permeabilized with 0·5% saponin, followed by intracellular staining for IFN-γ, IL-4 and IL-17A. Th1 T lymphocytes were defined as CD4+IFN-γ+, Th2 T lymphocytes as CD4+IL-4+ and Th17 T lymphocytes as CD4+IL-17A+ [31].
B lymphocyte subpopulations were defined as transitional (CD38highCD27−), pre-naive mature (CD38lowCD27−IgD+CD5+), naive mature (CD38lowCD27−IgD+CD5−), natural effector (CD38lowCD27+IgD+), IgG+CD27+ memory (CD38lowIgD−IgM−IgG+CD27+), IgA+CD27+ memory (CD38lowIgD−IgM-IgA+CD27+), IgG+CD27− memory (CD38lowIgD−IgM−IgG+CD27−), IgA+CD27− memory (CD38lowIgD−IgM−IgA+CD27−) and IgM+CD27+ memory (CD38lowIgD−IgM+CD27+) based on previous studies, with small adjustments [32].
For flow cytometric analyses, stained cells were measured using a fluorescence activated cell sorter (FACS) LSR-II (BD Biosciences) and data were analysed with BD FACSDiva software version 6·1.2 (BD Biosciences).
Cytokine analysis
Serum levels of IFN-γ, IL-4 and IL-17A were measured simultaneously using bead-based FlowCytomix simplex kits (Bender Medsystems GmbH, Vienna, Austria).
Statistical analysis
Subject characteristics are described as mean ± standard deviation (s.d.). The Kruskal–Wallis test was used to identify statistically significant differences between the four groups; when a difference was found the exact Mann–Whitney U-test was used to compare the different groups to each other. Statistics are displayed as median (range). All statistical analyses were performed with spss software version 15·0. A P-value < 0·05 (two-tailed) was considered statistically significant. Box-and-whisker plots display the 2·5–97·5 percentiles. Error bars are expressed as the standard error of the mean (s.e.m.).
Results
Patient characteristics
Newly diagnosed GD patients were hyperthyroid [fT4 35·0 mU/l (33·7–90·0 mU/l), GD diagnosis versus HC P = 0·001] with suppressed TSH levels [TSH 0·004 mU/l (0–0·004 mU/l), GD diagnosis versus HC P = 0·001] and increased TRAb levels [7·8 IU/l (2·6–167·8 IU/l), GD diagnosis versus HC P = 0·003; Fig. 1a–c]. In treated GD patients fT4 levels had normalized [24·2 mU/l (14·6–36·5 mU/l), GD treatment versus HC P = 0·15], while TSH levels were still suppressed [TSH 0 mU/l (0–1·09 mU/l), GD treatment versus HC P = 0·007] and TRAb levels were still slightly elevated [9·0 IU/l (4·2–25·0 IU/l), GD treatment versus HC P = 0·003; Fig. 1a–c]. Also in patients with recurrent GD, TRAb were slightly elevated [3·0 IU/l (0·1–3·7 IU/l), GD recurrent versus HC P = 0·02], while thyroid function was normal [fT4 14·6 mU/l (10·6–21·4 mU/l), GD recurrent versus HC P = 0·37] under anti-thyroid drug therapy (Fig. 1a–c).
A general flow cytometric blood analysis revealed no significant differences in cell numbers of the main leucocyte populations between the different groups of GD patients and HC (Fig. 2a). Within the lymphocyte compartment, total numbers of natural killer (NK) cells, B lymphocytes, CD4+ T lymphocytes and CD8+ T lymphocytes were comparable between the different groups of patients and HC (Fig. 2b).
Fig. 2.
