Abstract
While much is known about tolerogenic dendritic cell effects on forkhead box protein 3 (FoxP3)+ regulatory T cells, virtually nothing is known about their effects on another arm of immunoregulation that is mediated by a subpopulation of immunosuppressive B cells. These cells suppress rheumatoid arthritis, lupus and inflammatory bowel disease in mice, and functional defects have been reported in human lupus. We show that co-stimulation-impaired tolerogenic dendritic cells that prevent and reverse type 1 diabetes mellitus induce the proliferation of human immunosuppressive B cells in vitro. We also show that the suppressive properties of these B cells concentrate inside the CD19+CD24+ B cell population and more specifically inside the CD19+CD24+CD38+ regulatory B cell population. We discovered that B cell conversion into suppressive cells in vitro is partially dependent on dendritic cell production of retinoic acid and also that CD19+CD24+CD38+ B regulatory cells express retinoic acid receptors. Taken together, our data suggest a model whereby part of the immunosuppressive properties of human tolerogenic dendritic cells could be mediated by retinoic acid which, in addition to its known role in favouring T cell differentiation to FoxP3+ regulatory T cells, acts to convert B cells into immunosuppressive cells.
Keywords: antigen-presenting cells, autoimmunity, B cells, immunomodulation, immunosuppressive
Introduction
Historically, B lymphocytes have been considered primarily as antibody-producing and secondarily, as antigen-presenting cells [1,2]. Given their role in producing pathogenic antibodies, especially in rheumatic diseases and systemic lupus erythematosus (SLE) [3,4], B lymphocytes have been targeted for immunomodulation by therapeutic depletion and other methods [5–8]. The involvement of B lymphocytes in type 1 diabetes mellitus (T1D) aetiopathogenesis was shown initially in the non-obese diabetic (NOD) mouse strain, where B lymphocyte-deficient mice or those treated with anti-immunoglobulin (Ig)M antibodies were protected significantly from disease [9,10]. In T1D, which is a T cell-driven autoimmune disease targeting the insulin-producing beta cells of the pancreatic islets of Langerhans, the pathogenic role of B lymphocytes has rested so far largely in their ability to act as antigen-presenting cells [11–16], producers of autoreactive antibodies [17,18] and modulators of the type of T cells that enter and are active within the pancreatic and islet environment [19]. B lymphocyte depletion, by anti-CD20 antibodies, stably prevented and, in some instances, reversed T1DM in NOD mice [20,21]. These observations motivated a clinical trial of the human anti-CD20 antibody (Rituximab) to preserve residual beta cell mass in new-onset T1D patients. The results are suggestive of a mild but statistically significant maintenance of beta cell function compared to untreated individuals [22].
Despite the large body of evidence supporting a pathogenic role for B lymphocytes in autoimmunity, important and reproducible data have suggested strongly that B lymphocytes could also act as immune suppressor cells [23]. These seemingly disparate observations were recently reconciled with the identification of at least two B lymphocyte populations that are inherently immunosuppressive, whose frequency and, possibly, activity, may change over time and during perturbations in peripheral tolerance [23,24]. Thus, under normal immune homeostasis, immunosuppressive B lymphocytes, now termed ‘regulatory B cells’ (Bregs), exist to maintain normal tolerance as part of an extended network of tolerogenic cells that include dendritic cells (DC) and regulatory T cells (Tregs).
Even though a number of cell surface markers characterize seemingly different populations of Bregs (reviewed in [23]), much attention has focused on a rare splenic B lymphocyte population in mice, whose existence was confirmed recently in humans [25], that expresses CD19highCD1dhighCD5+ and can suppress experimental contact hypersensitivity (CHS) in an antigen-restricted and interleukin (IL)-10-dependent manner [24,26,27]. In mice, these cells represent about 1% of total splenic B cells. Adoptive transfer of these B lymphocytes in a contact hypersensitivity mouse model effectively reduced inflammation in recipient mice sensitized with the same, but not with a different, chemical indicating that the suppressive function was antigen-specific. These cells required IL-10 for their suppressive effect [24,26,27]. In addition to these IL-10-producing cells, termed ‘B10’ Bregs, immature B lymphocytes which are probably transitional B220highCD21+CD23+ in phenotype, have been shown to suppress the adoptive transfer of T1D into immunodeficient NOD mice with diabetogenic immune cells [20]. B lymphocyte receptor-stimulated B cells from NOD mice delayed T1D in syngeneic prediabetic recipients, an effect dependent upon IL-10 [28]. Whether Bregs are deficient in frequency or function (or both) in T1D or whether purified/expanded Bregs from peripheral blood could be therapeutic, analogous to CD4+CD25+ Tregs, remains to be established.
An unexpected outcome of a Phase I clinical trial, where co-stimulation-impaired, tolerogenic autologous DC were administered to established T1D patients, was an increased frequency of B220+CD11c– cells. Although B220 on its own does not identify any specific immune cell population, as it is expressed in activated T cells and CD27– B cells [29,30], this phenomenon provoked a suspicion that B cells could represent the bulk of these cells. Flow cytometric surface phenotyping of the B220+CD11c– cells [31] suggested that they represented a late transitional B cell population that shared some cell surface proteins (CD5+CD10+CD24+CD38intermediate IL-10+) with at least one population of human Bregs reported and characterized recently [23,32,33]). We therefore hypothesized that the ex-vivo generated tolerogenic DC promoted suppressive B cell activity in part by increasing the frequency of such cells. In support of this hypothesis were data showing that CD19+B220+CD11c– IL-10+ cells obtained from freshly obtained peripheral blood mononuclear cells (PBMC) of recipients of the tolerogenic DC significantly suppressed the proliferation of T cells in allogeneic mixed leucocyte reaction cultures in vitro [31]. However, these data did not establish causality, nor did they offer substantive mechanistic insights into how tolerogenic DC might promote suppressive B cell activity. Herein, we provide novel data which directly address these questions.
These data suggest that the networks of tolerance against autoimmunity are not limited to T cells, but include B cells where a suppressive phenotype can be imprinted and modulated by tolerogenic DC.
Materials and methods
Enrichment and purification of human B and T cells
PBMC were obtained from whole blood of healthy adult volunteers from the Central Blood Bank of Pittsburgh, according to acceptable standards as mandated by the local Ethics Boards. Blood was diluted 1:1:1 with sterile phosphate-buffered saline (PBS) and Ficoll-Paque PLUS (Stem Cell Technologies, Vancouver, Canada) and then layered on the bottom of a sterile polypropylene tube. The blood was then centrifuged at 250 g for 30 min and the PBMC layer was removed. The PBMC were further washed in PBS and frozen, used directly in experiments or further enriched into specific immune cell populations by fluorescence activated cell sorter (FACS) or magnet-assisted cell separation/enrichment. For some experiments, frozen PBMC were thawed, separated or FACS-sorted into specific cell populations. Only viable cells (>90% viable as assessed by the LIVE/DEAD reagent (Invitrogen, Grand Island, NY, USA) by flow cytometry of an aliquot of the thawed cells) were considered in experiments using frozen PBMC as a source of cells.
