Abstract
Extensive evidence suggests that the immune system exerts powerful effects on bone cells, particularly in chronic disease pathologies such as rheumatoid arthritis (RA). The chronic inflammatory state in RA, particularly the excessive production of T cell-derived proinflammatory cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-17, triggers bone erosions through the increased stimulation of osteoclast formation and activity. While evidence supports a role for IL-17 and TNF-α secreted by conventional CD4+ T cells in RA, recent evidence in animal models of RA have implicated γδ T cells as a major producer of pathogenic IL-17. However, the capacity of γδ T cells to influence osteoclast formation and activity in humans has not yet been investigated widely. To address this issue we investigated the effects of γδ T cells on osteoclast differentiation and resorptive activity. We have demonstrated that anti-CD3/CD28-stimulated γδ T cells or CD4+ T cells inhibit human osteoclast formation and resorptive activity in vitro. Furthermore, we assessed cytokine production by CD3/CD28-stimulated γδ T cells and observed a lack of IL-17 production, with activated γδ T cells producing abundant interferon (IFN)-γ. The neutralization of IFN-γ markedly restored the formation of osteoclasts from precursor cells and the resorptive activity of mature osteoclasts, suggesting that IFN-γ is the major factor responsible for the inhibitory role of activated γδ T cells on osteoclastogenesis and resorptive activity of mature osteoclasts. Our work therefore provides new insights on the interactions between γδ T cells and osteoclasts in humans.
Keywords: γδ T cells, CD4+ T cells, IFN-γ, osteoclast, osteoimmunology
Introduction
Bone tissue is constantly remodelled during life through the activity of bone matrix-producing osteoblasts and bone-resorbing osteoclasts [1]. Osteoclasts are large multi-nucleated cells that are generated from the fusion of monocytic-lineage precursor cells, the process of which relies on the availability on two essential factors: macrophage colony-stimulating factor (M-CSF), which binds the c-Fms receptor expressed on osteoclast precursors [2], and receptor activator of nuclear factor κB ligand (RANKL), which binds the RANK receptor [3].
In recent years a variety of studies have demonstrated that the immune system is involved intimately in the regulation of bone cells, with a particular focus on how T cells interact with osteoclasts and influence their formation and/or activity. In chronic inflammatory disease states, such as rheumatoid arthritis (RA), the prevalent effect of activated CD4+ T cells is stimulatory towards the formation and activity of osteoclasts, resulting in bone loss and degradation of joint architecture [4]. However, evidence surrounding the exact role of activated T cells for influencing osteoclast formation is currently conflicting, with numerous in-vitro studies reporting either pro- or anti-osteoclastogenic effects of activated CD4+ T cells [5,6]. Activated CD4+ T cells produce RANKL [4] and various cytokines, including interleukin (IL)-17 [7], tumour necrosis factor (TNF)-α [8] and IL-1β [9] that support osteoclast formation and activity through direct or indirect mechanisms. However, CD4+ T cells also produce a variety of cytokines with anti-osteoclastogenic effects, such as interferon (IFN)-γ [6], granulocyte–macrophage colony-stimulating factor (GM-CSF) [10], IL-4 [11] and IL-10 [12].
While research has focused predominantly on the role of CD4+ T cells in inflammatory arthritis, a recent study implicated a further subset of T cells, so-called γδ T cells, as important mediators of the disease pathology in the collagen-induced arthritis (CIA) model of inflammatory arthritis [13]. Through production of IL-17, γδ T cells drive bone loss by increasing the production of RANKL by osteoblasts and/or stromal cells and inducing sustained osteoclast formation. In humans, γδ T cells represent a small subset of T cells (up to 5% of the total peripheral blood T cells), which express a heterodimeric T cell receptor (TCR) composed of a particular γ and δ chain, in contrast to conventional CD4+ T cells that express a heterodimeric TCR composed of an α and a β chain [14]. In support of a role of γδ T cells in the disease pathology of human RA, γδ T cells are present in the inflamed joints of rheumatoid arthritis patients [15,16], and have been shown to be capable of producing IL-17 upon activation under extreme polarizing conditions [17,18]. Furthermore, the activation of γδ T cells and its potential relevance to human health is of great clinical interest, particularly as the major subset of γδ T cells in human peripheral blood (Vγ9Vδ2+) are activated by anti-resorptive nitrogen–bisphosphonate drugs (N-BPs) [19,20], which are used widely to treat a variety of bone diseases characterized by excessive osteoclast activity. However, currently the role of human γδ T cells for influencing osteoclast formation and activity has not been elucidated.
In this study we show that activated γδ T cells exert inhibitory effects on osteoclast formation and resorptive activity comparable to activated CD4+ T cells, which is mediated primarily through production of IFN-γ by activated γδ T cells. Despite producing a variety of pro-osteoclastogenic cytokines upon activation, freshly isolated γδ T cells consistently failed to produce IL-17 ex vivo, suggesting important functional differences between murine and human γδ T cells for influencing osteoclastogenesis in inflammatory conditions.
Materials and methods
T cell isolation and activation
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats obtained from healthy donors by density-gradient separation using Lymphoprep™ reagent (Axis-Shield, Dundee, UK). Buffy coats were provided by the Scottish National Blood Transfusion Service and all studies were approved by the North of Scotland Research Ethics Committee. γδ T cells and CD4+ T cells were positively isolated using magnetic-activated cell sorting (MACS®) (Miltenyi Biotec, Bergisch Gladbach, Germany) using anti-γδ-TCR and anti-CD4-TCR beads, respectively. To increase the purity of γδ and CD4+ T cell isolations, CD14+ cells were depleted prior to T cell isolation, using anti-CD14+ Dynabeads® (Invitrogen, Carlsbad, CA, USA). The purity of the isolated T cell subsets was assessed by flow cytometry, with routine purities of ≥95% obtained for γδ T cells and >98% for CD4+ T cells. Assessment of monocyte and B cell contamination in the γδ T cell cultures was investigated using anti-CD14-Pacific Orange (Invitrogen) and anti-CD19-phycoerythrin (PE)-CF594 (BD Biosciences, San Jose, CA, USA) antibodies, respectively, and was found routinely to be approximately 1% for both monocytes and B cells. γδ and CD4+ T cells were activated with anti-CD3/anti-CD28-coated T-Activator Dynabeads® (Invitrogen) at a bead-to-cell ratio of 1:1 for 24 h, prior to incubation with autologous osteoclast precursors or mature osteoclasts. Activation of T cells was assessed using an anti-human CD69-PE antibody (BD Biosciences) and subsequent flow cytometric analysis using a LSR II flow cytometer (BD Biosciences).