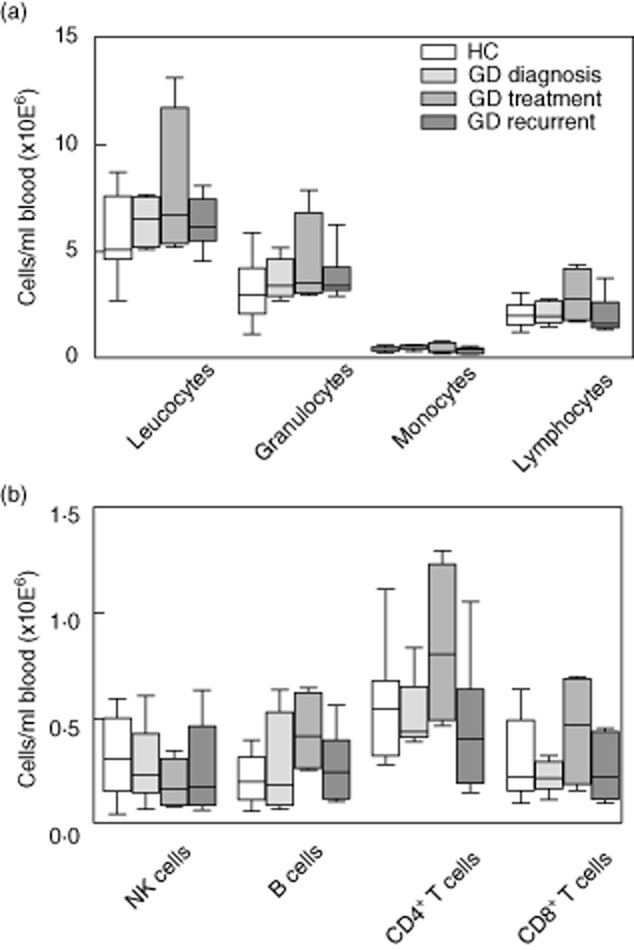
Absolute counts of leucocyte subpopulations (a) and lymphocyte subpopulations (b) in peripheral blood of healthy controls (HC) (n = 10), Graves' disease (GD) diagnosis (n = 5), GD treatment (n = 4), GD recurrent (n = 7). CD4 = CD4+ T lymphocytes; CD8 = CD8+ T lymphocytes. *P < 0·05.
GD is characterized by an activated T lymphocyte compartment
Newly diagnosed non-treated GD patients displayed elevated numbers of activated T lymphocytes compared to HC, as reflected by the significantly (P < 0·01) higher numbers of HLA-DR expressing CD4+ T lymphocytes and a trend towards increased numbers of CD25 expressing CD4+ T lymphocytes (Fig. 3a,b). Numbers of HLA-DR expressing CD4+ T lymphocytes normalized in GD patients treated with anti-thyroid drug therapy, while the trend of elevated CD4+CD25+ T lymphocyte numbers increased in this patient group (Fig. 3a,b). Similar trends in HLA-DR and CD25 expression were seen in CD8+ T lymphocytes (Fig. 3a,b).
Fig. 3.
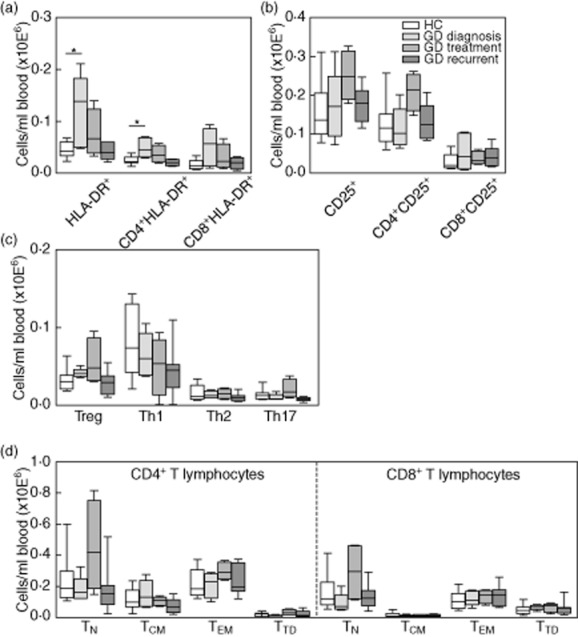
Absolute counts of human leucocyte antigen D-related (HLA-DR)+ T lymphocytes (a), CD25+ [forkhead box protein 3 (FoxP3)–] T lymphocytes (b), CD4+CD25+FoxP3+ regulatory T (Treg) lymphocytes, interferon (IFN)-γ-producing [T helper type 1 (Th1)] lymphocytes, interleukin (IL)-4-producing (Th2) lymphocytes and IL-17A-producing (Th17) lymphocytes (c) and naive and memory T lymphocyte subpopulations (d) in peripheral blood of healthy controls (HC) (n = 10), Graves' disease (GD) diagnosis (n = 5), GD treatment (n = 4) and GD recurrent (n = 7). CD4 = CD4+ T lymphocytes; CD8 = CD8+ T lymphocytes TN = naive T lymphocytes; TCM = central memory T lymphocytes; TEM = effector memory T lymphocytes; TTD = terminally differentiated T lymphocytes. *P < 0·05.
Absolute numbers of Tregs, Th1 (IFN-γ-producing), Th2 (IL-4-producing) and Th17 (IL-17A-producing) T lymphocytes were similar in all groups of GD patients and HC (Fig. 3c). In line with this, serum levels of IFN-γ, IL-4 and IL-17A did not differ between the study groups (data not shown).
Absolute numbers of CD4+ (Fig. 3d, left panel) and CD8+ (Fig. 3d, right panel) naive, central memory, effector memory and terminally differentiated T lymphocyte subpopulations were not significantly different in the different study groups. However, a trend towards increased numbers of naive T lymphocytes and CD31+ recent thymic emigrants (RTE) was seen in GD patients receiving anti-thyroid drug therapy (Fig. 3d, data not shown).