B cell enrichment
Depending on the experiment and the abundance of B cell populations required, specific cell subsets were obtained either by FACS-sorting from freshly collected PBMC or by FACS-sorting from PBMC enriched into CD19+ cells. We routinely used the EasySep Human B cell Enrichment System (Stem Cell Technologies) to enrich CD19+ B cells from freshly collected or previously frozen PBMC. When using these enriched CD19+ cells as the source of specific populations, a series of non-overlapping fluorophore-conjugated antibodies were added prior to sorting by FACS. In some experiments, freshly collected PBMC or enriched CD19+ cells were processed to capture IL-10-secreting cells using the human IL-10 secretion system (Miltenyi Biotec, Bergisch Gladbach, Germany) prior to cell sorting by FACS. Alternatively, where indicated, IL-10-secreting B cells were enriched directly from FACS-sorted CD19+B220+CD11c– cells (from freshly collected whole PBMC). The human Bregs reported by Blair et al. [32], characterized as CD19+CD24+/intermediate CD27+CD38+/intermediate, were FACS-sorted from freshly collected PBMC or from PBMC cell cultures following staining with antibodies listed in the figure legends. We used the LIVE/DEAD cell viability reagent (Invitrogen) in all flow cytometry and FACS-sorting to ensure that only live cells would be considered in the purification and in the analyses.
T cell enrichment
T cells were enriched routinely over a high-affinity CD3 negative selection column (R&D Systems, Minneapolis, MN, USA). Freshly obtained PBMC were loaded onto Ig and anti-Ig-coated beads. B cells bind to anti-Ig-coated beads by F(ab)-surface Ig interactions. Monocytes bind to Ig-coated beads via Fc interactions. The resulting column eluate contains highly enriched T cell populations (routinely >90% CD3+ enrichment). The T cells were used in proliferation assays in B cell co-culture as described below.
Generation of human DC in vitro
The methods for generating the two human DC populations (control and immunosuppressive) have been described elsewhere [31]. Control DC (cDC), which are phenotypically immature, were obtained from PBMC precursors after a 6-day culture in vitro in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 [31]. Tolerogenic co-stimulation impaired immunosuppressive DC (iDC) were generated similarly to cDC; however, the 6-day culture was supplemented with phosphorothioate-modified anti-sense oligonucleotides targeting the 5′ end of the CD40, CD80 and CD86 gene primary transcripts during the culture period [31]. Each of the anti-sense oligonucleotides were added to the culture at a final concentration of 3·3 mM. The sequences of each of the anti-sense oligonucleotides are: CD40: 5′-ACT GGG CGC CCG AGC GAG GCC TCT GCT GAC-3′; CD80: 5′-TTG CTC ACG TAG AAG ACC CTC CCA GTG ATG-3′; and CD86: 5′-AAG GAG TAT TTG CGA GCT CCC CGT ACC TCC-3′ [31]. On day 6 of the cDC and iDC cultures, the cells were harvested and checked for viability (trypan blue) and purity (forward- versus side-scatter plots and percentage of CD11c+ cells by flow cytometry) prior to further experimentation.
FACS and flow cytometry
FACS and flow cytometry were performed using a FACSCalibur or a FACSVantage workstation with Aria or Diva support (BD Biosciences, San Jose, CA, USA). For flow cytometry, the specific event acquisition gates were established using appropriate isotype antibody controls. Freshly obtained PBMC (1 × 105–2 × 106) or enriched CD19+ cells from freshly obtained PBMC were stained with human-specific antibodies, purchased from BD Biosciences unless noted otherwise. Antibodies for B cells were CD27 (clone M-T271), CD38 (clone HIT2), CD19 (clone SJ25C1), CD24 (clone ML5), CD5 (clone UCHT2), B220 (clone RA3-6B2), CD1d (clone CD1d142) and IL-10 (internal; JES3-19F1). We used the LIVE/DEAD cell viability reagent (Invitrogen) in all flow cytometry and FACS sorting to ensure that only live cells would be considered in the purification and in the analyses.
When FACS was used to enrich DC or when DC were characterized by flow cytometry, we used Fc-Block pretreatment (BD Biosciences) prior to antibody staining. We used clone B-ly6 (BD Biosciences) for CD11c-specific FACS and flow cytometry.
Detection and enrichment of RA-producing DC
To detect and enrich retinoic acid (RA)-producing DC from the GM-CSF/IL-4 cultures (cDC or iDC), we used the Aldefluor reagent (Stem Cell Technologies), a substrate of aldehyde dehydrogenases (ALDH) which are the rate-limiting enzymes for RA biosynthesis [34,35]. In the presence of bioactive enzyme, the substrate is converted into a fluorescent product and cells with such bioactivity are readily detectable to facilitate cell sorting or flow cytometry. Cells were stained with CD11c-specific antibodies and then co-treated as directed by the manufacturer with Aldefluor. The CD11c+Aldefluor+ cells were sorted by FACS, or their frequency was measured by flow cytometry.
Proliferation of B cell populations in the presence of DC in vitro
Freshly isolated PBMC (1 × 105–2 × 105), enriched CD19+ cells or specific B cell populations purified from freshly collected PBMC by FACS were placed into culture with or without an equal number of cDC, iDC or vehicle control in RPMI-1640 with 10% fetal bovine serum (FBS), supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 1× MEM-NEAA, 55 mM 2-mercaptoethanol and 100 μg/ml gentamicin (all purchased from Gibco-Invitrogen, Carlsbad, CA, USA). Proliferation of B cell populations was measured by flow cytometry [36–38] using a commercial 5-bromo-2-deoxyuridine (BrdU)+-containing kit (BrdU Flow Kit; BD Biosciences) in combination with antibodies to characterize the proliferating cells (antibodies as listed earlier). BrdU was added to individual wells on the final day of culture to a final concentration of 1 mM. We used the LIVE/DEAD cell viability reagent (Invitrogen) in all flow cytometry and FACS-sorting to ensure that only live cells would be considered in the purification and in the analyses. Where shown, absolute cell numbers were calculated by multiplying the frequency of the specific cell population inside the live total cell gate in the flow cytometry by the total number of cells in the culture well [determined by Coulter counter measurement (Z1 instrument, Beckman Coulter, CA, USA)].
In-vitro mixed leucocyte suppression assay
CD19+CD24+ cells, CD19+CD24+CD38+ B cells and CD19+CD24–CD38– cells FACS-purified directly from freshly procured PBMC or from 48–72 h cDC/iDC : CD19+ B cell co-cultures were added to allogeneic irradiated PBMC and syngeneic T cells in vitro for standard mixed leucocyte T cell proliferation assays (mixed leucocyte cultures: MLC) in RPMI-1640 with 10% FBS, supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 1× MEM-NEAA, 55 mM 2-mercaptoethanol and 100 μg/ml gentamicin (all purchased from Gibco-Invitrogen). Equal numbers (1 × 105–2 × 105 cells) of irradiated allogeneic PBMC were added to equal numbers of CD3+ T cells (T cells and B cells were from the same individual). B cell populations were added at a 1:10 ratio (to T cells). T cell proliferation was measured after 5 days by BrdU flow cytometry [36–38]. We used the LIVE/DEAD cell viability reagent (Invitrogen) to ensure that the measurements considered live cells. Where shown, cell numbers were calculated by multiplying the frequency of the specific cell population inside the live total cell gate in the flow cytometry by the total number of cells in the culture well determined by Coulter counter measurement.