Isolation of osteoclast precursors and generation of mature osteoclasts
Osteoclast precursors were isolated from buffy coats from healthy donors using Lymphoprep™ density-gradient separation and cultured in α-minimum essential medium (Sigma, St Louis, MO, USA) supplemented with 100 μg/ml L-glutamine (Sigma), 100 U/ml penicillin/streptomycin (Sigma) and 10% (v/v) heat-inactivated fetal bovine serum (FBS). Osteoclast precursors were expanded prior to generation of osteoclasts as reported previously [21]. Briefly, osteoclast precursors were treated with 20 ng/ml recombinant human (rh)M-CSF (R&D Systems, Abingdon, UK) for 5–7 days. To stimulate osteoclast formation, osteoclast precursors were cultured in a 96-well plate at 2 × 104 cells/well and treated with 2 ng/ml recombinant mouse (rm)RANKL (R&D Systems) for 5–6 days in the presence of 5 × 104 autologous unstimulated or activated γδ T cells or CD4+ T cells. Cells were subject to half-medium changes and supplemented with fresh cytokines every 48 h. In some experiments osteoclast precursor cells were incubated with 10% (v/v) conditioned media from γδ or CD4+ T cells stimulated for 24 h with anti-CD3/anti-CD28-coated T-Activator Dynabeads® (Invitrogen) and 10 U/ml of IL-2 (Sigma-Aldrich, Gillingham, UK).
For cytokine neutralization studies, the following antibodies were used: affinity-purified polyclonal goat anti-human IFN-γ antibody (10 μg/ml; R&D Systems), anti-human GM-CSF (1 μg/ml) and rat IgG2a isotype control (1 μg/ml) (both eBioscience, Vienna, Austria).
Osteoclast resorptive activity
Mature osteoclasts were generated on plastic for 5 days in the presence of 20 ng/ml rhM-CSF and 2 ng/ml rmRANKL, as reported previously [21]. Following this period, differentiated osteoclasts were detached with trypsin and plated onto dentine discs (obtained from elephant ivory) in a 96-well plate, at a density of 2 × 104 cells/well. Following a period of attachment (4 h), osteoclasts were co-cultured for 5 days with 5 × 104 autologous activated γδ T cells or CD4+ T cells in the presence of 20 ng/ml rhM-CSF and 2 ng/ml rmRANKL. In some experiments mature osteoclasts were cultured in the presence of 10% (v/v) conditioned media from γδ or CD4+ T cells generated as described above. Cultures were subject to half-medium changes with fresh cytokines and conditioned medium added every 48 h. To assess osteoclast resorptive activity, images of the dentine discs were captured using reflected light microscopy and the total resorbed surface area of dentine was quantified using in-house software to establish a grey threshold to determine the area resorbed by osteoclasts (in black) and non-resorbed surfaces (in white).
Immunohistochemistry
Osteoclast formation was assessed using immunohistochemical staining for expression of the vitronectin receptor (VNR) by mature osteoclasts using a mouse anti-CD51/CD61 antibody (AbD Serotec, Oxford, UK) and goat anti-mouse Alexa Fluor®-488 antibody (Molecular Probes, Carlsbad, CA, USA). Osteoclast formation was quantified by assessing multi-nucleated (≥3 nuclei) osteoclast number using fluorescence microscopy after nuclear counter-staining with 4′,6-diamidino-2-phenylindole (DAPI). To visualize polarized osteoclasts on dentine, the osteoclasts were fixed with 4% formaldehyde, prior to immunohistochemical staining for F-actin rings using tetramethylrhodamineisothiocyanate (TRITC)-phalloidin (Sigma-Aldrich) and quantification using fluorescence microscopy.
Cytokine production
Conditioned medium generated from activated γδ T cells and CD4+ T cells was assessed for levels of IL-4, IL-10, IFN-γ, TNF-α, IL-6 and IL-1β using a cytometric bead array (BD Biosciences) and analysed using a fluorescence activated cell sorter (FACS)Array flow cytometer. Production of IL-17 was determined using a R&D Systems Quantikine enzyme-linked immunosorbent assay (ELISA) kit, according to the manufacturer's instructions. Production of GM-CSF was assessed using a Milliplex Bead Assay (Millipore, Billerica, MA, USA) and a Bio-Plex 200 analyser (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data were analysed using the Kruskal–Wallis one-way analysis of variance on ranks (SigmaPlot®11·0), with inter-group comparisons analysed using the Wilcoxon matched-pairs rank test. P-values ≤0·05 were considered statistically significant.
Results
Activated γδ T cells inhibit osteoclast formation
To determine the effects of γδ T cells on osteoclast formation, purified γδ T cells (unstimulated or activated with anti-CD3/anti-CD28-coated Dynabeads®) were cultured with autologous M-CSF-dependent osteoclast precursors for 6 days in the presence of M-CSF and RANKL. Because VNR is also expressed on activated macrophages [22], we used multi-nuclearity (≥3 nuclei) in combination with VNR positivity as a determinant of osteoclast formation in our assays, as reported previously [23]. Co-cultures of osteoclast precursors and T cells were supplemented with exogenous RANKL as activated γδ or CD4+ T cells consistently failed to induce osteoclast formation in the absence of RANKL in our in-vitro assay system (data not shown). Osteoclast precursors were cultured with M-CSF alone to assess basal levels of spontaneous osteoclast formation.
The presence of unstimulated γδ T cells resulted in a non-statistically significant trend towards an increased number of VNR+ osteoclasts compared to RANKL + M-CSF alone, suggesting a potential stimulatory effect of unstimulated γδ T cells on osteoclast formation (Fig. 1a), whereas unstimulated CD4+ T cells had no stimulatory effect on osteoclast formation. Conversely, the addition of anti-CD3/CD28 stimulated γδ T cells or CD4+ T cells (Fig. 1b) resulted consistently in a significant inhibition of multi-nucleated VNR+ osteoclast formation (Fig. 1a). The marked inhibitory effect of activated T cells (both γδ and CD4+) on osteoclast formation was found to be independent of cell–cell contact, as the addition of 10% (v/v) conditioned medium from activated γδ T cells (Fig. 2a,c) or CD4+ T cells (Fig. 2b,c) was sufficient to markedly inhibit osteoclast formation. Furthermore, using Transwell inserts to isolate activated T cells from osteoclast precursors, we observed no decrease in the capacity of activated γδ T cells or CD4+ T cells for inhibiting osteoclast formation (Fig. S1).
Fig. 1.
