Abstract
Prognosis of patients with early inflammatory arthritis (EIA) is highly variable. The aim of this study was to compare, longitudinally and cross-sectionally, the levels of cytokine-expressing cells in peripheral blood (PB) from patients with EIA to those in established rheumatoid arthritis (RA) and healthy controls (HC). PB mononuclear cells from HC (n = 30), patients with EIA (n = 20) or RA (n = 38) were stimulated with phorbol myristate acetate (PMA)/ionomycin for 3 h, and stained for cell markers and cytokines. Serum cytokines and chemokines were measured by Luminex. Patients with EIA were reassessed at 6 and 12 months. The percentage of interleukin (IL)-17+interferon (IFN)-γ−CD4+ T cells [T helper type 17 (Th17)] was increased in RA and EIA versus HC. Serum IL-1β, IL-2, IL-4 IL-17 and macrophage inflammatory protein (MIP)-1α were increased in RA and EIA versus HC. IL-1Ra, IL-15 and IFN-α were increased in EIA versus HC. IL-6 and tumour necrosis factor (TNF)-α was increased in RA but not EIA versus HC. Disease activity scores in EIA patients improved over 12 months' treatment. Th17 percentage at baseline was correlated with both rheumatoid factor (RF) titre and functional deficit at 12 months. Baseline levels of serum granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-6 and IL-8 were correlated with Larsen score at 12 months. There were no significant changes in cytokine-expressing CD4+T cells over time, although the percentage of IL-6+ monocytes increased. IL-17+CD4+ T cells and serum IL-17 levels are increased in EIA. IL-6-expressing monocytes increase during the first year of disease, irrespective of disease-modifying anti-rheumatic drug (DMARD) therapy. We observed incomplete clinical responses, suggesting EIA patients need more intensive early therapy.
Keywords: early arthritis, IL-17, monocyte, Th17, treatment response
Introduction
The prognosis of patients with early inflammatory arthritis (EIA) is highly variable, ranging from persistent arthritis, development of rheumatoid arthritis (RA) or clinical remission [1]. Patients with very early RA have a distinct cytokine profile within synovial fluid (SF), which includes increased levels of interleukin (IL)-17 [2] and higher levels of IL-17+CD4+ T cells [3]. Multiple data from animal models and human studies suggest a role for IL-17 and IL-17+CD4+ T cells in chronic inflammation and joint damage progression in RA [4–9]. IL-17 antagonists have entered clinical trials in RA and a number of other inflammatory conditions, with early data suggesting clinically relevant responses [10,11]. However, IL-17 is not present universally in RA synovial tissue [12] and may be a predominant player in pathogenesis in only selected patients, or at only certain stages in the disease.
In RA, data suggest that patients with higher levels of IL-17+CD4+ T cells have higher levels of systemic inflammatory response [3] and synovial tissue IL-17 is associated with more rapid joint damage progression in synergy with tumour necrosis factor (TNF)-α [13]. Indeed, we have previously demonstrated increased levels of IL-17+interferon (IFN)-γ− CD4+ T cells (Th17 cells) in peripheral blood (PB) from patients with established RA versus healthy controls. Additionally, power Doppler ultrasound signal, used increasingly as a marker of active synovitis, was associated with increased levels of T helper type 17 (Th17) cells in RA SF, and IL-17+T cells were higher in synovial tissue of patients with active disease compared to patients in remission [14].
The purpose of this study was to compare levels of cytokine-expressing cells, including Th17 cells, in the PB of patients with EIA or established RA with healthy controls (HC). Patients with EIA were followed for 12 months to correlate changes in disease activity with PB cytokines and chemokines.
Methods
Study population
Peripheral blood (PB) was obtained from patients with EIA attending King's College Hospital. Patients with EIA all had documented swelling of at least one joint (either at their baseline visit or within the previous 2 years), and symptom duration of less than 2 years, prior to treatment with disease modifying agents. Within this group, a number of patients could be classified as RA by the 1987 American College of Rheumatology (ACR) criteria [15]; the remainder were termed ‘undifferentiated arthritis’. Patients with undifferentiated arthritis could not be classified as either RA, or another inflammatory arthritis, e.g. spondylarthropathy. A second cohort of patients with established RA (median duration 72 months), according to the 1987 ACR criteria attending Guy's & St Thomas' Rheumatology Department, was also recruited. Healthy controls were recruited from hospital/university students and members of staff. For established RA patients, clinical data including age, disease duration, medication, presence or absence of immunoglobulin (Ig)M rheumatoid factor or erosions were obtained from review of the medical notes. Patients with early arthritis were assessed at baseline, 6 and 12 months (clinical examination, peripheral blood cytokine profiling, rheumatoid factor, anti-citrullinated peptide antibodies and X-rays of hands and feet) and assigned to a final diagnostic category (RA or non-RA) based on fulfilment of the 1987 ACR criteria at 12 months. Disease activity was assessed by disease activity score 28 (DAS28) on the day of sample collection. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were determined on the day of sample collection in the clinical laboratory. Ethical approval was obtained from the Bromley and Brent NHS research ethics committees. All subjects gave informed consent.