Gene expression analysis
Two × 106 FACS-sorted CD19+CD24+CD38+ B cells from freshly collected PBMC of healthy adults were prepared for real-time, semi-quantitative reverse transcription–polymerase chain reaction (RT–PCR) to detect the steady state expression or RA receptors. Total RNA was isolated using the RNEasy mRNA Isolation System (Qiagen, Valencia, CA, USA). cDNA was synthesiszed using the SuperScript III System (Invitrogen) and then real-time PCR was conducted with the iQ SYBR Green Mix (Bio-Rad, Hercules, CA, USA) in an iCycler. Relative steady-state mRNA levels were calculated based on the 2Δ-ΔCt method after correction for beta actin gene expression levels.
The primer sequences used were identical to those used by Ballow et al. [39], as follows: RAR-α1 forward 5′-AGGCGCTCTGACCACTCTCCA-3′, reverse 5′-CCCACTTCAAAGCACTTCTG-3′; RAR-α2 forward 5′-ATGTACGAGAGTGTGGAAGTCGGG-3′, reverse 5′-CCCACTTCAAAGCACTTCTG-3′; RAR-β2 forward 5′-TGGATGTTCTGTCAGTGAGTCCT-3′, reverse 5′-CCCACTTCAAAGCACTTCTG-3′; RAR-γ1 forward 5′-GCCACCAATAAGGAGCGACTC-3′, reverse 5′-CCCACTTCAAAGCACTTCTG-3′; and RAR-γ2 forward 5′-GCGATGTACGACTGTATGGAAACG-3′, reverse 5′ CCCACTTCAAAGCACTTCTG-3′.
Treatment of B cells and DC : B cell co-cultures with RA or selective pan-RAR antagonist ER50891 in vitro
Purified, lipopolysaccharide (LPS)-free all-trans RA (RA; Sigma Aldrich, St Louis, MO, USA) was added to 2 × 106 freshly-collected, cultured PBMC from normal human adult donors at 20 nM final concentration in 24-well plates. Cells were incubated in RPMI-1640 with 10% FBS, supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 1× MEM-NEAA, 55 mM 2-mercaptoethanol and 100 μg/ml gentamicin (all purchased from Gibco-Invitrogen) at 37 degrees for 24–72 h, depending on the particular experiment. The cells were then washed extensively and CD19+CD24+CD38+ B cell frequency was measured by flow cytometry. Similarly, 2 × 106 CD19+ B cells were added to equal numbers of iDC in the presence or absence of the pan-RAR selective antagonist ER50891 (Tocris Biosciences, Minneapolis, MN, USA) at a final concentration of 1 μM for 72 h. The B cells and/or DC were subsequently isolated by magnet assistance for further analysis.
Statistical analysis of the data
Statistically relevant differences among means (Student's t-test, analysis of variance: anova) and medians (paired Wilcoxon's test) were ascertained using GraphPad Prism version 4 software (GraphPad, La Jolla, CA, USA). In all statistical analyses, a P-value < 0·05 was considered to represent statistically significant differences.
Results
The immunosuppressive subpopulation in the DC-sensitive CD19+B220+CD11c– IL-10+ B cells consists of CD19+CD24+ B cells and, in particular, suppressive CD19+CD24+CD27+CD38+ B cells
We have shown previously that T1D patients treated with cDC or iDC exhibit an increase in the frequency of B220+CD11c– cells in the peripheral blood [31].
Flow cytometry of these cells [31] suggested that they represented a late transitional B cell population that shared some cell surface proteins (CD5+CD10+CD24+CD38intermediate) with at least one population of human Bregs recently reported and characterized [23,32,33]). Thus, we hypothesized that the increase in the frequency of B220+CD11c– cells in DC recipients was a consequence of, and reflected an increase in, the number of constituent suppressive immunoregulatory B cell populations that express B220 on the surface, even though B220 on its own does not define B cells [29,30]. We discovered subsequently that a population of CD19+B220+CD11c– IL-10+ cells accounted for an average of 48% of the B220+CD11c– cells (V. D. C., B. P. and N. G., unpublished data) and, more importantly, that the CD19+B220+CD11c– IL-10+ population was immunosuppressive in vitro [31].
To date, two human B cell populations with immunosuppressive ability in vitro have been characterized, mainly by cell surface markers [23,25,26,32,40]. Although both populations produce IL-10, their surface phenotypes are different. ‘B10’ Bregs express the CD1d and CD5 markers [25,26], whereas the other suppressive cells are characterized specifically as CD19+CD24+/intermediateCD38+/intermediate [23,32,40]. We first asked if the suppressive properties of the CD19+B220+CD11c– IL-10+ B cells shown in [31] were concentrated in either or both of the currently characterized Bregs (CD19+CD1d+CD5+ or CD19+CD24+CD27+CD38+ B cells [23,25,26,32,40]), or if other novel CD19+ cell populations inside the parental CD19+B220+CD11c– IL-10+ cell population possessed suppressive ability. Using flow cytometry (Supplementary Fig. S1 shows the approach), we determined that CD19+CD24+CD27+CD38+ cells accounted for 19·85% (median) of FACS-sorted CD11c–B220+CD19+ IL-10+ cells from freshly acquired PBMC (Fig. 1a; n = 6 healthy unrelated adult individuals). We did not detect any B10 Bregs (CD19+CD1d+CD5+ IL-10+ cells) [25] inside the CD11c–B220+CD19+ IL-10+ population (not shown).
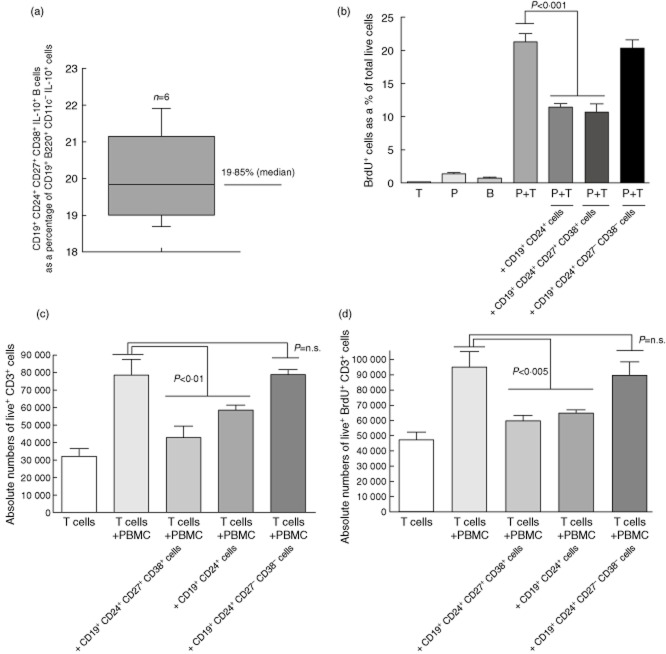
Fig. 1.