GD is characterized by increased transitional B lymphocytes and decreased memory B lymphocytes
Transitional B lymphocyte numbers were significantly (P = 0·01) increased in newly diagnosed untreated GD patients when compared to HC. Also a trend towards increased numbers of pre-naive mature and naive mature B lymphocytes was found (Fig. 4a). In contrast, a trend towards decreased memory B lymphocyte numbers was found. In GD patients receiving anti-thyroid drug therapy, transitional (P = 0·03) and pre-naive mature (P = 0·04) B lymphocyte numbers were increased compared to HC, while the trend towards decreased memory B lymphocytes as seen in the newly diagnosed group was normalized (Fig. 4a). In patients treated for recurrent GD, numbers of all B lymphocyte subpopulations were comparable to HC (Fig. 4a).
Fig. 4.
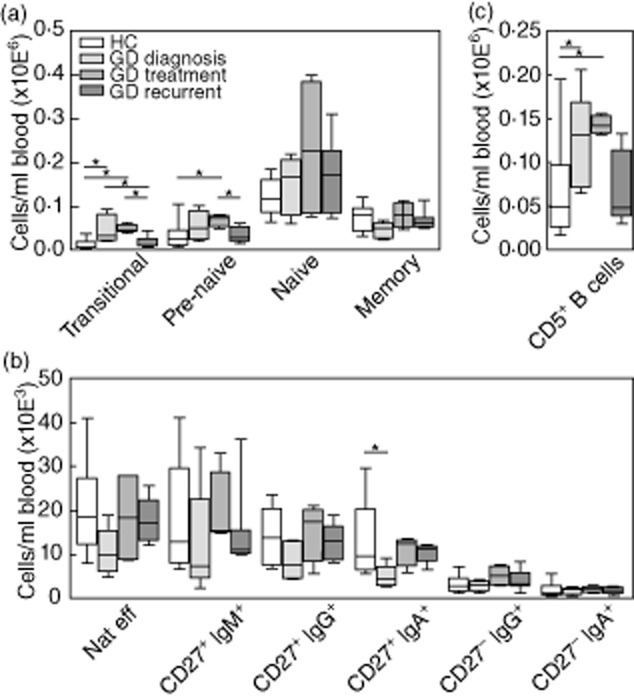
Absolute counts of transitional, pre-naive mature, naive mature and memory B lymphocytes (a), memory B lymphocyte subpopulations (b) and CD5+ B lymphocytes (c) in peripheral blood of healthy controls (HC) (n = 10), GD diagnosis (n = 5), GD treatment (n = 4) and GD recurrent (n = 7). *P < 0·05.
More detailed analysis of the major memory subpopulations showed a tendency towards decreased natural effector, CD27+IgM+, CD27+IgG+ and CD27+IgA+ memory B lymphocyte numbers in newly diagnosed GD compared to HC, treated GD and recurrent GD, being significant only for the CD27+IgA+ memory B lymphocyte population (GD diagnosis versus HC, P = 0·005, Fig. 4b). The examined CD27− memory B lymphocyte populations did not differ between the study groups.
GD has been associated previously with increased percentages of CD5+ B lymphocytes [33]. In line with this, we found increased CD5+ B lymphocyte numbers in newly diagnosed and treated GD patients (GD diagnosis versus HC P = 0·04; GD treatment versus HC P = 0·02, Fig. 4c). In humans, CD5 is expressed mainly on transitional and pre-naive mature B lymphocytes [34], which is in line with the increased numbers of transitional and pre-naive mature B lymphocytes we observed in these GD groups.
Increased transitional and pre-naive mature B lymphocytes correlate with fT4 plasma levels
Transitional B lymphocytes represent recent bone marrow (BM) emigrants [35]. The increased transitional B lymphocyte numbers in peripheral blood of GD patients may therefore result from enhanced B lymphocyte development and/or BM output in these patients. It has been demonstrated that thyroid hormones are capable of stimulating pro-B cell proliferation [36]. It could therefore be that thyroid hormones enhance B lymphocyte development and/or BM output in GD, resulting in increased numbers of transitional B lymphocytes in the blood. In support of this hypothesis, we found a positive correlation between fT4 plasma levels and total CD5+ (Fig. 5a; P < 0·01), transitional (Fig. 5b; P < 0·01) and pre-naive mature (Fig. 5c; P < 0·05) B lymphocyte numbers in our total study cohort (GD patients and HC).
Fig. 5.