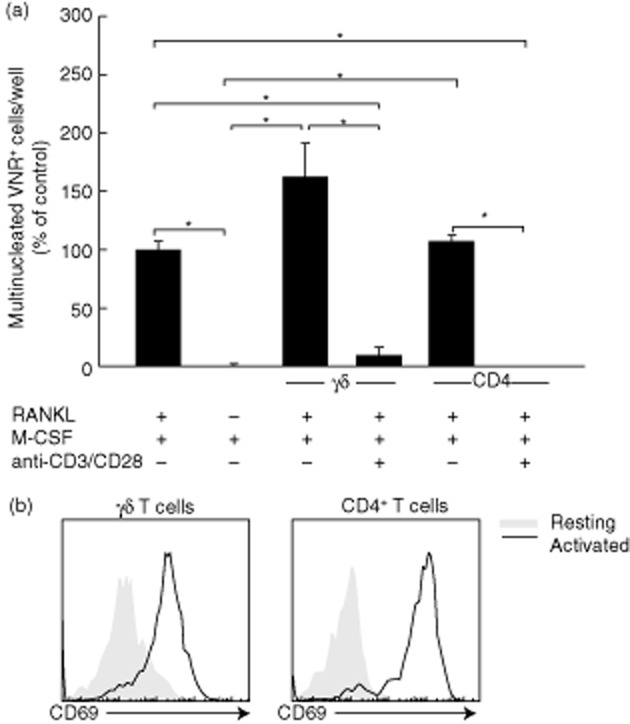
Activated γδ T cells and CD4+ T cells inhibit osteoclast formation. (a) Quantification of osteoclast formation following incubation of osteoclast precursor cells with resting or activated γδ or CD4+ T cells. Macrophage colony-stimulating factor (M-CSF)-dependent osteoclast precursors were cultured in the presence of M-CSF and receptor activator of nuclear factor κB-ligand (RANKL) and co-cultured with resting or anti-CD3/CD28-stimulated γδ T cells or CD4+ T cells, for 5 days. Osteoclast formation was assessed using immunohistochemical staining for vitronectin receptor (VNR) and counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). Multi-nucleated (≥3 nuclei) VNR+ cells per well were quantified using fluorescence microscopy. Data shown are the mean ± standard error of the mean from three experiments from independent donors. *P < 0·05. (b) Activation of γδ T cells and CD4+ T cells following activation with anti-CD3/CD28 T activator beads. Purified T cells were stimulated with anti-CD3/CD28 beads (1:1 ratio) for 24 h, prior to staining with anti-human CD69-phycoerythrin (PE) antibody and flow cytometric analysis. Data shown are from one experiment and are representative of two further experiments from independent donors.
Fig. 2.
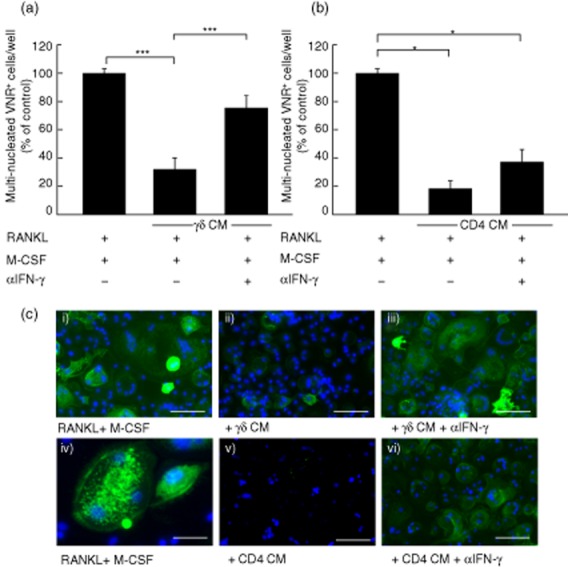
The blockade of interferon (IFN)-γ partially overcomes the inhibitory effect of activated γδ T cells, but not CD4+ T cells. Macrophage colony-stimulating factor (M-CSF)-dependent osteoclast precursors were cultured in the presence of receptor activator of nuclear factor κB-ligand (RANKL) and co-cultured with 10% (v/v) conditioned medium (CM) from activated γδ T cells (a) or 10% (v/v) CM from activated CD4+ T cells (b), in the presence or absence of α-IFN-γ. Following immunohistochemical staining for vitronectin receptor (VNR), cells were counterstained with DAPI and multinucleated (≥3 nuclei) VNR+ cells per well were quantified using fluorescence microscopy. Data shown are the mean ± standard error of the mean from three experiments from independent donors. *P < 0·05; ***P < 0·001. (c) Representative images showing anti-VNR staining (green) and nuclei (blue) of osteoclast precursor cells treated with M-CSF and RANKL (i), and higher magnification view in (iv); 10% (v/v) CM from activated γδ T cells alone (ii) or in the presence of α-IFN-γ (iii); 10% (v/v) CM from activated CD4+ T cells alone (v) or in the presence of α-IFN-γ (vi). Scale bars are 50 μM (i–iii; v–vi) and 20 μM (iv).
Cytokine production by activated γδ T cells and activated CD4+ T cells
To identify potential mediators of the inhibitory effects of T cells on osteoclast formation we assessed the levels of bone-active cytokines produced by unstimulated γδ and CD4+ T cells and following 24 h stimulation with anti-CD3/anti-CD28-coated Dynabeads (Table 1).
Table 1.
Cytokine production from activated γδ and CD4+ T cells
| Cytokine concentration (pg/ml) | Cytokine concentration (pg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| Resting γδ | Activated γδ | P | n | Resting CD4 | Activated CD4 | P | n | |
| IFN-γ | 21·8 ± 8·0 | 1124·6 ± 513·3 | <0·001 | 10 | 3·0 ± 2·2 | 1605·1 ± 423·9 | <0·001 | 8 |
| IL-4 | 3·8 ± 1·6 | 10·0 ± 2·6 | n.s. | 8 | 3·0 ± 1·8 | 107·6 ± 37·9 | <0·001 | 8 |
| IL-10 | 5·5 ± 0·9 | 30·7 ± 8·1 | <0·05 | 9 | 2·5 ± 1·3 | 630·5 ± 173·9 | <0·001 | 8 |
| TNF-α | 33·4 ± 8·5 | 1050·4 ± 256·9 | <0·001 | 10 | 4·4 ± 2·1 | 2630·1 ± 440·4 | <0·001 | 8 |
| IL-1β | 12·6 ± 2·8 | 54·9 ± 11·9 | <0·05 | 6 | 2·4 ± 0·8 | 68·4 ± 21·8 | <0·05 | 6 |
| IL-6 | 151·7 ± 33·3 | 430·1 ± 131·1 | <0·05 | 10 | 14·2 ± 4·4 | 327·6 ± 94·3 | <0·05 | 8 |
| IL-17 | 4·3 ± 4·2 | 13·5 ± 10·9 | n.s. | 16 | 9·7 ± 9·7 | 354·6 ± 118·5 | <0·001 | 14 |
| GM-CSF | 0·7 ± 0·4 | 209·1 ± 60·9 | <0·05 | 3 | n.d. | 4412·5 ± 1575·9 | – | 3 |
The production of pro- and anti-osteoclastogenic cytokines was determined for resting γδ and CD4 T cells, and following anti-CD3/anti-CD28 stimulation for 24 h. Production of interferon (IFN)-γ, interleukin (IL)-10, tumour necrosis factor (TNF)-α, IL-1β, IL-6 and granulocyte– macrophage colony-stimulating factor (GM-CSF) was assessed using a multiplexed cytometric bead array. IL-17 production was assessed by enzyme-linked immunosorbent assay (ELISA). Data shown are mean ± standard error of the mean from independent experiments from n number of independent donors (n.s. = not significant; n.d. = not determined).