Frequency and suppressive character in vitro of CD19+CD24+ B cells from freshly collected peripheral blood mononuclear cells (PBMC) from normal healthy humans. (a) The graph summarizes the median and the range of the % CD19+CD24+CD27+CD38+ B cells [human regulatory B cell (hBreg) constituent] in CD19+B220+CD11c– IL-10+ cells in freshly obtained PBMC of six normal healthy adults. (b) CD19+CD24+ B cells are suppressive in allogeneic mixed lymphocyte reaction (MLR) in vitro. The graph summarizes the proliferation of T cells in co-cultures consisting of column-enriched PBMC-derived T cells, PBMC-derived flow-sorted CD19+CD24+CD27–CD38– B cells, flow-sorted CD19+CD24+CD38+ B cells, flow-sorted CD19+CD24–CD38– B cells and allogeneic irradiated human PBMC. The suppressive ability of the CD19+CD24+ B cells (represented by the 5th bar from the left of the graph) is compared to that of the characterized CD19+CD24+CD38+ Bregs (represented by the 6th bar from the left). The bars in the graph shows the mean 5-bromo-2-deoxyuridine (BrdU)+ cells as a percentage of total viable cells in quintiplicate co-cultures. The error bars show the standard error of the mean (s.e.m.). Statistically relevant differences are shown in the graph as analysed using two-way analysis of variance (anova). These data are representative of six independently conducted experiments on separate occasions. T: T cells alone; P: irradiated allogeneic PBMC; B: CD19+CD24+ cells alone. (c) The bars in the graph shows the absolute numbers of viable CD3+ T cells in co-cultures established as described in (b). The error bars show the s.e.m. of quintiplicate co-cultures. Statistically relevant differences are shown in the graph as analysed using two-way anova. These data are representative of three independently conducted experiments on separate occasions. (d) The bars in the graph show the absolute numbers of proliferating (BrdU+) live CD3+ T cells in co-cultures established as described in (b). The error bars show the s.e.m. of quintiplicate co-cultures. Statistically relevant differences are shown in the graph as analysed using two-way anova. These data are representative of three independently conducted experiments on separate occasions.
CD19+ cells enriched from freshly collected PBMC were then FACS-sorted into three B cell populations: (i) CD11c–CD19+CD24+, (ii) CD11c–CD19+CD24+CD27–CD38– and (iii) CD11c–CD19+CD24+CD27+CD38+ cells (sorting approach shown in Supplementary Fig. S2a and purity of the sorted cells shown in Supplementary Fig. S2b,c). Unlike the CD11c–CD19+CD24+CD27+CD38+ cells, the CD11c–CD19+CD24+CD27–CD38– cells were unable to suppress T cell proliferation in allogeneic MLC (Fig. 1b,c). Unexpectedly, FACS-sorted CD11c–CD19+CD24+ cells exhibited statistically similar suppressive ability as the CD19+CD24+CD27+CD38+ B cells (Fig. 1b,c). In all instances, the lower T cell frequency (Fig. 1c) in the MLC was due to decreased proliferation and absolute numbers of live CD3+ T cells (Fig. 1c,d) and not to an increase in the numbers of dead cells (including T cells) or changes in B cell frequency (Supplementary Fig. S3 and S4).
Human iDC increase the frequency of suppressive CD19+CD24+CD38+ B cells in vitro
We hypothesized that iDC could directly affect the frequency of the suppressive CD19+CD24+CD27+CD38+ B cells and that a potentially significant increase in their number could account for the increased frequency of B220+CD11c– cells in the PBMC of iDC recipients [31]. To test this, freshly collected PBMC from healthy adults were enriched into CD19+ cells. Of these cells, 2 × 106 were then cultured in the presence of an equal number of autologous cDC, iDC (generated from the same PBMC) or PBS vehicle for 3 days. The frequency of CD19+CD24+CD38+ cells in those co-cultures was then measured by flow cytometry. Figure 2a shows that, in the presence of iDC, the frequency of CD19+CD24+CD38+ B cells was increased significantly. Furthermore, the frequency of CD27+ cells inside the CD19+CD24+CD38+ population was increased substantially. This increase in frequency was due specifically to an increase in the proliferation of CD19+CD24+CD38+ cells, especially the CD27+ subpopulation (measured as the frequency and absolute number of BrdU+ cells; Fig. 2a,b). Interestingly, exposure of the CD19+ B cells to the iDC increased significantly the numbers of viable cells in general (Fig. 2a, P2 peak in the LIVE/DEAD histogram at the top). When comparing the segregation of the individual cell surface markers used to identify the B cells, the only discernible difference is in the generation of two peaks representing the CD19+ population in the presence of cDC or iDC (Fig. 2c). There are no other significant differences in the segregation of the other markers used (CD24, CD27, CD38; Fig. 2c). Specificity of the antibodies and non-specific antibody binding was controlled by the appropriate isotypes (Supplementary Fig. S5).
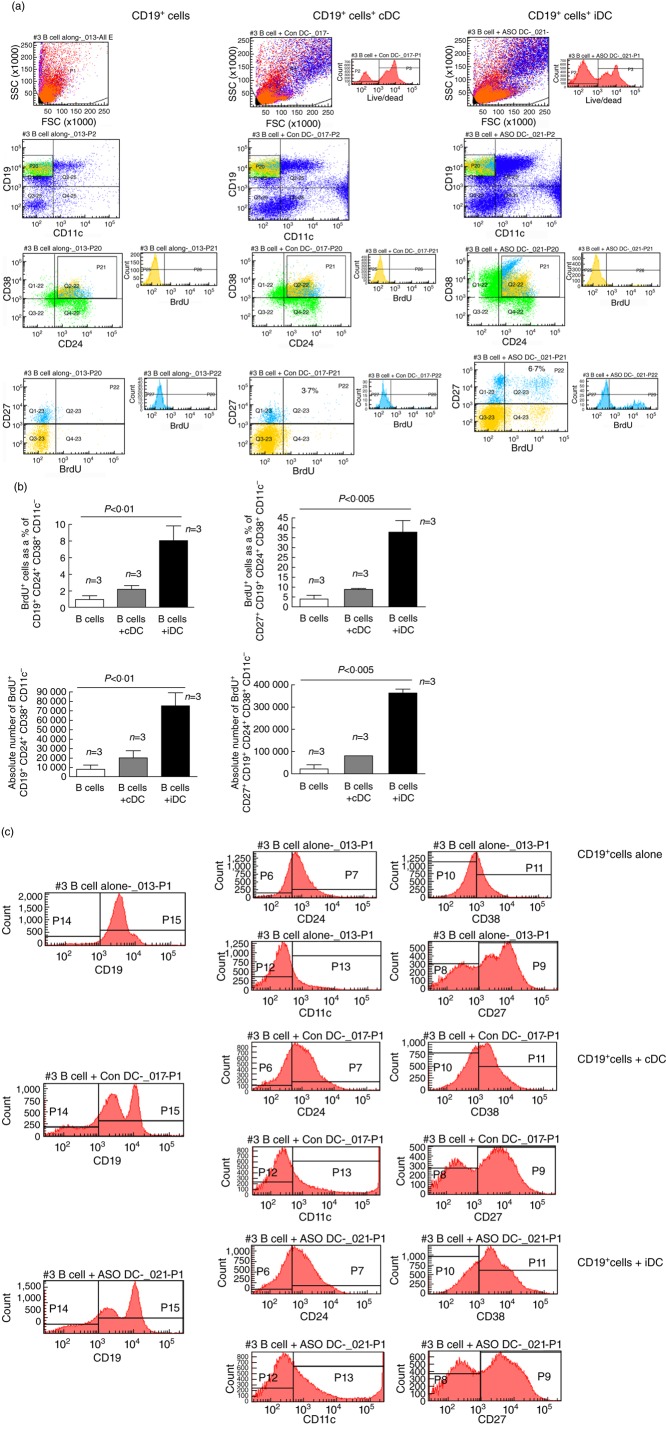
Fig. 2.