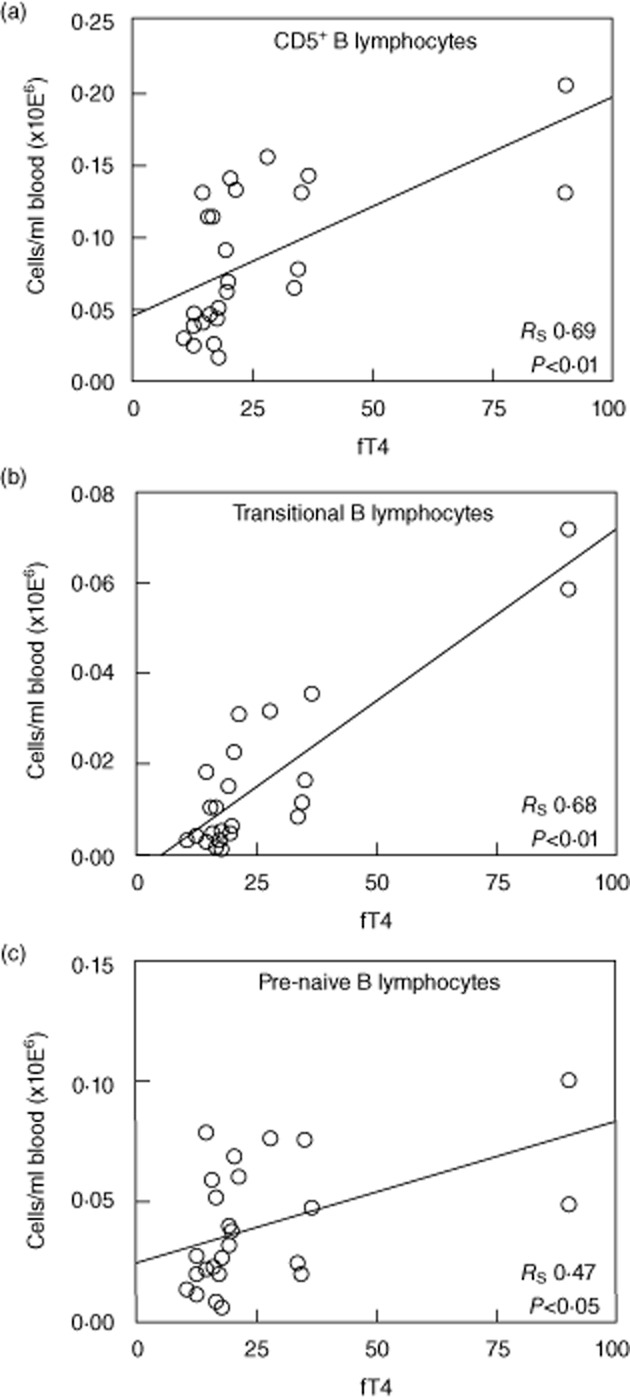
Correlation between free thyroxine (fT4) plasma levels and total CD5+ B lymphocytes (a), transitional B lymphocytes (b) and pre-naive B lymphocytes (c).
Discussion
In this study, combined immunophenotypic analysis of both the B and T lymphocyte compartments in peripheral blood from GD patients was performed and correlated with parameters of disease. We confirm the presence of increased activated T lymphocyte and CD5+ B lymphocyte numbers in peripheral blood from GD patients. Importantly, we demonstrate that the increase of CD5+ B lymphocytes in the blood of GD patients is largely attributable to increased numbers of transitional and pre-naive mature B lymphocytes. Moreover, the fT4 plasma levels in GD correlated positively with this increase in transitional and pre-naive mature B lymphocyte numbers.
The peripheral blood T lymphocyte compartment of the newly diagnosed untreated GD patients in our study showed increased numbers of activated CD4+ T lymphocytes (HLA-DR+ and CD25+), similar to other studies [5,7,11]. HLA-DR+ T lymphocyte numbers were normalized to the level found in HC in GD patients receiving anti-thyroid drug therapy. In concordance with this, a reduction of HLA-DR expression in thyrocytes has been described upon initiation of anti-thyroid drug therapy [37]. In contrast, CD25+ T lymphocyte numbers did not normalize in GD patients receiving anti-thyroid drug therapy. Previous studies have reported increased percentages of both HLA-DR+ and CD25+ T lymphocytes in GD patients receiving anti-thyroid drug therapy [7,11]. It is possible that differences in duration of treatment or methodological differences (percentage of cells versus absolute cell numbers) account for the discrepancies between studies. Nevertheless, all available data so far point to the presence of an activated T lymphocyte compartment in GD, and this present study shows that this is only marginally influenced by anti-thyroid drug therapy. These data suggest that T cell directed therapy could be attempted in GD.