Following activation, γδ T cells and CD4+ T cells were found to produce significant levels of IFN-γ, IL-10, TNF-α, IL-1β, IL-6 and GM-CSF compared to unstimulated cells. GM-CSF production was not detected in unstimulated CD4+ T cells, but was increased markedly following activation, with levels increased markedly (∼20-fold) compared to activated γδ T cells. While IL-4 production was not increased significantly in γδ T cells, CD4+ T cells increased IL-4 production significantly following activation. The marked production of IFN-γ, IL-4, IL-17 and IL-10 by activated CD4+ T cells reflects the heterogeneous nature of cells in this population, i.e. T helper type 1 (Th1), Th2, Th17 and regulatory T cells (Treg). Interestingly, we observed marked production of IL-17 in activated CD4+ T cells, but not activated γδ T cells, suggesting that γδ T cells isolated from healthy volunteers do not produce IL-17 ex vivo following anti-CD3/CD28 stimulation. Of further note, unstimulated γδ T cells produced increased levels of IL-6 (∼10-fold higher) compared to unstimulated CD4+ T cells, which may explain the tendency of unstimulated γδ T cells to enhance RANKL-induced osteoclastogenesis (Fig. 1a). This is consistent with a previous study documenting the heterogeneity of γδ T cells for producing IL-6 in healthy donors [24].
IFN-γ is the primary mediator of the anti-osteoclastogenic effect of activated γδ T cells
Following the observation that high levels of IFN-γ are produced by activated γδ and CD4+ T cells and this is reported to inhibit osteoclastogenesis [6], we sought to determine if neutralization of IFN-γ could prevent the inhibitory effects of activated T cell conditioned medium. The addition of an anti-IFN-γ antibody restored osteoclast formation significantly in the presence of activated γδ T cell conditioned medium (Fig. 2a,c), suggesting that the soluble factor responsible for the inhibitory effects of activated γδ T cell conditioned medium on osteoclastogenesis is IFN-γ. Anti-IFN-γ treatment only partially overcame the inhibitory effects of activated CD4+ T cell conditioned medium on osteoclastogenesis (Fig. 2b,c) suggesting that, while IFN-γ mediated some inhibitory effects of activated CD4+ T cells, there are further inhibitory factors produced by CD4+ T cells that potently inhibit osteoclastogenesis.
A further potential mechanism through which activated T cells may inhibit osteoclast formation is through production of GM-CSF which, in the presence of RANKL, induces osteoclast precursors to differentiate down the dendritic cell lineage [10]. However, the neutralization of GM-CSF did not overcome the inhibitory effects of conditioned medium from activated γδ T cells (Fig. S2a) or CD4+ T cells (Fig. S2b) on osteoclast formation. This suggests that IFN-γ is the primary mediator of the inhibitory effects of activated γδ T cells on osteoclastogenesis, while diversion of osteoclast precursor differentiation towards the dendritic cell lineage by GM-CSF had no effect in our in-vitro assay system.
Given the powerful inhibitory effects of γδ T cells stimulated with anti-CD3/CD28 beads for affecting osteoclast formation, we then sought to determine whether γδ T cells activated with N-BPs [such as zoledronic acid (ZOL)] had similar inhibitory effects on osteoclast formation. ZOL-expanded Vγ9Vδ2 T cells consistently inhibited RANKL-induced osteoclast formation, and even more pronounced effects on osteoclast formation were observed following the addition of 10% (v/v) conditioned medium obtained from these expanded cultures (Fig. S3). The inhibitory effects of ZOL-activated Vγ9Vδ2 T cells (or conditioned medium from these cultures) on osteoclast formation could be markedly prevented following neutralization of IFN-γ, indicating that IFN-γ is also the major soluble factor responsible for the inhibitory effects of ZOL-activated Vγ9Vδ2 T cells on osteoclast formation.
Activated γδ T cells inhibit osteoclast resorptive activity
To assess whether activated γδ T cells can influence osteoclast resorption, we seeded mature osteoclasts onto dentine discs, co-cultured them with γδ T cells or CD4+ T cells for 6 days and assessed resorptive capacity. Both activated γδ (Fig. S4a) and CD4+ T cells (Fig. S4b) reduced the osteoclast resorption area significantly by ∼60 and 70%, respectively. To assess whether the observed inhibitory effects of activated T cells were again due to the release of soluble inhibitors we conducted similar experiments using activated T cell-derived conditioned medium. In the presence of either activated γδ T cell or CD4+ T cell conditioned medium we observed markedly reduced resorptive activity compared to control osteoclasts (Fig. 3a,b).
Fig. 3.