The frequency and absolute cell numbers of suppressive B cells is increased in co-culture with control DC (cDC) and immunosuppressive DC (iDC) in vitro. (a) Flow cytometry was conducted as shown. The frequency of human suppressive B cells (CD19+CD24+CD38+CD27+/–) measured after a 2-day co-culture of thawed normal human donor peripheral blood mononuclear cells (PBMC) with autologous cDC or iDC generated from the same PBMC donor. The quadrant plots at the top of the figure show the frequency of CD19+CD11c– cells as a percentage of total viable cells in culture. The quadrant plots in the lower middle of the figure show the CD24+CD38+/intermediate cell frequency as a percentage of CD19+CD11c– cells. The quadrant plots and histograms at the bottom serve to distinguish whether the differences in the frequencies of the B cells analysed in the preceding quadrants are due to changes in the expression of single surface molecules (CD27) or to proliferative/differentiation events [5-bromo-2-deoxyuridine (BrdU)+] in response to provision of the DC in the co-culture. These data are representative of the outcome of triplicate co-cultures on at least two independent occasions using PBMC from two unrelated healthy adults. The events coloured green represent the CD38+CD24+ cells inside the CD19+CD11c– parental population. The events in yellow represent the BrdU+ cells and the events in light blue represent the CD27+ cells. (b) A graphical summary of the frequency (upper graphs) and the absolute number (lower graphs) of CD19+CD24+CD38+CD11c– B cells detected in 2- day co-cultures of freshly collected PBMC and control DC (cDC) or immunosuppressive DC (iDC). The graphs at the top summarize the frequency of proliferative (BrdU) cells as a percentage of CD19+CD24+CD38+CD11c– B cells (top left) and as a percentage of CD27+CD19+CD24+CD38+CD11c– B cells (top right). The graphs at the bottom summarize the absolute cell numbers of CD19+CD24+CD38+CD11c– B cells (bottom left) and of CD27+CD19+CD24+CD38+CD11c– B cells (bottom right). The bars in the graphs show the mean of triplicate co-cultures and the error bars the standard error of the mean (s.e.m.). In all three graphs, the differences among the means were stastitically significant, as shown above each graph [two-way analysis of variance (anova)]. (c) Other than the acquisition of a biphasic CD19+ expression pattern, the segregation of surface proteins on the co-cultured cells was not significantly different among control, cDC and iDC conditions after 2 days. The data are representative of at least four independent co-cultures of thawed PBMC with cDC, iDC or media control.
Human cDC and iDC express the rate-limiting enzyme for RA biosynthesis and produce RA in vitro
Gene chip-based expression analysis of the autologous DC used in the Phase I trial [31] revealed that the rate-limiting enzyme for RA biosynthesis, ALDH1A2, was expressed in cDC and iDC generated from PBMC of normal adults (data not shown). To confirm the gene chip data and to demonstrate that cDC and iDC produce RA, we employed a reagent (Aldefluor) that reacts with RA-producing cells to identify and measure the frequency of RA-producing cells by flow cytometry.
In Fig. 3a we show that cDC and iDC generated in vitro from the PBMC of two genetically discordant healthy adults react with Aldefluor. iDC are more reactive with Aldefluor compared to cDC on a per-cell basis [based on mean fluorescence intensity (MFI) measurements]. Furthermore, the frequency of iDC that are Aldefluor+CD11c+ is higher than cDC that are Aldefluor+CD11c+ in DC generated from the PBMC of six unrelated healthy adults (summarized in the graph in Fig. 3b). To ensure that Aldefluor positivity was concentrated specifically inside the CD11c+ population, we repeated the flow cytometry by first gating CD11c+ cells and then measuring the frequency and MFI of Aldefluor+ cells inside the CD11c+ cell gate (Supplementary Fig. S6). This analysis confirmed our findings shown in Fig. 3a,b. Taken together, these data suggest that the increased Aldefluor reactivity in iDC compared to the cDC, even though both populations produce RA, is a consequence of more RA production by iDC compared to cDC on a per cell basis (MFI of Aldefluor in cDC versus iDC in Supplementary Fig. S6).
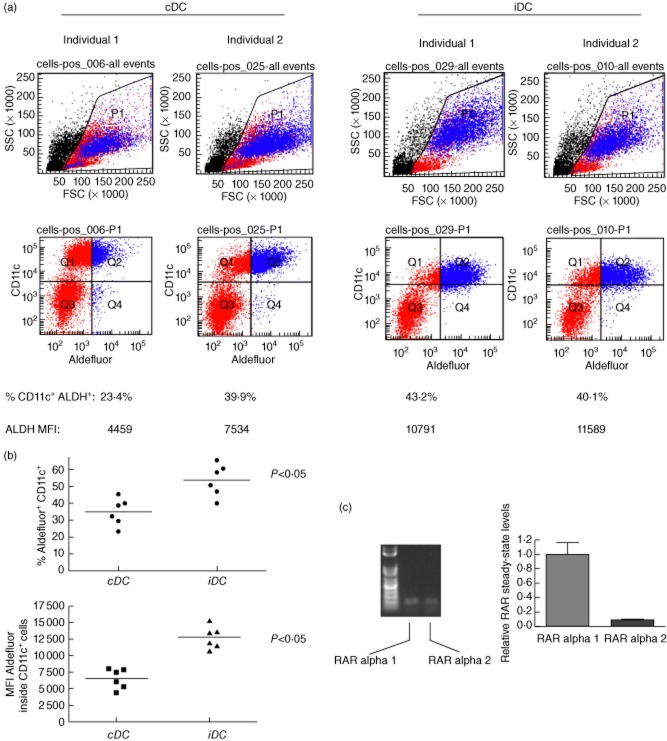
Fig. 3.