An increased percentage of naive T lymphocytes and RTEs has been described in euthyroid GD patients receiving anti-thyroid drug therapy compared to HC [15]. Moreover, it has been demonstrated that TSH enhances human T lymphocyte development via functional TSHR expression on human thymocytes, suggesting that TRAb might also enhance T lymphocyte development and thus thymic output of naive T lymphocytes (van der Weerd et al. submitted) [38,39]. In line with this, our study revealed a trend towards increased naive T lymphocyte and RTE numbers in GD patients receiving anti-thyroid drug therapy compared to HC, albeit not statistically significant. Such a trend, however, was not observed in newly diagnosed GD patients. As the newly diagnosed GD patients were significantly older than the other groups (Table 1), we cannot formally exclude an age-dependent effect on naive T lymphocyte numbers in this group. However, only naive CD8+ T lymphocyte numbers correlated significantly with age (rs = −0·6; P < 0·01), while naive CD4+ T lymphocyte numbers (rs = −0·35; P = 0·08) and RTE numbers (rs = −0·26; P = 0·19) did not. Overall, these observations support the notion that circulating naive T lymphocyte and RTE populations are increased in patients receiving anti-thyroid drug therapy. Moreover, in our study, peripheral blood naive T lymphocyte and RTE numbers did not correlate with TRAb or fT4 levels. We therefore suggest that the increased naive T lymphocyte and RTE numbers merely reflect an effect of anti-thyroid drug therapy rather than being part of the pathogenesis of GD.
The peripheral blood B lymphocyte compartment of patients with GD has been shown previously to contain an increase in CD5+ B lymphocytes [24], which is in line with our observation. CD5 expression on peripheral blood B lymphocytes is confined specifically to transitional and pre-naive mature B lymphocytes [34]. Transitional B lymphocytes represent recent BM emigrants that subsequently differentiate via the intermediate pre-naive mature B lymphocyte stage into CD5− naive mature B lymphocytes [34,35]. In this study we have demonstrated that the increase in CD5+ B lymphocytes in GD is attributable to an increase in transitional and pre-naive mature B lymphocyte numbers. In contrast to this, memory subpopulations showed a tendency to decreased cell numbers in GD.
Autoantibody-mediated autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and Sjögren's syndrome (SS) have also been associated with increased percentages of CD5+ and transitional B lymphocytes, although absolute cell numbers were normal in these studies [33,34,40–42]. Several mechanisms have been suggested to explain this increase in transitional B lymphocytes in autoimmune diseases. Disturbed negative selection in the BM might account for increased BM emigration by immature B lymphocytes and subsequent peripheral blood entrance as transitional B lymphocytes [34,40,43]. In addition, chronic inflammation might enhance transitional B lymphocyte numbers, as it has been demonstrated that especially IL-4 facilitates survival of transitional and pre-naive mature B lymphocytes [34,40]. Moreover, factors such as B lymphocyte activating factor (BAFF) and macrophage migration inhibitory factor (MIF), both crucial survival factors for naive B lymphocytes [35,44], have been found elevated in GD serum [45,46]. In concordance with this, BAFF inhibition reduced total peripheral B lymphocyte numbers in a hyperthyroid GD mouse model [47]. These factors may thus contribute to the expansion of transitional and pre-naive mature B lymphocytes in GD.
In addition, elevated thyroid hormone levels in GD may also contribute to the increase in transitional B lymphocyte numbers. It has been demonstrated that hypothyroid hyt+/+ mice have decreased numbers of developing B lymphocyte progenitors in the BM which could be restored by thyroid hormone supplementation (own observations, data not shown) [48]. In line with this, reduced total BM cell numbers have been demonstrated in T3Rα−/− mice, and thyroid hormones have been found to stimulate pro-B lymphocyte proliferation, thereby increasing total numbers of B lymphocyte progenitors [36,49]. Moreover, hypothyroidism in humans is also associated with decreased bone marrow output [50]. Based on these data we hypothesized that increased thyroid hormone levels in GD influence bone marrow activity and thereby contribute to the increased transitional and pre-naive B lymphocyte numbers that we found in the peripheral blood from GD patients. This hypothesis is supported further by the significant positive correlation that we observed between fT4 levels and the number of total CD5+, transitional and pre-naive mature B lymphocytes.
Despite the important pathogenic role of autoantibodies in GD, we observed a slight reduction in memory B lymphocytes and plasma cells involved in Ig production in newly diagnosed GD patients compared to HC. It has, however, been shown that intra-thyroidal B lymphocytes consist mainly of marginal zone and memory B lymphocytes [21,22]. This suggests that redistribution of memory cells into thyroid tissue may be responsible for the reduction in peripheral blood memory B lymphocyte numbers in newly diagnosed GD.