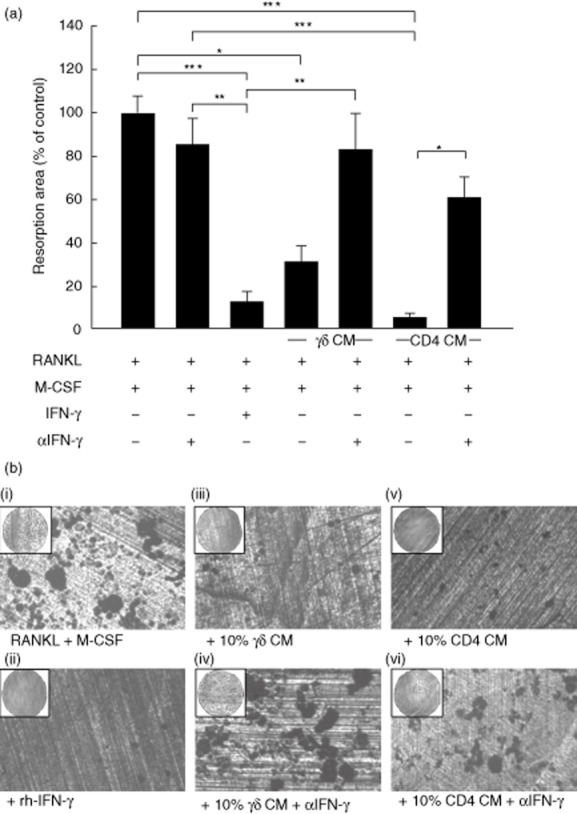
Activated γδ T cells and CD4+ T cells inhibit the resorptive activity of mature osteoclasts. (a) Mature osteoclasts were seeded onto dentine discs and cultured with 10% (v/v) activated γδ T cell conditioned medium (CM) or activated CD4+ T cell CM, in the presence or absence of α-interferon (IFN)-γ, for 6 days. 100 U/ml recombinant human (rh)-IFN-γ (R&D Systems) was used as a positive control. Resorptive area was quantified using reflected light microscopy and in-house software. Data shown are the mean ± standard error of the mean from three experiments from independent donors. *P < 0·05. (b) Representative images showing osteoclast resorption pits on dentine discs: receptor activator of nuclear factor κB-ligand (RANKL) and M-CSF alone (i); rhIFN-γ (ii); 10% (v/v) CM from activated γδ T cells alone (iii), or in the presence of α-IFN-γ (iv); 10% (v/v) CM from activated CD4+ T cells alone (v), or in the presence of α-IFN-γ (vi).
Due to the powerful inhibitory role of γδ T cell-derived IFN-γ on osteoclastogenesis we assessed whether IFN-γ was responsible for the inhibitory effects on osteoclast resorption. Neutralization of IFN-γ resulted in the almost complete restoration of the resorption area in osteoclasts treated with activated γδ T cell conditioned medium and markedly overcame the inhibitory effects of CD4+ T cell conditioned medium (Fig. 3a,b), suggesting that IFN-γ is the predominant soluble factor responsible for the inhibitory effect of both activated γδ and CD4+ T cell conditioned medium on resorption of mature osteoclasts. The marked inhibitory effect on osteoclast resorption was paralleled by a profound reduction in F-actin ring number in cultures treated with either activated γδ T cell conditioned medium or activated CD4+ T cell conditioned medium (Fig. 4a,b), which was comparable in efficacy to rhIFN-γ treatment, and thereby demonstrates the importance of F-actin ring formation for osteoclastic resorptive activity. In agreement with the quantified resorption data, this inhibitory effect of γδ T cell conditioned medium on F-actin ring number was completely abolished when IFN-γ was neutralized (Fig. 4a,b).
Fig. 4.
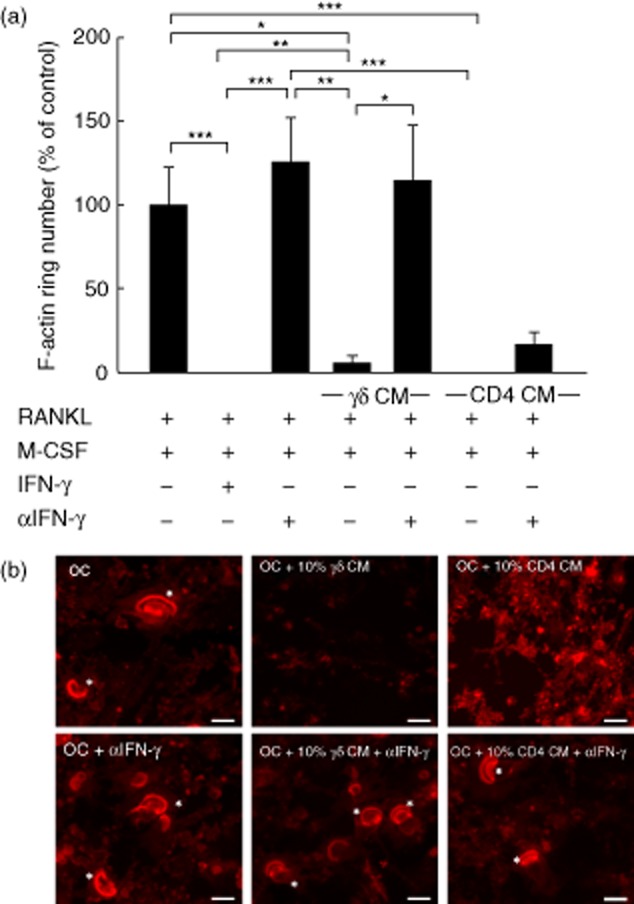
F-actin ring formation in osteoclasts is disrupted by interferon (IFN)-γ derived from activated γδ T cells. Mature osteoclasts were seeded onto dentine discs, supplemented with receptor activator of nuclear factor κB-ligand (RANKL) and macrophage colony-stimulating factor (M-CSF), and treated with 10% (v/v) conditioned medium (CM) from activated γδ or CD4+ T cells, in the presence or absence of α-IFN-γ, for 6 days. (a) F-actin ring number was quantified by fluorescence microscopy following immunohistochemical staining using tetramethylrhodamineisothiocyanate (TRITC)-phalloidin. Data shown are the mean ± standard error of the mean from three experiments from independent donors. *P < 0·05; **P < 0·05; ***P < 0·001). (b) Representative images of osteoclasts (left panels) treated with CM from activated γδ (middle panels) or CD4+ T cells (right panels), in the presence or absence of α-interferon (IFN)-γ. Asterisks indicate the F-actin rings. Images are from one experiment and are representative of two further experiments from independent donors. Scale bar = 50 μM.
Discussion
Numerous studies have reported that activated T cells are capable of influencing osteoclastogenesis either positively or negatively, with a recent focus on the stimulatory nature of the IL-17-producing Th17 subset of CD4+ T cells on the pathological bone loss associated with chronic inflammatory disease states [7,25]. IL-17 stimulates RANKL expression by osteoblasts and stromal cells indirectly, thereby increasing osteoclastogenesis, and thus Th17 cells may play a pathological role in bone loss associated with chronic inflammatory disease states, such as RA. However, in the murine model of human RA, collagen-induced arthritis (CIA), γδ T cells were identified as a major cell type responsible for IL-17 production [13]. As yet, the contribution of γδ T cells to chronic autoimmune conditions in humans, particularly their capacity to influence osteoclastogenesis, is unknown.
In this study, we identified that activated γδ T cells inhibit the formation of mature osteoclasts from monocytic lineage precursors via a cell-contact independent mechanism. γδ T cells were found to increase production of a variety of bone-active cytokines with both pro- and anti-osteoclastogenic effects following anti-CD3/CD28 stimulation, but failed consistently to produce IL-17. This was in marked contrast to CD4+ T cells, which produced abundant IL-17 upon activation. Using neutralizing antibodies we determined that the inhibitory effect of activated γδ T cells on osteoclastogenesis was mediated predominantly via IFN-γ, while IFN-γ played a relatively minor role in the inhibitory effect of activated CD4+ T cells. Finally, we identified that activated γδ and CD4+ T cells inhibit the resorptive activity of mature osteoclasts, which is mediated predominantly by production of IFN-γ.