Human control dendritic cells (cDC) and immunosuppressive DC (iDC) produce rheumatoid arthritis (RA). (a) cDC and iDC area Aldefluor-reactive. cDC and iDC generated from peripheral blood mononuclear cells (PBMC) from two unrelated healthy adult individuals were stained with Aldefluor and CD11c-specific antibodies. Shown are representative flow cytometric profiles. At the bottom are the values of percentage of CD11c+Aldefluor+ cells and the geometric mean fluorescence intensity (MFI), corresponding to the frequency of CD11c+Aldefluor+ cells at the end of the cDC/iDC generation process and the mean Aldefluor reactivity on a per cell basis, respectively. These data are representative of four independently established cDC/iDC cultures from PBMC of six unrelated, healthy adult individuals. The events coloured red indicate the CD11c+ cells and those coloured blue the Aldefluor-reactive cells. (b) A graphic representation of the frequency of CD11c+Aldefluor+ cDC and iDC generated from PBMC of six unrelated, healthy adult individuals. The data are shown as % Aldefluor+CD11c+ cells of the total cultured cells generated in vitro. The median percentage of CD11c+Aldefluor+ cells between cDC and iDC cultures is statistically significant (P < 0·05) by Mann–Whitney U-test. (c) Steady-state retinoic acid receptors (RAR) alpha 1 and alpha 2 mRNA is readily detectable in flow-sorted human regulatory B cells (Bregs). Total RNA was harvested from 2 × 106 flow-sorted CD19+CD24+CD38+ B cells from freshly collected PBMC of healthy adults, reverse-transcribed and the cDNA was then amplified in semi-quantitative polymerase chain reaction (PCR using RAR isoform-specific primers. Only the alpha 1 and alpha 2 isoforms were amplifiable in cDNA. The gel shows two consistently amplifiable PCR products representing RAR alpha 1 and alpha 2 and the graph on the right shows the relative steady-state mRNA levels of the two detectable transcripts as a fold expression/under-expression compared to the alpha 1 isoform. The bars represent the means of mRNA from cells of triplicate flow sorts and the error bars the standard error of the mean (s.e.m.). The differences in relative expression levels are statistically significant (P < 0·05, Student's t-test).
Human CD19+CD24+CD38+ Bregs express RA receptors
That cDC and iDC produced RA (Fig. 3a) and the evidence that RA is part of a mechanism that determines the generation of Tregs and possibly Bregs in the periphery [41–47], compelled us to propose that Breg biology might be regulated by RA. This would crucially depend upon Bregs expressing receptors for RA. As the frequency of the CD19+CD24+CD38+ Bregs is rare in freshly collected PBMC, protein-based quantitation of RA receptor isoforms less abundant than the major alpha isoform is challenging (e.g. Western blotting). We chose instead to measure steady-state mRNA to determine RA receptor expression and to then compare the relative expression levels of the isoforms using real-time semiquantitative RT–PCR. We established that only RAR alpha 1 and alpha 2 were amplifiable by RT–PCR from total RNA of purified CD19+CD24+CD38+ Bregs (Fig. 3c). Following subsequent RT–quantitative PCR (qPCR) amplifications, when setting the absolute expression levels of RAR alpha 1 to a value of 1, it became apparent that RAR alpha 2, even as it is expressed when compared to RAR alpha 1, is expressed at significantly lower relative levels (Fig. 3c). RAR beta and gamma were undetectable in all attempts to reverse-transcribe and then amplify from total RNA.
RA treatment of CD19+ B cells in vitro increases the frequency of CD19+CD24+CD38+ Bregs
Considering that cDC and iDC produced RA and that CD19+CD24+CD38+ Bregs expressed RAR alpha, we asked if RA could be responsible, at least in part, for the proliferation of the CD19+CD24+CD38+ Bregs when CD19+ B cells were cultured with DC (Fig. 2). In Fig. 4a and the summary graph (Fig. 4b) we show the frequency of CD19+CD24highCD38high (cells represented inside the P15 gate of the FACS quadrant plots) in freshly collected PBMC from two of six healthy adult individuals after 3 days of culture in the presence/absence of RA. Even though there is significant variability in the frequency of these cells in freshly collected PBMC among the six healthy, unrelated adult individuals (Fig. 4c), when RA was added to CD19+ cells enriched from the PBMC of all individuals, the frequency of CD19+CD24+CD38+ cells was increased significantly after 3 days (Fig. 4c). This increase, however, was not due to proliferation, as the frequency and numbers of viable BrdU+ cells that are represented in the CD19+CD24+CD38+ population were not statistically different among control and RA-treated cultures of CD19+ cells from two individuals (data not shown).
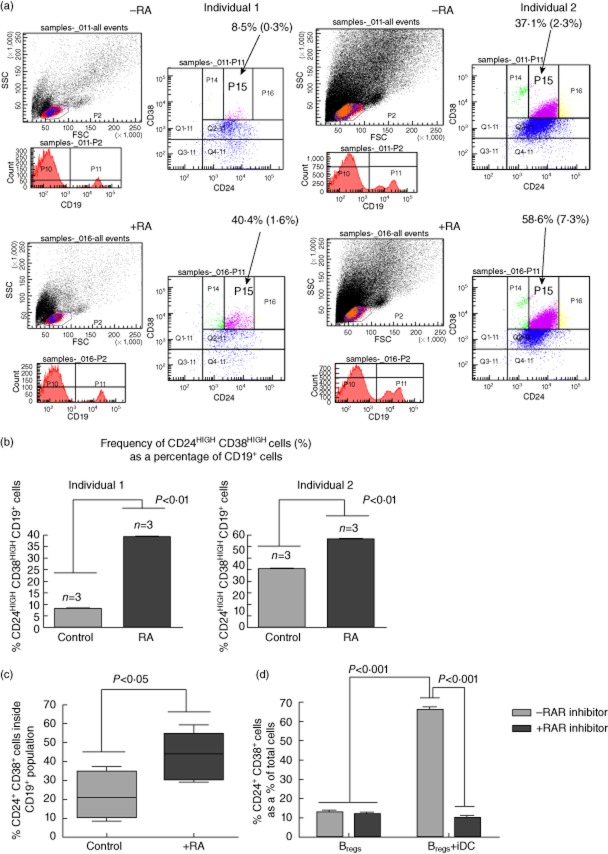
Fig. 4.