Cell culture
PB mononuclear cells (PBMC) were isolated by density gradient centrifugation using Lymphoprep (PAA Laboratories, Pasching, Austria) and cultured for 3 or 4 h at a concentration of 1 × 106/ml in RPMI-1640 medium supplemented with 1% penicillin/streptomycin, 1% glutamine and 10% heat-inactivated fetal calf serum (PAA Laboratories). Cultures were stimulated with either phorbol myristate acetate (PMA) (50 ng/ml; Sigma-Aldrich, St Louis, MO, USA) and ionomycin (750 ng/ml; Sigma-Aldrich) or lipopolysaccharide (LPS) (50 ng/ml; Sigma-Aldrich) in the presence of GolgiStop (Becton Dickinson, Franklin Lakes, NJ, USA).
Flow cytometry
Cells were stained for 30 min at 4°C with anti-CD14-allophycocyanin/cyanin 7 (APC/Cy7) (Biolegend, San Diego, CA, USA), fixed in 2% paraformaldehyde, then permeabilized with Saponin 0·5% (Sigma-Aldrich) and stained for 30 min at 4°C with anti-CD3-phycoerythrin (PE)/Cy7 (both Biolegend), anti-CD4-peridinin chlorophyll (PerCP)/Cy5·5 (Becton Dickinson), anti-IFN-γ-fluorescein isothiocyanate (FITC), anti-TNF-α-APC (eBiosciences, San Diego, CA, USA), anti-IL-6-FITC and anti-IL-17-PE (both Biolegend) or the appropriate isotype controls. Cells were acquired on a FACSCanto II (Becton Dickinson) using bead compensation and analysed using FlowJo (Treestar Inc, Ashland, OR, USA). Live cells were gated using forward- and side-scatter; monocytes and CD4+ T cells were identified by CD14 and CD3/CD4 expression respectively.
Serum cytokine and chemokine analysis
Cell-free serum was stored at −80°C prior to analysis of 25 cytokines and chemokines using a multiplex detection kit according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA): granulocyte–macrophage colony-stimulating factor (GM-CSF), IFN-α, IFN-γ, IL-1 receptor antagonist (Ra), IL-1β, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p40/p70), IL-13, IL-15, IL-17, monocyte chemoattractant protein (MCP)-1/CCL2, macrophage inflammatory protein (MIP)-1α/CCL3, MIP-1β/CCL4, regulated on activation, normal T expressed and secreted (RANTES/CCL5), monokine induced by IFN-γ (MIG/CXCL9), IFN-γ-induced protein (IP-10/CXCL10) and TNF-α. Analysis was performed on undiluted serum samples using a Luminex 100 system (Luminex Corporation, Austin, TX, USA). Minimum cytokine detection levels with these assays ranged between 3 and 40 pg/ml.
Clinical assessments and drug therapy in EIA patients
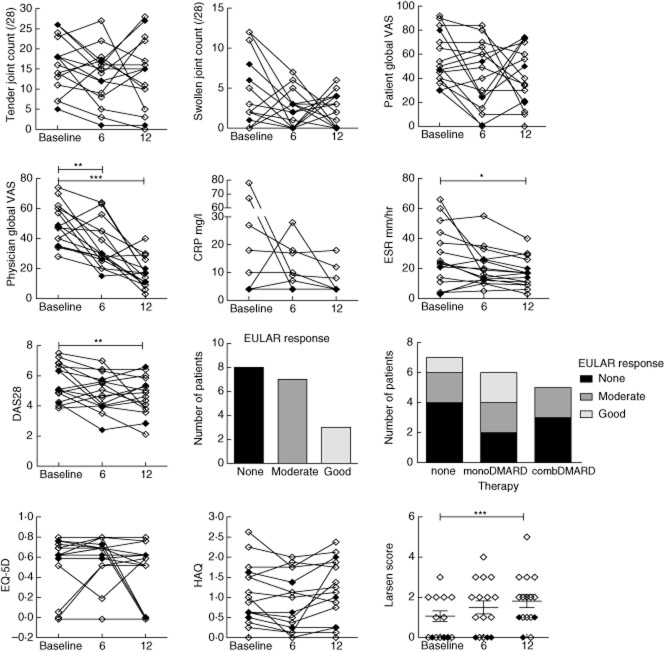
Disease activity was assessed in EIA patients at baseline, 6 and 12 months by the following: tender and swollen joint count (28 joints), visual analogue scores for patient and physician global, ESR and CRP. DAS28 was calculated at each visit. Ultrasound was not used to assess joint swelling. Function was assessed by the health assessment questionnaire (HAQ), and quality of life using EQ-5D questionnaires. Joint damage was assessed by standard radiographs of hands and wrists, read using the Larsen scoring method [16]. The X-ray reader was blinded to experimental and laboratory data.
No specific treatment strategy was specified in the observational study of EIA patients. Eleven of 16 EIA patients received disease-modifying anti-rheumatic drug (DMARD) therapy following their baseline visit: six received monotherapy (either sulphasalazine or methotrexate) and five received combination therapy with methotrexate and hydroxychloroquine. Five patients received steroids or non-steroidal anti-inflammatory drugs (NSAIDs) alone, with some patients refusing DMARDs. All but two patients received corticosteroids (oral, intramuscular or intra-articular). The treating rheumatologists were blinded to all experimental data, and therefore decisions to start or change therapy were independent of cytokine expression results.