To date, the effect of T cells on osteoclast formation has focused predominantly on conventional T cell subsets, such as CD4+ T cells, while the effects of non-conventional T cell subsets, such as γδ T cells, for influencing osteoclastogenesis are currently ill-defined, particularly in humans. We have found that activation of γδ T cells with anti-CD3/CD28 stimulation results in potent inhibitory effects on osteoclastogenesis, with similar inhibitory effects observed with activated CD4+ T cells. The inhibitory effects of activated CD4+ T cells on osteoclastogenesis are consistent with previous studies [6,26], and highlight further the fundamental importance of route of T cell activation for influencing osteoclastogenesis. Using murine cells, Wyzga et al. demonstrated that the influence of activated CD4+ T cells on osteoclastogenesis was crucially dependent upon the mechanism of activation, with anti-CD3 stimulation resulting in an inhibitory effect on osteoclastogenesis, whereas concanavalin A or phytohaemagglutinin treatment resulted in stimulatory effects. Thus, it is likely that the numerous conflicting studies documenting both stimulatory [5] and inhibitory effects [6] of activated T cells on osteoclastogenesis reflect the use of a variety of stimulation protocols, as well as the utilization of both murine and human assay systems.
By analysing cytokine production in both unstimulated and anti-CD3/CD28 activated γδ T cells and CD4+ T cells we observed that, unlike their murine counterparts, purified human γδ T cells failed consistently to produce detectable IL-17 ex vivo following stimulation but produced abundant levels of IFN-γ, thereby displaying a marked Th1 bias, as reported previously [27]. IL-17 production has been reported recently in the predominant subset of γδ T cells in human peripheral blood, Vγ9Vδ2 T cells (also referred to as Vγ2Vδ2), although typically only <1% of cells from healthy donors demonstrated IL-17 production following non-specific activation with phorbol myristate acetate (PMA) plus ionomycin [17]. Therefore, although it is apparent that Vγ9Vδ2 T cells can acquire the ability to produce IL-17 following culture with a variety of Th17 differentiation-promoting cytokines [such as IL-1β, IL-6, IL-23 and transforming growth factor (TGF-β)] [18], it is evident that freshly isolated human γδ T cells from healthy donors produce IFN-γ preferentially, with potential IL-17 release probably influenced by the method of stimulation and culture conditions. Thus, it is possible that in the local microenvironment of inflamed joints the abundance of proinflammatory cytokines, coupled with the liberation of TGF-β from bone matrix due to excessive osteoclast activity, may provide a suitable environment for the generation of IL-17-producing γδ T cells.
In support of a pathological role for γδ T cells in human autoimmune disease, γδ T cells have been reported in the synovial fluid of RA patients [15,16,28], and an increased prevalence of IL-17-producing γδ T cells have been detected very recently in the peripheral blood of patients with ankylosing spondylitis [28]. Consistent with our findings, Kenna et al. demonstrated that anti-CD3/CD28 stimulation of γδ T cells resulted in abundant IFN-γ production in the absence of increased IL-17 production in healthy controls, yet in ankylosing spondylitis patients the pattern was reversed, with a profound reduction in IFN-γ release and a marked increase in IL-17 production, which was potentiated by IL-23 treatment [29]. However, recent studies in RA patients have suggested that IL-17 production is due exclusively to CD4+ Th17 cells [30], with no IL-17-producing γδ T cells resident in synovial fluid or infiltrating the rheumatoid synovium [31]. Therefore, despite compelling evidence for a pathological IL-17-producing role of γδ T cells in murine experimental arthritis, to date the evidence for a pathological role of γδ T cells in stimulating bone loss associated with chronic inflammatory diseases is currently lacking. Further studies are therefore warranted to determine the contribution of γδ T cell-derived IL-17 to chronic human autoimmune diseases.
During this study IFN-γ was revealed as the major mediator of the inhibitory effects of activated γδ T cells on osteoclast formation and resorption. By stimulating the degradation of the TNF receptor-associated factor 6 (TRAF-6) protein through activation of the ubiquitin–proteasome system, IFN-γ potently inhibits RANK signalling required for osteoclast formation [6]. Further inhibitory effects of IFN-γ on osteoclast formation have also been postulated, including stimulation of apoptosis through Fas/FasL interactions [32] and decreased expression of RANKL and c-Fms [33]. Interestingly, despite producing similar levels of IFN-γ as activated γδ T cells, neutralization of IFN-γ in activated CD4+ T cell cultures had only a partial effect on recovering osteoclastogenesis, suggesting that activated CD4+ T cells are able to suppress osteoclastogenesis via multiple mechanisms, such as through production of the well-characterized inhibitors IL-4 [34], and/or IL-10 [35].
The net contribution of IFN-γ to bone metabolism in vivo is currently an area of intensive debate, with conflicting reports documenting both pro- and anti-osteoclastogenic effects of IFN-γ. The anti-osteoclastogenic effects of IFN-γ in vivo are supported by the finding that IFN-γ−/− mice experience enhanced bone resorption during collagen-induced arthritis [36]. Conversely, a number of studies have reported pro-osteoclastogenic effects of IFN-γ, as IFN-γ is an effective treatment for osteopetrosis in humans [37], and IFN-γ−/− mice are protected from ovariectomy-induced bone loss [38]. These data suggest that IFN-γ has multiple roles in pathological bone diseases and further supports the notion that there exists a complex interplay between the immune system and regulation of bone homeostasis, particularly in pathological inflammatory disease states.
The profound inhibitory effect of IFN-γ derived from activated γδ and CD4+ T cells on resorption by mature osteoclasts is supported by a previous study showing that IFN-γ decreases expression of the secreted protease cathepsin K in RANKL-treated RAW 264·7 cells (a murine pre-osteoclastic cell line) [39]. As cathepsin K plays an important role in the degradation of bone matrix proteins during bone resorption, this provides a direct link by which T cell-derived IFN-γ may directly impair resorption by mature osteoclasts. The inhibitory effects on resorption may be due partially to an inhibition of further osteoclast formation during the culture period, rather than direct inhibition of osteoclast resorptive function per se. However, the profound inhibitory effect of rhIFN-γ on resorption observed in our assays is strongly indicative of a direct effect of IFN-γ on resorptive activity by mature osteoclasts, rather than an inhibitory effect on further osteoclast formation in our assay system. Furthermore, the importance of IFN-γ to the efficacy of CD4+ T cells for inhibiting resorption was in marked contrast to its relative lack of effect for inhibiting osteoclastogenesis. Taken together, this suggests that IFN-γ is specifically inhibiting osteoclast resorptive function via a direct effect on the resorptive capacity of mature osteoclasts.