Peripheral blood mononuclear cells (PBMC) exposure to rheumatoid arthritis (RA) in vitro results in increased frequency of CD19+CD24+CD38+regulatory B cells (Bregs). (a) Freshly collected PBMC from unrelated healthy adults were treated with all-trans RA (RA) or phosphate-buffered saline (PBS) (control) for a period of 3 days followed by flow cytometric determination of CD19+CD24+/highcd38+/highBregs. The two panels (left and right side; top and bottom) show representative flow cytometric data of triplicate cultures of control and RA-treated freshly collected PBMC. Shown are the flow cytometric data from single cultures of two unrelated healthy individuals. Quadrant Q2-11 represents a parental CD24+CD38+ population of CD19+ cells. The highly suppressive Bregs cluster inside the P15 region. The values shown represent the frequency of CD24+CD38+ cells as a percentage of total CD19+ cells and the values inside the parentheses (*) represent the frequency of CD24+CD38+ cells as a percentage of total cells in culture. The frequency of Bregs inside P15 is summarized in the graphs in (b). The events coloured blue indicate the CD24+CD38+ cells, those in purple the cd38high population, those in green the cd24low and those in yellow the cd24high cells. The indication ‘–RA’ and ‘+RA’ in each of the panels is used to distinguish the data from cultures treated with (+) or without (–) retinoic acid. The panels on the left (top and bottom) represent the outcome in cells from individual 1 and the panels on the right (top and bottom) represent the outcomes in cells from individual 2. The top panels (left and right) represent the outcome in untreated cells and the bottom panels (left and right) represent the outcome in RA-treated cells. (b) Graphic summary of effects of RA on the frequency of CD19+CD24highCD38high B cells in PBMC from the two individuals shown in (a). The bars show the mean CD24highCD38high cell frequency [cells inside the P15 fluorescence activated cell sorter (FACS) gate shown in (a)] as a percentage of CD19+ cells from freshly collected PBMC treated with phosphate-buffered saline (PBS) (control) or all-trans RA after 3 days as described in the Methods. The error bars represent the standard error of the mean (s.e.m.) of triplicate wells. The differences between the means in each of the graphs is statistically significant (*P < 0·01, Student's t-test). These data are representative of three independent experiments with triplicate cultures. (c) The graph summarizes the medians and the range in the frequency of CD24+CD38+Bregs as a percentage of CD19+ cells in culture in control or RA-exposed PBMC cultures from six unrelated healthy individuals. When comparing the medians in a paired Wilcoxon test, the differences in Breg frequency between control and RA-exposed cultures is statistically significant (P < 0·05). (d) Co-culture of immunosuppressive DC (iDC) with CD19+ B cells from thawed syngeneic PBMC in the presence of a retinoic acid receptor (RAR) antagonist interferes with the expansion of CD19+CD24+CD38+ B cells in vitro. The bars represent the frequency of CD19+CD24+CD38+ B cells as a percentage of the total cells co-cultures of CD19+CD24+CD38+B cells with iDC or media alone. The presence of the RAR antagonist is indicated according to the bar design shown in the legend at the top right. The bars represent the mean and the error bars the standard error of the mean (s.e.m.) of quintiplicate cultures. Two-way analysis of variance (anova) identified statistically significant differences in Breg frequency among the cultures and the P-values are shown in the graph. This experiment was conducted on two separate occasions.
iDC fail to promote increased CD19+CD24+CD38+ B cell frequency in the presence of an RAR antagonist in vitro
In order to determine whether the iDC-induced proliferation of CD19+CD24+CD38+ B cells was due exclusively to RA, or if additional mechanisms are involved we co-cultured 2 × 105 iDC in the presence of an equal number of syngeneic CD19+ B cells enriched from thawed, viable PBMC in the presence or absence of 1 mM of ER-50891, a pan-RAR selective antagonist [48] for 72 h. In Fig. 4d we demonstrate that the frequency of CD19+CD24+CD38+ Bregs is significantly lower in iDC co-cultures in the presence of the RAR inhibitor. The inhibitor did not alter viability, as the % of live cells were statistically indistinguishable between inhibitor and control-treated cultures (data not shown).
Discussion
We have shown that administration into humans of autologous DC generated ex vivo under conditions promoting and stabilizing a tolerogenic state is safe and without any detectable side effects [31]. In the same study, we also discovered that administration of autologous DC with tolerogenic ability generated under ‘conventional’ GM-CSF/IL-4 conditions resulted in an increased frequency of B220+CD11c– cells, preferentially in iDC recipients. We also demonstrated the suppressive ability of B cells whose frequency increased in the DC recipients. The surface phenotype of these B cells, however, as assessed by flow cytometry, suggested that they were heterogeneous in character and thus could consist, in principle, of more than one suppressive subpopulation. How tolerogenic DC could mobilize one or more populations of Bregs is the question we have begun to address.
Herein, we demonstrate that the tolerogenic DC used in our Phase I trial, when co-cultured with freshly obtained PBMC, induce an increase in frequency of CD19+CD24+CD38+ B cells in vitro, which are suppressive in allogeneic MLC. By virtue of their surface phenotype these cells are most probably identical, if not related to the Bregs reported by Mauri and colleagues [32,40]. The increase in their frequency appears to be due to proliferation of existing CD19+CD24+CD38+ Bregs as well as conversion of CD19+ B cells in PBMC into CD19+CD24+CD38+ Bregs. We also show that suppression of T cells in allogeneic MLC in vitro is conferred by the CD19+CD24+ constituent of the B220+CD11c– population from freshly obtained PBMC and more specifically by the known CD19+CD24+CD38+ Bregs. In the process of identifying mechanisms of how the tolerogenic DC could promote suppressive B cell activity, we have discovered that CD19+CD24+CD38+ Bregs express retinoic acid (RA) receptors. We demonstrate further that the generation of CD19+CD24+CD38+ Bregs from CD19+ B cells in the presence of tolerogenic DC in vitro is most probably dependent, at least in part, on DC-produced RA.
In humans, Bregs were first identified mainly as CD5+B1a cells, CD21+CD23– marginal zone cells or CD1d+CD21+CD23+ T2-marginal zone precursor B cells [33]. Mauri and colleagues narrowed down the core phenotype of at least one Breg population to CD19+CD24+/intermediateCD38+/intermediate which produces IL-10 [23,32]. Even though IL-10 production appears to define all suppressive B cells identified thus far, including the B220+CD19+CD11c– population we reported [31], IL-10-producing B cells are not necessarily regulatory [49]. In fact, IL-10 expression may be transient as Bregs seem to transition through an IL-10-expressing phase to finally rest as immunoglobulin-secreting cells that might not rely on IL-10 for suppressive ability [50]. In our clinical trial [31], we discovered that the suppressive B cell population whose frequency was increased in cDC and iDC recipients did not rely on IL-10 for suppression in vitro [31]. Those reported B cells represent a heterogeneous population. Herein, we confirm that the bulk of suppressive activity inside those B cells is concentrated inside the already characterized CD19+CD24+CD38+ B cell population [32] which constitutes about 20% of the CD19+B220+CD11c– IL-10+ population, on average, in a small sample of normal individuals. We also discovered that CD19+CD24+ cells are as suppressive as the Bregs reported by Mauri and colleagues [32,40]. These cells could represent either a novel and distinct suppressive cell type, a less-differentiated population from which the CD19+CD24+/intermediateCD38+/intermediate B cells emerge under currently unknown conditions, or a phenotypically metastable population that modulates between CD27+/CD38+ and CD27–/CD38– states without any functional difference. Whether the increase in frequency of the suppressive CD19+B220+CD11c– IL-10+ B cells in tolerogenic DC recipients as reported in [31] represents an effect of DC on B cells to induce the differentiation of suppressor precursors to become CD19+CD24+ suppressive cells, or to specifically induce the proliferation of pre-existing suppressive CD19+CD24+ cells with a plasticity in CD27 and CD38 expression, is currently unknown. Nevertheless, in view of our data, if RA is one of the mediators of DC effects on the generation of Bregs, both proliferation of existing Bregs and differentiation of precursors could be operational.