Statistical methods
Values are expressed as mean and standard deviations for normally distributed data, or median and interquartile range for non-parametric data. Data were assessed for normality using the D'Agostino and Pearson omnibus normality test. Comparisons between patients and healthy controls were made using unpaired t-tests or Mann–Whitney U-tests for parametric and non-parametric data, respectively. Changes over time were analysed using either analysis of variance (anova) with Bonferroni's post-test or Kruskal–Wallis with Dunn's post-test. Correlation coefficients were obtained using Spearman's method. Linear and logistic regression were used to assess prediction of outcome at 12 months. Data were analysed using Prism version 5 (GraphPad Software, Inc., La Jolla, CA, USA) and spss version 18 (IBM, Armonk, NY, USA). For all tests, P-values of less than 0·05 were considered significant.
Results
Subject characteristics
Twenty patients with EIA, 38 patients with established RA and 30 healthy controls were recruited. There were no significant differences in age or sex between the three groups. Clinical characteristics of patients and controls are shown in Table 1.
Table 1.
Characteristics of patients and healthy controls
| Healthy controls | RA | EIA | |
|---|---|---|---|
| Number of patients | 30 | 38 | 20 |
| Female sex, n (%) | 18 (60) | 27 (71) | 15 (75) |
| Age in years, mean (s.d.) | 51 (20) | 55 (16) | 51 (14) |
| Duration of symptoms in months, median (IQR) | 72 (24, 111) | 9 (5, 17) | |
| Rheumatoid factor +ve, n% | 28 (72) | 11 (55) | |
| ESR, mm/h, median (IQR) | 20 (11·5, 33) | 22 (12, 36) | |
| DAS28, mean (s.d.) | 4·6 (1·5) | 5·3 (1·3) | |
| C-reactive protein mg/l, mean (s.d.) | 14 (14·6) | 27·6 (60·0) | |
| Erosive disease, n (%) | 22 (58) | 11 (55) |
Patients with early inflammatory arthritis (EIA) were recruited from an early arthritis clinic, and patients with established rheumatoid arthritis (RA) were recruited from general rheumatology clinics. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and disease activity score 28 (DAS28) were measured on the day blood was drawn. Disease activity assessments were not performed in healthy subjects. IQR: interquartile ratio; s.d.: standard deviation.
Peripheral blood from EIA and RA patients show comparable changes in IL-17+CD4+ T cells versus healthy controls
We first examined whether IL-17 or IFN-γ producing CD4+ T cells were enhanced in the PB of patients with EIA and established RA versus healthy controls. The percentage of ex-vivo (PMA/ionomycin/GolgiStop stimulated for 3 h) CD3+CD4+IL-17+IFN-γ− (Th17) cells was low in healthy controls [median 0·40, interquartile range (IQR) 0·24–0·65%], but elevated significantly in patients with RA (median 0·59, IQR 0·38–1·55%) and EIA (median 0·81, IQR 0·15–1·46%; P = 0·0067 by Kruskal–Wallis test, P < 0·05 for RA and EIA versus HC by Dunn's post-test). The percentage of cells expressing both IL-17 and IFN-γ was increased in patients with RA but not EIA versus HC. The percentage of CD4+ T cells expressing IFN-γ but not IL-17 was high in all groups, with no differences observed between healthy controls and patients with either EIA or RA (Fig. 1).
Fig. 1.
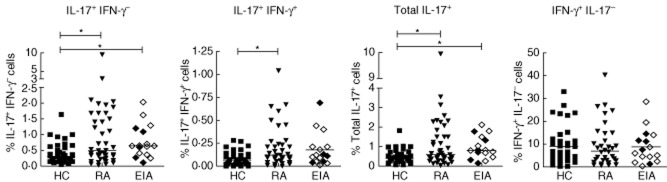
Interleukin (IL)-17+CD4+ T cell frequencies are increased in early inflammatory arthritis (EIA) and rheumatoid arthritis (RA). Peripheral blood mononuclear cells (PBMC) were isolated by density gradient separation and plated at a density of 1 × 106 with phorbol myristate acetate (PMA) and ionomycin in the presence of GolgiStop for 3 h prior to staining for CD3, CD4, IL-17 and interferon (IFN)-γ. Symbols represent individual patients and lines median values. Between-group comparisons were performed using the Kruskal–Wallis test with Dunn's post-test (versus healthy controls). A number of patients within the EIA group could be classified at baseline as RA using the 1987 criteria, shown by open symbols; those with undifferentiated arthritis (UA) are designated by closed symbols within the EIA group. *P < 0·05.
PBMCs from healthy controls and patients with EIA were also stimulated with LPS for 4 h and the percentage of TNF-α or IL-6 expressing CD14+ monocytes analysed, but no differences were observed (data not shown).
T cell and stromal cytokines and chemokines are increased in EIA and RA
Sera were available from 12 healthy controls, 17 patients with established RA and 14 patients with EIA at baseline, for analysis of cytokine and chemokine levels using a 25-plex Luminex assay. Serum IL-1β, IL-2, IL-4, IL-17 and MIP-1α were all increased in RA and EIA versus HC. Some cytokines were differentially elevated in either RA or EIA: IL-1Ra, IL-15 and IFN-α were increased in EIA versus HC, and IL-6 and TNF-α were increased in RA but not EIA versus HC (Fig. 2). No differences were observed in serum levels of GM-CSF, IFN-γ, IL-2R, IL-5, IL-7, IL-8, IL-10, IL-12 (p40/p70), IL-13, IP-10, MCP-1, MIG, MIP-1β or RANTES. Cytokines and chemokines were above the detection limits in the great majority of serum samples.