The importance of γδ T cells for influencing bone biology is currently poorly understood, but may be of specific interest in the context of therapeutic administration of anti-resorptive aminobisphosphonates drugs (N-BPs). We and others have shown previously that N-BPs, such as ZOL, potently induce an IFN-γ-producing phenotype in γδ T cells in vitro [20] and in vivo [40,41]. By inducing the accumulation of the γδ-TCR agonists isopentenyl pyrophosphate (IPP) and dimethylallyl diphosphate (DMAPP) [42] in monocytic-lineage cells [43], N-BPs selectively activate the major subset of γδ T cells in peripheral blood, so-called Vγ9Vδ2 T cells [19]. Thus N-BPs, by virtue of their high bone affinity and relatively selective targeting of osteoclasts, potentially induce an IFN-γ-effector phenotype in Vγ9Vδ2 T cells specifically in the local bone microenvironment, which may be of relevance in vivo given the inhibitory effects of ZOL-induced IFN-γ production by Vγ9Vδ2 T cells on osteoclast formation that we observed in this study. Such a mechanism may further potentiate the anti-resorptive effects of N-BPs by inhibiting osteoclast formation and resorption through the direct effects of γδ T cell-derived IFN-γ. This further highlights the important links between the immune system and bone resorbing cells such as osteoclasts, and suggests that pharmacological manipulation of innate immune cells, for example with N-BPs, may have important consequences for host immunity and bone health.
In conclusion, this study demonstrates that activated human γδ T cells are capable of potent inhibitory effects on osteoclast formation, and the resorptive activity of mature osteoclasts, mediated via production of IFN-γ.
Acknowledgments
This work was supported by funding from the Oliver Bird Foundation (A. P.) and Arthritis Research UK (K. T.). The authors would like to thank Dr H. M. Wilson (University of Aberdeen) for critical comments on the manuscript.
Disclosure
The authors declare no financial or commercial conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. The inhibitory effect of activated γδ T cells and CD4+ T cells on osteoclast formation is independent of cell–cell contact. Quantification of osteoclast formation following incubation of osteoclast precursor cells with anti-CD3/CD28-activated γδ or CD4+ T cells for 5 days, in the presence of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB-ligand (RANKL). Activated γδ T cells and activated CD4+ T cells were co-cultured with osteoclast precursor cells to permit cell contact, or cells were separated using a Transwell insert into which the activated T cells were placed. Osteoclast formation was assessed using immunohistochemical staining for vitronectin receptor (VNR) and counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). Multi-nucleated (≥3 nuclei) VNR+ cells per well were quantified using fluorescence microscopy. Data shown are the mean ± standard deviation from two experiments from independent donors.
Fig. S2. The blockade of granulocyte–macrophage colony-stimulating factor (GM-CSF) does not overcome the inhibitory effect of activated γδ T cells or CD4+ T cells on osteoclastogenesis. Osteoclast precursors were cultured in the presence of receptor activator of nuclear factor κB-ligand (RANKL) + macrophage colony-stimulating factor (M-CSF) and treated with 10% (v/v) conditioned medium (CM) from activated γδ T cells (a), or 10% (v/v) CM from activated CD4+ T cells (b), in the presence or absence of αGM-CSF or isotype control [immunoglobulin (Ig)G1], for 5–6 days. Following immunohistochemical staining for vitronectin receptor (VNR) and nuclear counterstaining with 4′,6-diamidino-2-phenylindole (DAPI), multi-nucleated (≥3 nuclei) VNR+ cells per well were quantified using fluorescence microscopy. Data shown are the mean ± standard error of the mean from three experiments from independent donors. *P < 0·05; ***P < 0·001.
Fig. S3. Zoledronate-activated γδ T cells inhibit osteoclast differentiation via interferon (IFN)-γ. Peripheral blood mononuclear cells (PBMCs) were seeded in a 24-well plate at a density of 1 × 106 cells/ml (1 ml/well) in α-minimum essential medium (MEM) supplemented with 1 μM zoledronic acid (ZOL) plus 10 U/ml interleukin (IL)-2 and were cultured for 7 days. Cells were subjected to half-medium changes every 3 days. After 7 days γδ T cells were positively isolated using γδ T cell magnetic affinity cell sorter (MACS) beads. Macrophage colony-stimulating factor (M-CSF)-expanded osteoclast precursors were treated with M-CSF and receptor activator of nuclear factor κB-ligand (RANKL) for 5 days, in the presence or absence of autologous ZOL-activated γδ T cells. In some wells osteoclast precursors were incubated with 10% (v/v) conditioned medium from 72 h cultures of PBMCs treated with ZOL, as described above. To assess the contribution of interferon (IFN)-γ to the inhibitory effect of γδ T cells (or conditioned medium) on osteoclast formation, affinity-purified polyclonal goat anti-human IFN-γ antibody (10 μg/ml) was added to the cultures. Data shown are the mean ± standard deviation from two experiments from independent donors.
Fig. S4. Activated γδ T cells and CD4+ T cells inhibit the resorptive activity of mature osteoclasts. Mature osteoclasts were seeded onto dentine discs and cultured with resting or activated γδ T cells (a), or resting or activated CD4+ T cells (b), for 6 days. The resorptive area was quantified using reflected light microscopy and in-house software to determine resorption pits. Data shown are the mean ± standard error of the mean from three experiments from independent donors. ***P < 0·001.