In conclusion, combining detailed immunophenotypic analysis of B and T lymphocyte subpopulations in peripheral blood, we confirm the presence of an activated T lymphocyte compartment in GD patients, which is not affected significantly by anti-thyroid drug therapy. Moreover, we demonstrate an increase in transitional and pre-naive mature CD5+ B lymphocyte numbers in the peripheral blood of GD patients, which correlated positively with fT4 plasma levels. This suggests that fT4 plasma levels may contribute to the increase in transitional and pre-naive mature B lymphocytes.
Acknowledgments
The authors thank Sandra de Bruin-Versteeg for her assistance with the figures, Menno van Zelm for assistance with B lymphocyte staining and analyses, the endocrinologists and nuclear physicians who helped us with GD patients and Hans van Toor and Yolanda de Rijke for measuring T3, T4 and TSH levels in mouse and human sera. This work was supported by the ‘Nederlandse vereniging van Graves' patienten (NVGP)’ and internal grants from the Departments of Internal Medicine and Immunology. F. J. T. S. is supported in part by Kika, ZonMW and AICR. This work was supported by internal grants from the departments of Internal Medicine and Immunology.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Cooper DS. Hyperthyroidism. Lancet. 2003;362:459–468. doi: 10.1016/S0140-6736(03)14073-1. [DOI] [PubMed] [Google Scholar]
- 2.Armengol MP, Juan M, Lucas-Martin A, et al. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am J Pathol. 2001;159:861–873. doi: 10.1016/S0002-9440(10)61762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Skowronek I, Sierocinska-Sawa J, Szewczyk L, Korobowicz E. Interaction of lymphocytes and thyrocytes in Graves' disease and nonautoimmune thyroid diseases in immunohistochemical and ultrastructural investigations. Horm Res. 2009;71:350–358. doi: 10.1159/000223420. [DOI] [PubMed] [Google Scholar]
- 4.Klecha AJ, Barreiro Arcos ML, Frick L, Genaro AM, Cremaschi G. Immune-endocrine interactions in autoimmune thyroid diseases. Neuroimmunomodulation. 2008;15:68–75. doi: 10.1159/000135626. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa N, Eguchi K, Ueki Y, et al. Expression of adhesion molecules on infiltrating T cells in thyroid glands from patients with Graves' disease. Clin Exp Immunol. 1993;94:363–370. doi: 10.1111/j.1365-2249.1993.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marazuela M, Postigo AA, Acevedo A, Diaz-Gonzalez F, Sanchez-Madrid F, de Landazuri MO. Adhesion molecules from the LFA-1/ICAM-1,3 and VLA-4/VCAM-1 pathways on T lymphocytes and vascular endothelium in Graves' and Hashimoto's thyroid glands. Eur J Immunol. 1994;24:2483–2490. doi: 10.1002/eji.1830241034. [DOI] [PubMed] [Google Scholar]
- 7.Gessl A, Wilfing A, Agis H, et al. Activated naive CD4+ peripheral blood T cells in autoimmune thyroid disease. Thyroid. 1995;5:117–125. doi: 10.1089/thy.1995.5.117. [DOI] [PubMed] [Google Scholar]
- 8.Ludgate ME, McGregor AM, Weetman AP, et al. Analysis of T cell subsets in Graves' disease: alterations associated with carbimazole. BMJ (Clin Res Ed) 1984;288:526–530. doi: 10.1136/bmj.288.6416.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia N, Zhou S, Liang Y, et al. CD4+ T cells and the Th1/Th2 imbalance are implicated in the pathogenesis of Graves' ophthalmopathy. Int J Mol Med. 2006;17:911–916. [PubMed] [Google Scholar]
- 10.Bossowski A, Urban M, Stasiak-Barmuta A. Analysis of changes in the percentage of B (CD19) and T (CD3) lymphocytes, subsets CD4, CD8 and their memory (CD45RO), and naive (CD45RA) T cells in children with immune and non-immune thyroid diseases. J Pediatr Endocrinol Metab. 2003;16:63–70. doi: 10.1515/jpem.2003.16.1.63. [DOI] [PubMed] [Google Scholar]
- 11.Gessl A, Waldhausl W. Elevated CD69 expression on naive peripheral blood T-cells in hyperthyroid Graves' disease and autoimmune thyroiditis: discordant effect of methimazole on HLA-DR and CD69. Clin Immunol Immunopathol. 1998;87:168–175. doi: 10.1006/clin.1998.4524. [DOI] [PubMed] [Google Scholar]