Fig. 2.
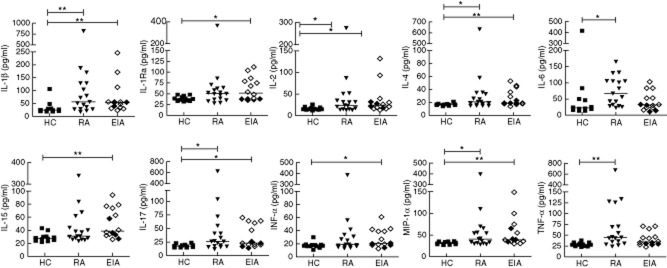
Serum cytokines and chemokines. Sera from patients with early inflammatory arthritis (EIA), rheumatoid arthritis (RA) and healthy controls (HC) were frozen at −80°C until thawed for analysis using a multiplex assay. Cytokines and chemokines with significant differences versus HC are shown. Symbols represent individual patients and lines median values. Between-group comparisons were performed using the Kruskal–Wallis test with Dunn's post-test (versus healthy controls). Within the EIA group, patients classified as RA are shown by open symbols, and undifferentiated arthritis (UA) by closed symbols. *P < 0·05; **P < 0·01.
Most EIA patients were classifiable as RA at baseline but full disease suppression was not achieved at 12 months
At baseline, 12 patients with EIA were classifiable as RA using the 1987 criteria, and a further two patients were classified as RA at subsequent visits [if the 2010 European League Against Rheumatism (EULAR)/ACR criteria [17] for RA were applied then one additional patient could be classified as RA at baseline]. Patients classifiable as RA using the 1987 criteria had significantly higher age, ESR and Larsen scores at baseline but there were no differences in tender or swollen joint count, CRP, DAS28, HAQ or EQ-5D (Table 2). Patients with undifferentiated arthritis could not be classified as either RA or another inflammatory arthritis, e.g. spondylarthropathy. Longitudinal data were available for 16 patients at baseline, 6 and 12 months' follow-up, while two patients attended only at baseline, and two others at baseline and 12 months. Disease activity was high at baseline in the majority of patients.
Table 2.
Characteristics of rheumatoid arthritis (RA) and undifferentiated arthritis (UA) patients at baseline within the early inflammatory arthritis (EIA) cohort
| RA at baseline (n = 14) | UA at baseline (n = 6) | |
|---|---|---|
| Female sex n (%) | 10 (71) | 5 (83) |
| Age (years) | 56 ± 12 | 40 ± 13* |
| Symptom duration (months) | 10 ± 6 | 12 ± 8 |
| Rheumatoid factor titre | 38 (16, 147) | 13·5 (10, 165) |
| Anti-CCP titre (n = 9) | 52 (1, 340)† | 2 (2, 2·7) |
| Tender joint count | 16·5 (12·5, 19·3) | 12 (4·5, 23·75) |
| Swollen joint count | 2·0 (0·0, 6·5) | 2·0 (0·0, 6·5) |
| Assessor global score | 48 (35, 61) | 49 (37, 62) |
| ESR | 28 (13, 46) | 14 (4, 22)* |
| DAS28 | 5·5 (4·7, 6·8) | 4·6 (3·3, 6·0) |
| C-reactive protein | 4 (4, 23) | 4 (4, 128) |
| Modified HAQ | 0·94 (0·35, 1·56) | 0·82 (0·43, 1·69) |
| Larsen score | 2 (0, 2) | 0 (0, 0·5)* |
| EQ-5D total | 0·62 (0·40, 0·74) | 0·69 (0·44, 0·80) |
Patients in the EIA cohort were classified as either UA or RA based on fulfilment of the 1987 American College of Rheumatology (ACR) criteria for RA. Values are presented as mean ± standard deviation or median (interquartile values).
P < 0·05 versus RA at baseline.
Anti-cyclic citrullinated peptide (CCP) titre was not available for all patients at baseline. DAS28: disease activity score 28; ESR: erythrocyte sedimentation rate; HAQ: health assessment questionnaire.
Responses to therapy were variable, but Physician Global scores, ESR and DAS28 fell between baseline and 12 months (Fig. 3). When patients receiving DMARDs were considered separately, mean DAS28 fell from 5·3 to 4·2 (compared to 5·3–4·5 for the whole cohort). Seven patients achieved moderate and three achieved good EULAR responses. Despite partial improvements in disease activity, overall health scores assessed by the EQ-5D and function did not improve. Larsen scores also increased significantly over time (even in those receiving DMARDs), suggesting that disease suppression was inadequate (Fig. 3).
Fig. 3.
Changes in clinical measures over 1 year of follow-up in early inflammatory arthritis (EIA). Sixteen patients had complete data available for visits at baseline, 6 and 12 months. Symbols and lines represent individual patients. Changes over time were analysed using repeated-measures analysis of variance (anova) with Bonferroni post-test versus baseline. Patients classified as rheumatoid arthritis (RA) are shown by open symbols and undifferentiated arthritis (UA) by closed symbols. *P < 0·05; **P < 0·01; ***P < 0·001.