References
- 1.Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature. 1965;206:489–490. doi: 10.1038/206489a0. [DOI] [PubMed] [Google Scholar]
- 2.Hattersley G, Owens J, Flanagan AM, Chambers TJ. Macrophage colony stimulating factor is essential for osteoclast formation in vitro. Biochem Biophys Res Commun. 1991;177:526–531. doi: 10.1016/0006-291x(91)92015-c. [DOI] [PubMed] [Google Scholar]
- 3.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 4.Kong YY, Feige U, Sarosi L, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 5.Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 6.Takayanagi H, Ogasawara K, Hida S, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 7.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-α induces osteoclastogenesis of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:14181–14188. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1β and tumor necrosis factor-α, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteobalstic cells. Bone. 1999;25:255–259. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 10.Grcevic D, Lukic IK, Kovacic N, Ivcevic S, Katavic V, Marusic A. Activated T lymphocytes suppress osteoclastogenesis by diverting early monocyte/macrophage progenitor lineage commitment towards dendritic cell differentiation through down-regulation of receptor activator of nuclear factor-kappa and c-Fos. Clin Exp Immunol. 2006;146:146–158. doi: 10.1111/j.1365-2249.2006.03181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein NC, Kreutzmann C, Zimmermann SP, et al. Interleukin-4 and interleukin-13 stimulate the osteoclast inhibitor osteoprotegerin by human endothelial cells through the STAT6 pathway. J Bone Miner Res. 2008;23:750–758. doi: 10.1359/jbmr.080203. [DOI] [PubMed] [Google Scholar]
- 12.Kim YG, Lee CK, Nah SS, Mun SH, Yoo B, Moon HB. Human CD4+ CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2007;357:1046–1052. doi: 10.1016/j.bbrc.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 13.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayday AC, Saito H, Gillies SD, et al. Structure, organization and somatic rearrangement of T cell gamma genes. Cell. 1985;40:259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- 15.Brennan FM, Londei M, Jackson AM, et al. T cells expressing γδ chain receptors in rheumatoid arthritis. J Autoimmun. 1988;1:319–326. doi: 10.1016/0896-8411(88)90002-9. [DOI] [PubMed] [Google Scholar]
- 16.Keystone EC, Rittershaus C, Wood N, et al. Elevation of a γδ T cell subset in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 1991;84:78–82. [PMC free article] [PubMed] [Google Scholar]
- 17.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirement for the differentiation of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol. 2010;184:7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caccamo N, La Mendola C, Orlando V, et al. Differentiation, phenotype, and function of interleukin-17-producing human Vg9Vd2 T cells. Blood. 2011;118:129–137. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 19.Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 20.Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced γδ-T-cell proliferation and activation in vitro. J Bone Miner Res. 2004;19:278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- 21.Henriksen K, Karsdal MA, Taylor A, Tosh D, Coxon FP. Generation of human osteoclasts from peripheral blood. Methods Mol Biol. 2012;816:159–175. doi: 10.1007/978-1-61779-415-5_11. [DOI] [PubMed] [Google Scholar]
- 22.Hermann P, Armant M, Brown E, et al. The vitronectin receptor and its associated CD47 molecule mediates proinflammatory cytokine synthesis in human monocytes by interaction with soluble CD23. J Cell Biol. 1999;144:767–775. doi: 10.1083/jcb.144.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujikawa Y, Sabokbar A, Neale S, Athanasou NA. Human osteoclast formation and bone resorption by monocytes and synovial macrophages in rheumatoid arthritis. Ann Rheum Dis. 1996;55:816–822. doi: 10.1136/ard.55.11.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewitt RE, Lissina A, Green AE, Slay ES, Price DA, Sewell AK. The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood γδ T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol. 2005;139:101–111. doi: 10.1111/j.1365-2249.2005.02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyzga N, Varghese S, Wikel S, Canalis E, Sylvester FA. Effects of activated T cells on osteoclastogenesis depends on how they are activated. Bone. 2004;35:614–620. doi: 10.1016/j.bone.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 27.DeBarros A, Chaves-Ferreira M, d'Orey F, Ribot J, Silva-Santos B. CD70–CD27 interactions provide survival and proliferative signals that regulate T cell receptor-driven activation of human γδ peripheral blood lymphocytes. Eur J Immunol. 2011;41:195–201. doi: 10.1002/eji.201040905. [DOI] [PubMed] [Google Scholar]
- 28.Meliconi R, Pitzalis C, Kingsley GH, Panayi GS. γδ T cells and their subpopulations in blood and synovial fluids from rheumatoid arthritis and spondyloarthritis. Clin Immunol Immunopathol. 1991;59:165–172. doi: 10.1016/0090-1229(91)90090-w. [DOI] [PubMed] [Google Scholar]
- 29.Kenna TJ, Davidson SI, Duan R, et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γδ T cells in patients with active ankylosing sponylitis. Arthritis Rheum. 2012;64:1420–1429. doi: 10.1002/art.33507. [DOI] [PubMed] [Google Scholar]
- 30.Pollinger B, Junt T, Metzler B, et al. Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011;186:2602–2612. doi: 10.4049/jimmunol.1003370. [DOI] [PubMed] [Google Scholar]
- 31.Ito Y, Usui T, Kobayashi S, et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis but not in rheumatoid arthritis. Arthritis Rheum. 2009;60:2294–2303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- 32.Kohara H, Kitaura H, Fujimura Y, et al. IFN-γ directly inhibits TNF-α-induced osteoclastogenesis in vitro and in vivo and induces apoptosis mediated by Fas/Fas ligand interactions. Immunol Lett. 2011;137:53–61. doi: 10.1016/j.imlet.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Ji JD, Park-Min KH, Shen Z, et al. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-γ in human osteoclast precursors. J Immunol. 2009;183:7223–7233. doi: 10.4049/jimmunol.0900072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shioi A, Teitelbaum SL, Ross FP, et al. Interleukin-4 inhibits murine osteoclast formation in vitro. J Cell Biochem. 1991;47:272–277. doi: 10.1002/jcb.240470313. [DOI] [PubMed] [Google Scholar]
- 35.Xu LX, Kukita T, Kukita A, Otsuka T, Niho Y, Iijima T. Interleukin-10 selectively inhibits osteoclastogenesis by inhibiting differentiation of osteoclast progenitors into preosteoclast-like cells in rat bone marrow culture system. J Cell Physiol. 1995;165:624–629. doi: 10.1002/jcp.1041650321. [DOI] [PubMed] [Google Scholar]
- 36.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-γ receptor-deficient mice. J Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 37.Key LL, Rodriguiz RM, Steve WM, et al. Long-term treatment of osteopetrosis with recombinant human interferon gamma. N Engl J Med. 1995;332:1594–1599. doi: 10.1056/NEJM199506153322402. [DOI] [PubMed] [Google Scholar]
- 38.Gao Y, Grassi F, Ryan MR, et al. IFN-γ stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang M, Martinez AF, Jacobs J, Balkan W, Troen BR. RANK ligand and interferon gamma differentially regulate cathepsin gene expression in pre-osteoclastic cells. Biochem Biophys Res Commun. 2005;328:756–763. doi: 10.1016/j.bbrc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Thompson K, Roelofs A, Jauhiainen M, Monkkonnen H, Monkkonnen J, Rogers MJ. Activation of γδ T cells by bisphosphonates. Adv Exp Med Biol. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- 41.Welton JL, Morgan MP, Marti S, et al. Monocytes and γδ T cells control the acute-phase response to intravenous zoledronate: insights from a phase IV safety trial. J Bone Miner Res. 2013;28:464–471. doi: 10.1002/jbmr.1797. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 43.Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