- 12.Weetman AP. Cellular immune responses in autoimmune thyroid disease. Clin Endocrinol (Oxf) 2004;61:405–413. doi: 10.1111/j.1365-2265.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 13.Fournier C, Chen H, Leger A, Charreire J. Immunological studies of autoimmune thyroid disorders: abnormalities in the inducer T cell subset and proliferative responses to autologous and allogeneic stimulation. Clin Exp Immunol. 1983;54:539–546. [PMC free article] [PubMed] [Google Scholar]
- 14.Calder EA, Irvine WJ, Davidson NM, Wu F. T, B and K cells in autoimmune thyroid disease. Clin Exp Immunol. 1976;25:17–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Armengol MP, Sabater L, Fernandez M, et al. Influx of recent thymic emigrants into autoimmune thyroid disease glands in humans. Clin Exp Immunol. 2008;153:338–350. doi: 10.1111/j.1365-2249.2008.03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marazuela M, Garcia-Lopez MA, Figueroa-Vega N, et al. Regulatory T cells in human autoimmune thyroid disease. J Clin Endocrinol Metab. 2006;91:3639–3646. doi: 10.1210/jc.2005-2337. [DOI] [PubMed] [Google Scholar]
- 17.Pan D, Shin YH, Gopalakrishnan G, Hennessey J, De Groot LJ. Regulatory T cells in Graves' disease. Clin Endocrinol (Oxf) 2009;71:587–593. doi: 10.1111/j.1365-2265.2009.03544.x. [DOI] [PubMed] [Google Scholar]
- 18.Roura-Mir C, Catalfamo M, Sospedra M, Alcalde L, Pujol-Borrell R, Jaraquemada D. Single-cell analysis of intrathyroidal lymphocytes shows differential cytokine expression in Hashimoto's and Graves' disease. Eur J Immunol. 1997;27:3290–3302. doi: 10.1002/eji.1830271228. [DOI] [PubMed] [Google Scholar]
- 19.Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto's thyroiditis (Th1) and Graves' disease (Th2) Neuroimmunomodulation. 2004;11:209–213. doi: 10.1159/000078438. [DOI] [PubMed] [Google Scholar]
- 20.Hidaka Y, Okumura M, Shimaoka Y, Takeoka K, Tada H, Amino N. Increased serum concentration of interleukin-5 in patients with Graves' disease and Hashimoto's thyroiditis. Thyroid. 1998;8:235–239. doi: 10.1089/thy.1998.8.235. [DOI] [PubMed] [Google Scholar]
- 21.Segundo C, Rodriguez C, Garcia-Poley A, et al. Thyroid-infiltrating B lymphocytes in Graves' disease are related to marginal zone and memory B cell compartments. Thyroid. 2001;11:525–530. doi: 10.1089/105072501750302813. [DOI] [PubMed] [Google Scholar]
- 22.Segundo C, Rodriguez C, Aguilar M, et al. Differences in thyroid-infiltrating B lymphocytes in patients with Graves' disease: relationship to autoantibody detection. Thyroid. 2004;14:337–344. doi: 10.1089/105072504774193159. [DOI] [PubMed] [Google Scholar]
- 23.Mori H, Amino N, Iwatani Y, et al. Increase of peripheral B lymphocytes in Graves' disease. Clin Exp Immunol. 1980;42:33–40. [PMC free article] [PubMed] [Google Scholar]
- 24.Iwatani Y, Amino N, Kaneda T, et al. Marked increase of CD5+ B cells in hyperthyroid Graves' disease. Clin Exp Immunol. 1989;78:196–200. [PMC free article] [PubMed] [Google Scholar]
- 25.Weetman AP, Yateman ME, Ealey PA, et al. Thyroid-stimulating antibody activity between different immunoglobulin G subclasses. J Clin Invest. 1990;86:723–727. doi: 10.1172/JCI114768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raikow RB, Dalbow MH, Kennerdell JS, et al. Immunohistochemical evidence for IgE involvement in Graves' orbitopathy. Ophthalmology. 1990;97:629–635. doi: 10.1016/s0161-6420(90)32548-4. [DOI] [PubMed] [Google Scholar]
- 27.Werner SC, Wegelius O, Fierer JA, Hsu KC. Immunoglobulins (E,M,G) and complement in the connective tissues of the thyroid in Graves's disease. N Engl J Med. 1972;287:421–425. doi: 10.1056/NEJM197208312870901. [DOI] [PubMed] [Google Scholar]
- 28.Hegedus L. Treatment of Graves' hyperthyroidism: evidence-based and emerging modalities. Endocrinol Metab Clin North Am. 2009;38:355–371. doi: 10.1016/j.ecl.2009.01.009. ix. [DOI] [PubMed] [Google Scholar]
- 29.Laurberg P. Remission of Graves' disease during anti-thyroid drug therapy. Time to reconsider the mechanism? Eur J Endocrinol. 2006;155:783–786. doi: 10.1530/eje.1.02295. [DOI] [PubMed] [Google Scholar]