Baseline immune function correlates with both conventional laboratory measures and outcome at 12 months
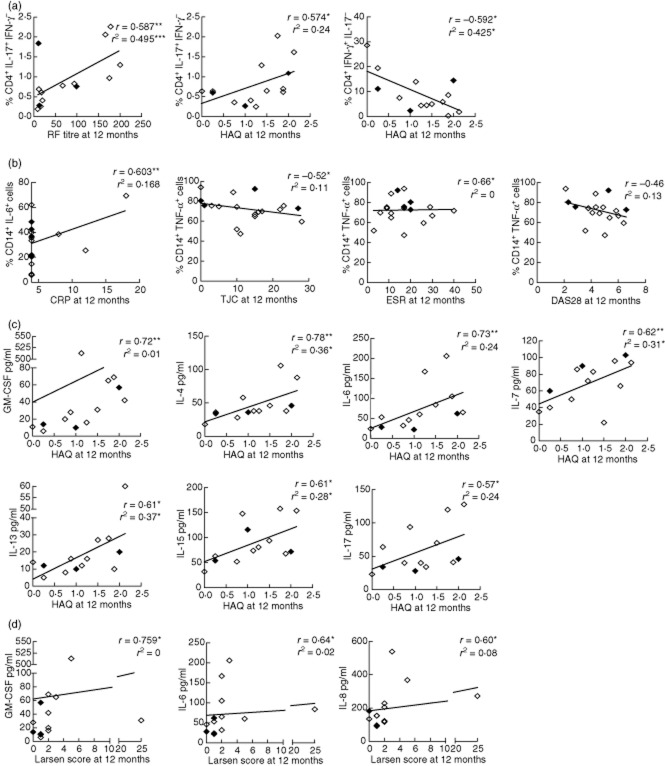
The baseline percentage of IL-17+IFN-γ−CD4+ cells was correlated with RF titre at baseline (r = 0·53, P = 0·03). Conversely, the percentage of IFN-γ+CD4+ T cells at baseline was correlated negatively with both initial patient global assessment (r = −0·673, P < 0·01) and baseline CRP (r = −0·573, P < 0·01). We investigated whether baseline cytokine expression by T cells and monocytes could be used to predict outcome at 1 year. The baseline percentage of IL-17+IFN-γ−CD4+ T cells remained correlated positively with RF titre (r = 0·587, P < 0·01) and also HAQ (r = 0·574, P < 0·03) at 12 months. The association between IL-17+IFN-γ−CD4+ T cells and RF titre was significant on linear regression (R2 = 0·495, β = 0·704, P = 0·005) and the relationship with HAQ approached significance (R2 = 0·24, β = 0·245, P = 0·07). Conversely, the percentage of IFN-γ+IL-17−CD4+ T cells at baseline was correlated negatively with HAQ at 12 months (r = −0·592, P < 0·05), and remained significant on linear regression (R2 = 0·425, β = −0·652, P = 0·01, Fig. 4a). Multiple linear regression for HAQ at 12 months including the percentage of both IFN-γ+IL-17−CD4+ T cells and IL-17+IFN-γ−CD4+ T cells showed that the percentage of IFN-γ+CD4+ T cells was an independent predictor of lower HAQ at 12 months (R2 = 0·576, β = −0·584, P = 0·01). The percentage of IL-6+ monocytes at baseline was correlated with CRP at 12 months (r = 0·603, P < 0·01), but baseline percentage of TNF-α+ monocytes was correlated negatively with both ESR (r = −0·66, P < 0·01) and tender joint count (r = −0·52, P < 0·05) with a trend to a negative association with DAS28 at 12 months (r = −0·46, P = 0·05). None of these factors remained significant on linear regression (Fig. 4b). Baseline cytokine expression in T cells or monocytes did not predict disease activity or damage at 12 months (data not shown), but there were correlations between HAQ at 12 months and a number of serum cytokines at baseline, including GM-CSF, IL-4, IL-6, IL-7, IL-13, IL-15 and IL-17. These relationships remained significant upon linear regression for serum levels of IL-4 (R2 = 0·36, P < 0·01), IL-7 (R2 = 0·31, P < 0·05) and IL-15 (R2=−0·28, P < 0·05, Fig. 4c). Baseline levels of serum GM-CSF, IL-6 and IL-8 were correlated with Larsen score at 12 months; however, none of these factors remained significant on linear regression, and the data are heavily skewed by a single data point (Fig. 4d). Logistic regression was performed for HAQ progression, high disease activity at 12 months and increase in Larsen score over 12 months using baseline clinical and laboratory variables, and no significant relationships were seen (data not shown).
Fig. 4.
Twelve-month outcome and baseline serum and cytokine expression. Twelve-month clinical data [rheumatoid factor (RF) titre, swollen joint count (SJC), tender joint count (TJC), disease activity score 28 (DAS28), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), Larsen score and health assessment questionnaire (HAQ)] were correlated with cytokine expression and serum cytokine levels at baseline (Spearman's correlation, r). Parameters with significant correlations are plotted. The line of best fit using linear regression is shown (r2). Patients classified as rheumatoid arthritis (RA) are shown by open symbols, and undifferentiated arthritis (UA) by closed symbols. *P < 0·05; **P < 0·01.