- 30.Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of graves ophthalmopathy. Med Clin North Am. 2012;96:311–328. doi: 10.1016/j.mcna.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Weerd K, Dik WA, Schrijver B, et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes. 2012;61:401–408. doi: 10.2337/db11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkowska MA, Driessen GJ, Bikos V, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dono M, Cerruti G, Zupo S. The CD5+ B-cell. Int J Biochem Cell Biol. 2004;36:2105–2111. doi: 10.1016/j.biocel.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182:4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 35.Berkowska MA, van der Burg M, van Dongen JJ, van Zelm MC. Checkpoints of B cell differentiation: visualizing Ig-centric processes. Ann NY Acad Sci. 2011;1246:11–25. doi: 10.1111/j.1749-6632.2011.06278.x. [DOI] [PubMed] [Google Scholar]
- 36.Foster MP, Montecino-Rodriguez E, Dorshkind K. Proliferation of bone marrow pro-B cells is dependent on stimulation by the pituitary/thyroid axis. J Immunol. 1999;163:5883–5890. [PubMed] [Google Scholar]
- 37.Zantut-Wittmann DE, Tambascia MA, da Silva Trevisan MA, Pinto GA, Vassallo J. Antithyroid drugs inhibit in vivo HLA-DR expression in thyroid follicular cells in Graves' disease. Thyroid. 2001;11:575–580. doi: 10.1089/105072501750302886. [DOI] [PubMed] [Google Scholar]
- 38.Murakami M, Hosoi Y, Negishi T, et al. Thymic hyperplasia in patients with Graves' disease. Identification of thyrotropin receptors in human thymus. J Clin Invest. 1996;98:2228–2234. doi: 10.1172/JCI119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wortsman J, McConnachie P, Baker JR, Jr, Burman KD. Immunoglobulins that cause thymocyte proliferation from a patient with Graves' disease and an enlarged thymus. Am J Med. 1988;85:117–121. doi: 10.1016/0002-9343(88)90516-5. [DOI] [PubMed] [Google Scholar]
- 40.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youinou P, Mackenzie L, le Masson G, et al. CD5-expressing B lymphocytes in the blood and salivary glands of patients with primary Sjogren's syndrome. J Autoimmun. 1988;1:185–194. doi: 10.1016/0896-8411(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 42.Plater-Zyberk C, Maini RN, Lam K, Kennedy TD, Janossy G. A rheumatoid arthritis B cell subset expresses a phenotype similar to that in chronic lymphocytic leukemia. Arthritis Rheum. 1985;28:971–976. doi: 10.1002/art.1780280903. [DOI] [PubMed] [Google Scholar]
- 43.Dorner T, Jacobi AM, Lee J, Lipsky PE. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J Immunol Methods. 2011;363:187–197. doi: 10.1016/j.jim.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Sapoznikov A, Pewzner-Jung Y, Kalchenko V, Krauthgamer R, Shachar I, Jung S. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat Immunol. 2008;9:388–395. doi: 10.1038/ni1571. [DOI] [PubMed] [Google Scholar]
- 45.Vannucchi G, Covelli D, Curro N, et al. Serum BAFF concentrations in patients with Graves' disease and orbitopathy before and after immunosuppressive therapy. J Clin Endocrinol Metab. 2012;97:E755–759. doi: 10.1210/jc.2011-2614. [DOI] [PubMed] [Google Scholar]
- 46.van der Gaag R, Broersma L, Mourits MP, et al. Circulating monocyte migration inhibitory factor in serum of Graves' ophthalmopathy patients: a parameter for disease activity? Clin Exp Immunol. 1989;75:275–279. [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert JA, Kalled SL, Moorhead J, et al. Treatment of autoimmune hyperthyroidism in a murine model of Graves' disease with tumor necrosis factor-family ligand inhibitors suggests a key role for B cell activating factor in disease pathology. Endocrinology. 2006;147:4561–4568. doi: 10.1210/en.2006-0507. [DOI] [PubMed] [Google Scholar]
- 48.Montecino-Rodriguez E, Clark RG, Powell-Braxton L, Dorshkind K. Primary B cell development is impaired in mice with defects of the pituitary/thyroid axis. J Immunol. 1997;159:2712–2719. [PubMed] [Google Scholar]
- 49.Arpin C, Pihlgren M, Fraichard A, et al. Effects of T3R alpha 1 and T3R alpha 2 gene deletion on T and B lymphocyte development. J Immunol. 2000;164:152–160. doi: 10.4049/jimmunol.164.1.152. [DOI] [PubMed] [Google Scholar]
- 50.Stagi S, Azzari C, Bindi G, et al. Undetectable serum IgA and low IgM concentration in children with congenital hypothyroidism. Clin Immunol. 2005;116:94–98. doi: 10.1016/j.clim.2005.03.003. [DOI] [PubMed] [Google Scholar]