IL-6 expression by monocytes increases over time in EIA, but there are no changes in cytokine expression by CD4+ T cells
Sixteen patients attended for follow-up visits and had repeat analyses of T cell and monocyte cytokine expression. There were no significant changes in the percentages of IL-17+ or IFN-γ+CD4+ T cells or TNF-α+ monocytes over time, although considerable variation was observed in individuals (Fig. 5a–c). There was a significant elevation in the percentage of IL-6+CD14+ monocytes in response to LPS at 12 months versus baseline (Fig. 5d) in patients with or without DMARD therapy. There was little change in the levels of serum cytokines and chemokines between baseline and 6 months, except IP-10 and IL-2R, both of which fell at 6 months versus baseline, but were not elevated significantly versus healthy controls at baseline (data not shown).
Fig. 5.
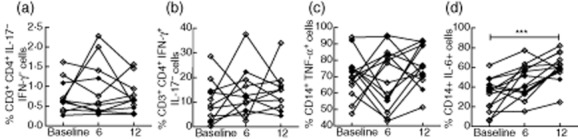
Interleukin (IL)-6 but not tumour necrosis factor (TNF)-α expression by monocytes increases over 12 months in early inflammatory arthritis (EIA). Peripheral blood mononuclear cells (PBMC) were isolated by density gradient separation and plated at a density of 1 × 106 with either phorbol myristate acetate (PMA)/ionomycin for 3 h (a,b) or lipopolysaccharide (LPS) for 4 h (c,d) prior to staining for CD3, CD4, IL-17 and interferon (IFN)-γ, or CD14, TNF-α and IL-6, respectively. Sixteen patients had complete data available for visits at baseline, 6 and 12 months. Symbols and lines represent individual patients. Patients classified as rheumatoid arthritis (RA) are shown by open symbols, and undifferentiated arthritis (UA) by closed symbols. Changes over time were analysed using repeated-measures analysis of variance (anova) or Friedman test with Dunn's multiple comparison post-test versus baseline. ***P < 0·001.
Discussion
This study shows that the levels of peripheral IL-17+IFN-γ−CD4+ T cells and serum IL-17 are increased in patients with treatment-naive EIA compared to healthy controls, to a similar extent as observed in established RA. These data are in agreement with several other studies showing that serum IL-17 levels or Th17 cell frequency are elevated in RA peripheral blood compared with healthy controls [3,14,18–21]. However, conflicting data exist [22,23], including a recent study which showed that patients with early RA within a cohort of EIA had lower levels of Th17 cells [24]. Some of this variation may be due to laboratory protocols: we stimulated PBMC for only a short period (3 h), where others have used isolated CD4+ cells with stimulation protocols ranging from 3 to 16 h. In addition, other studies recruited patients with very early disease (within weeks of symptom onset), while our cohort had median symptom duration of 9 months and may therefore be more consistent with patients with established disease, although they were not receiving DMARDs at baseline. These data suggest that Th17 cells are involved in inflammatory arthritis, regardless of disease stage or treatment, although treatment effects (both increases and decreases) have been observed in other studies [3,25–28].
We also identified altered serum cytokine and chemokine levels in our patient groups; IL-1β, IL-2, IL-4, IL-17 and MIP-1α were increased in both RA and EIA versus HC, and IL-1Ra, IL-15 and IFN-α in EIA versus HC. There was considerable variation between patients in both groups. A previous study investigated cytokine and chemokine levels in patients prior to and at onset of RA. IL-17 expression was increased in pre-patients (up to 5 years before symptom onset), although this difference was not statistically significant [29]. IL-17 levels decreased following disease onset, while MCP-1, MIP-1α, GM-CSF and G-CSF were increased significantly in pre-patients compared with control subjects, and a number of other cytokines became increased significantly versus controls in the 3 years prior to disease onset (IL-2R, IL-1β, IL-9, IL-10, eotaxin, GM-CSF). Other studies have also demonstrated increased IL-15 in early arthritis, which may be associated with disease severity [30,31]. The increase in serum IL-4 demonstrated in EIA and RA is perhaps surprising. Data from animal models show that IL-4 ameliorates CIA in DBA/1 mice, and synovial fluid mononuclear cells (SFMC) from RA patients treated with IL-4 in vitro are more resistant to Th1 activation [32]. A number of disease-modifying agents (methotrexate and gold) promote Th2 cells while suppressing Th1 cells [33,34]. In addition, many women experience improvement of their arthritis during pregnancy (reviewed in [35]), and the switch towards a Th2 profile in pregnancy may be at least partially responsible [36]. Synovial fluid from patients with RA has shown either low or absent IL-4 [37], although Raza et al. demonstrated a distinct cytokine profile in synovial fluid (SF) which included IL-4 [2]. In general, the chemokines and cytokines found to be elevated in our EIA cohort may represent an activated immune system, which continues during established RA, although there are likely to be treatment effects in patients with long-standing disease.
Despite the finding of significant increases in both serum IL-17 and IL-17+CD4+ T cells in peripheral blood, we were unable to demonstrate relationships between active disease (joint counts, ESR, global assessments, CRP or DAS28), in agreement with our previous work [14]. This is in contrast with Leipe et al., who reported a positive correlation between Th17 cells in peripheral blood and CRP in patients with early RA and psoriatic arthritis [3], all of whom were also treatment-naive, but had shorter symptom duration than our cohort. We were unable to demonstrate a decrease in IL-17+CD4+ T cells following treatment, again in contrast with Leipe et al., although they tested pre- and post-anti-TNF-α, which has a more potent effect on disease activity. These conflicting results suggest that the role of peripheral blood Th17 cells as a biomarker for either disease activity or treatment response is not yet convincing. However, we show a relationship between the baseline percentage of IL-17+CD4+ T cells with RF titre at both baseline and 12 months and HAQ at 12 months, which may indicate an association with more severe disease. We also demonstrate negative correlations between IFN-γ+CD4+ T cell frequency and both initial patient global assessment and CRP levels and HAQ at 12 months, which fits with existing data showing a negative correlation between IFN-γ and joint damage progression in both RA [13] and experimental models of arthritis [38,39]. We also identified a number of serum cytokines, including IL-6, that were correlated with either Larsen score or HAQ, which supports a previous finding that plasma IL-6 is associated with X-ray progression [40].
During the first year of follow-up we were unable to demonstrate significant changes in either cytokine expression by CD4+ T cells or the majority of serum cytokines/chemokines, despite reductions in ESR, physician global scores and DAS28. Mean DAS28 in EIA patients at baseline was 5·3 versus 4·5 after 12 months despite use of DMARDs ± steroids, similar to data from the methotrexate monotherapy arm in a large clinical trial involving patients with early RA [41]. However, we noted an increase in IL-6 expression by monocytes over time in the EIA cohort, which may explain the observed increase in serum IL-6 between EIA and established RA patients. Recently published data from other European early arthritis cohorts [42,43] have detected increased IL-6, particularly in patients with very early RA compared to undifferentiated arthritis [43]. As the majority of patients in our cohort had RA, we had insufficient power to detect a difference between these two groups. In common with our data, the study by Cascao et al. was unable to demonstrate changes in cytokines and chemokines following treatment with steroids or methotrexate [43]. It is possible that conventional treatment for early arthritis with DMARDS, with or without steroids, is insufficient to switch off the underlying immune dysregulation. Further work is needed in larger cohorts to establish if more intensive treatment improves clinical response and normalizes the cytokine profile, and if cytokine profiles can be used to guide treatment decisions.
Our study is limited by relatively low numbers, and by the relative high proportion of patients classifiable as RA at enrolment. This has prevented detailed regression analysis for predictors of either RA, response to treatment or persistent high disease activity at 1 year. Similarly, the relatively low numbers of patients may have inadequate power to demonstrate small differences in either cytokine expression or serum levels of cytokines or chemokines. Despite this we have been able to demonstrate that IL-17+CD4+ cells are elevated in EIA, and that the percentage of IL-6+ monocytes increases over time. IL-17 and IL-6 are associated with poor function and damage, respectively. In addition, multiple chemokines and cytokines are elevated in serum during EIA, and are not altered significantly by conventional DMARD therapy or corticosteroids.
Conclusion
IL-17 expressing CD4+ T cells and serum IL-17 are increased in peripheral blood from patients with EIA. IL-6 expressing monocytes increased significantly during follow-up, despite treatment with DMARD. We also observed incomplete clinical responses, suggesting that more intensive therapy is required early in the disease process in most patients.
Acknowledgments
This research was funded by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's & St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Hazes JMW, Luime JJ. The epidemiology of early inflammatory arthritis. Nat Rev Rheumatol. 2011;7:381–390. doi: 10.1038/nrrheum.2011.78. [DOI] [PubMed] [Google Scholar]
- 2.Raza K, Falciani F, Curnow SJ, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–795. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 4.Lubberts E, Joosten LA, Oppers B, et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 5.Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 6.Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- 7.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 8.Koenders MI, Lubberts E, van de Loo FAJ, et al. Interleukin-17 acts independently of TNF-alpha under arthritic conditions. J Immunol. 2006;176:6262–6269. doi: 10.4049/jimmunol.176.10.6262. [DOI] [PubMed] [Google Scholar]
- 9.Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–805. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 10.Genovese MC, Van den Bosch F, Roberson SA, et al. LY2439821, a humanized anti-IL-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase 1 randomized, double-blind, placebo-controlled, proof of concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 11.Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 12.Stamp LK, Easson A, Petterson L, Highton J, Hessian PA. Monocyte derived interleukin (IL)-23 is an important determinant of synovial IL-17A expression in rheumatoid arthritis. J Rheumatol. 2009;36:2403–2408. doi: 10.3899/jrheum.081304. [DOI] [PubMed] [Google Scholar]
- 13.Kirkham BW, Lassere MN, Edmonds JP, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 14.Gullick NJ, Evans HG, Church LD, et al. Linking power doppler ultrasound to the presence of Th17 cells in the rheumatoid arthritis joint. PLoS ONE. 2010;5:e12516. doi: 10.1371/journal.pone.0012516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Edmonds J, Saudan A, Lassere M, Scott D. Introduction to reading radiographs by the Scott modification of the Larsen method. J Rheumatol. 1999;26:740–742. [PubMed] [Google Scholar]
- 17.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 18.Metawi S, Abbas D, Kamal M, Ibrahim M. Serum and synovial fluid levels of interleukin-17 in correlation with disease activity in patients with RA. Clinical Rheumatol. 2011;30:1201–1207. doi: 10.1007/s10067-011-1737-y. [DOI] [PubMed] [Google Scholar]
- 19.Shen H, Goodall JC, Gaston JSH. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 20.Niu Q, Cai B, Huang Z-, Shi Y-, Wang L- Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int. 2012;32:2731–2736. doi: 10.1007/s00296-011-1984-x. [DOI] [PubMed] [Google Scholar]
- 21.van Hamburg JP, Asmawidjaja PS, Davelaar N, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 22.Jandus C, Bioley G, Rivals J-P, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–2317. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 23.Yamada H, Nakashima Y, Okazaki K, et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1299–1304. doi: 10.1136/ard.2007.080341. [DOI] [PubMed] [Google Scholar]
- 24.Arroyo-Villa I, Bautista-Caro M-B, Balsa A, et al. Frequency of Th17 CD4+ T cells in early rheumatoid arthritis: a marker of anti-CCP seropositivity. PLoS ONE. 2012;7:e42189. doi: 10.1371/journal.pone.0042189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue C, You X, Zhao L, et al. The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatol Int. 2010;30:1553–1557. doi: 10.1007/s00296-009-1179-x. [DOI] [PubMed] [Google Scholar]
- 26.Kageyama Y, Kobayashi H, Kato N. Infliximab treatment reduces the serum levels of interleukin-23 in patients with rheumatoid arthritis. Mod Rheumatol. 2009;19:657–662. doi: 10.1007/s10165-009-0217-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen D-Y, Chen Y-M, Chen H-H, Hsieh C-W, Lin C-C, Lan J-L. Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-alpha therapy. Arthritis Res Ther. 2011;13:R126. doi: 10.1186/ar3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aerts NE, De Knop KJ, Leysen J, et al. Increased IL-17 production by peripheral T helper cells after tumour necrosis factor blockade in rheumatoid arthritis is accompanied by inhibition of migration-associated chemokine receptor expression. Rheumatology. 2010;49:2264–2272. doi: 10.1093/rheumatology/keq224. [DOI] [PubMed] [Google Scholar]
- 29.Kokkonen H, Söderström I, Rocklöv J, Hallmans G, Lejon K, Rantapää Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:383–391. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 30.González-Álvaro I, Ortiz AM, Alvaro-Gracia J, et al. Interleukin 15 levels in serum may predict a severe disease course in patients with early arthritis. PLoS ONE. 2011;6:e29492. doi: 10.1371/journal.pone.0029492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamana A, Ortiz AM, Alvaro-Gracia J, et al. Characterization of serum interleukin-15 in healthy volunteers and patients with early arthritis to assess its potential use as a biomarker. Eur Cytokine Netw. 2010;21:186–194. doi: 10.1684/ecn.2010.0203. [DOI] [PubMed] [Google Scholar]
- 32.van Roon JA, Van Roy JL, Duits A, Lafeber FP, Bijlsma JW. Proinflammatory cytokine production and cartilage damage due to rheumatoid synovial T helper-1 activation is inhibited by IL-4. Ann Rheum Dis. 1995;54:836–840. doi: 10.1136/ard.54.10.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Constantin A, Loubet-Lescoulié P, Lambert N, et al. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis Rheum. 1998;41:48–57. doi: 10.1002/1529-0131(199801)41:1<48::AID-ART7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Kim TS, Kang BY, Lee MH, Choe YK, Hwang SY. Inhibition of interleukin-12 production by auranofin, an anti-rheumatic gold compound, deviates CD4+ T cells fro the Th1 to the Th2 pathway. Br J Pharmacol. 2001;134:571–578. doi: 10.1038/sj.bjp.0704298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Da Silva JAP, Spector TD. The role of pregnancy in the course and aetiology of rheumatoid arthritis. Clin Rheumatol. 1992;11:189–194. doi: 10.1007/BF02207955. [DOI] [PubMed] [Google Scholar]
- 36.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 37.Miossec P, Naviliat M, D'Angeac AD, Sany J, Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor β in rheumatoid synovitis. Arthritis Rheum. 1990;33:1180–1187. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- 38.Page C, Smale S, Carty S, et al. Interferon-gamma inhibits interleukin-1beta-induced matrix metalloproteinase production by synovial fibroblasts and protects articular cartilage in early arthritis. Arthritis Res Ther. 2010;12:R49. doi: 10.1186/ar2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams AS, Richards PJ, Thomas E, et al. Interferon-γ protects against the development of structural damage in experimental arthritis by regulating polymorphonuclear neutrophil influx into diseased joints. Arthritis Rheum. 2007;56:2244–2254. doi: 10.1002/art.22732. [DOI] [PubMed] [Google Scholar]
- 40.Knudsen L, Klarlund M, Skjødt H, et al. Biomarkers of inflammation in patients with unclassified polyarthritis and early rheumatoid arthritis. Relationship to disease activity and radiographic outcome. J Rheumatol. 2008;35:1277–1287. [PubMed] [Google Scholar]
- 41.Choy EHS, Smith CM, Farewell V, et al. Factorial randomised controlled trial of glucocorticoids and combination disease modifying drugs in early rheumatoid arthritis. Ann Rheum Dis. 2008;67:656–663. doi: 10.1136/ard.2007.076299. [DOI] [PubMed] [Google Scholar]
- 42.Gottenberg J-E, Dayer J-M, Lukas C, et al. Serum IL-6 and IL-21 are associated with markers of B cell activation and structural progression in early rheumatoid arthritis: results from the ESPOIR cohort. Ann Rheum Dis. 2012;71:1243–1248. doi: 10.1136/annrheumdis-2011-200975. [DOI] [PubMed] [Google Scholar]
- 43.Cascão R, Moura R, Perpétuo I, et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res Ther. 2010;12:R196. doi: 10.1186/ar3168. [DOI] [PMC free article] [PubMed] [Google Scholar]