DC generated from PBMC progenitors in the presence of GM-CSF/IL-4 are known to be tolerogenic [51,52] and produce RA [53]. Mechanistically, evidence suggests that RA alone, as well as DC producing RA, maintain the balance of T cells in favour of immunosuppressive forkhead box protein 3 (FoxP3)+ Tregs at the expense of proinflammatory T helper 17 (Th17) T cells [54,55]. RA-producing DC are also therapeutic in animal models of inflammatory bowel disease, largely by stabilizing in vivo the frequency of FoxP3+ Tregs. Less is known about the effects of RA on B cells, although studies suggest that it is important in the maturation of IgA-producing B cells [47]. Exposure of PBMC to RA in vitro yields an increase in the frequency of CD19+CD24+CD38+ B cells. Thus, we propose that RA is one direct mediator of iDC action to promote the expansion of Bregs, largely through proliferation, although the effect of RA addition to CD19+ B cells does not result in an expansion of Bregs as large as when the B cells are cultured with iDC. We believe therefore that RA is one, but not the only, mediator of DC action on Bregs and/or their precursors.
The findings of Maseda et al. [50] suggest further that B10 Bregs emerge from a transitional and/or memory population consequent to antigen exposure and B cell receptor (BCR) activation and that the BCR repertoire is polyclonal. Furthermore, those data show that B10 Bregs come to rest as Ig-producing cells. This finding is intriguing, and raises the possibility that T1D-related autoantibodies may not be a consequence of only a series of proinflammatory islet-directed B cell-mediated pathogenic events, but they could also be a consequence of an immunosuppressive counter-regulation involving Bregs which, as demonstrated by Maseda et al., produce Ig.
Very recently, Volchenkov and colleagues discovered that immature DC, generated in the presence of dexamethasone and 1α,25-dihydroxyvitamin D3, gave concomitant rise to Treg and Breg frequency in vitro [56]. These findings strengthen our conclusion that immunosuppressive DC act through regulatory T cells and Bregs [57]. It is tempting to speculate that tripartite DC : Breg : Treg communication occurs in vivo in regulating tolerance. B cells can interact with FoxP3+ Tregs; B cells facilitate early accumulation of FoxP3+ Tregs in the central nervous system of mice with experimental autoimmune encephalomyelitis (EAE). In two important studies, the authors demonstrated that IL-10 producing Bregs were necessary to restore Tregs and to promote recovery from EAE independently of IL-10, but through glucocorticoid-induced TNF receptor (GITR) ligand [58] and B7 signalling [59]. Adoptive transfer of LPS-activated B cells expressing a glutamic acid decarboxylase (GAD)–IgG fusion protein into NOD diabetic mice was shown to stimulate a rapid increase in CD4+CD25+ Treg numbers [60]. Furthermore, protection from diabetes by splenocytes from diabetes-free, B cell-administered NOD mice was contingent on the presence of CD4+CD25+ T cells [61]. Also, CD40L-activated B cells have been shown recently to generate CD4+ and CD8+ Tregs from naive precursors [62,63] and a novel Breg population was shown to differentiate T cells into a regulatory IL-10+CD4+ population that account partially for an improvement in lupus [64]. In NOD mice, iDC administration increased the frequency of CD4+CD25+ T cells [65], and when the kinetics of Treg and Breg frequencies were compared, there appeared to be a concomitant co-regulation in response to iDC in vivo (N. G., unpublished observations). Whether the two regulatory cell populations respond independently or in an interactive manner to iDC, or physiologically to endogenous tolerogenic DC, is currently unknown. Another question that is germane is whether Bregs sensitive to tolerogenic DC are antigen-specific or polyclonal. This aspect of tolerogenic DC action is currently under study.
These findings, along with the very recently reported discovery of a method to expand Bregs in vitro [66], also usher in a potential new therapeutic approach to T1D immunotherapy that involves Bregs and molecules which stabilize their suppressive ability, including RA.
Acknowledgments
The authors would like to thank Robert Lakomy and Alexis Styche for excellent assistance with the flow cytometry analyses and the flow-sorting. This work was supported by grants from the RiMed Foundation (to M. T. and V. D. C.) and in part by NIH NIDDK DK063499 (to M. T.) and JDRF 17-2007-1066 (to N. G.).
Disclosure
NG and MT are on the Scientific Advisory Board and hold equity in the form of common stock of DIAVACS, a biotechnology entity that has licensed the intellectual property pertaining to iDC from the University of Pittsburgh.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's website:
Fig. S1. Flow cytometry approach used to measure and flow sort the B cell populations described in the manuscript either from freshly collected peripheral blood mononuclear cells (PBMC) or from CD19+ cells enriched from PBMC by magnetic column assistance. The forward-/side-scatter plots represent the starting cell populations prior to flow sorting into more pure populations. The ending populations are highlighted in magenta colour.
Fig. S2. (a) The method used to fluorescence activated cell sorter (FACS) CD19+ B cells from either freshly acquired or thawed peripheral blood mononuclear cells (PBMC) into the different B cell populations used in suppression assays and in dendritic cell (DC) co-cultures or in experiments assessing the role of rheumatoid arthritis (RA) is shown at the top. Below the solid line, we show typical controls used to establish the gates in order to acquire specific and pure cell populations. (b) Flow cytometric analysis of the purity of FACS-sorted CD19+CD24+CD27+CD38+ B cells from CD19+ cells enriched from freshly collected or thawed PBMC. The inset at the top left shows the forward-/side-scatter profiles of the FACS-sorted CD19+CD24+CD27+CD38+ B cells and the quadrant plots show the purity. (c) Flow cytometric analysis of the purity of FACS-sorted CD19+CD24+CD27–CD38– B cells from CD19+ cells enriched from freshly collected or thawed PBMC. The inset at the top left shows the forward-/side-scatter profiles of the FACS-sorted CD19+CD24+CD27–CD38– B cells and the quadrant plots show the purity.
Fig. S3. The graph summarizes the absolute numbers of 5-bromo-2-deoxyuridine (BrdU)+CD19+ cells in the mixed leucocyte culture (MLC) regulatory B cell (Breg) suppression assays represented by Fig. 1b. The bars represent the mean BrdU+CD19+ absolute cell numbers and the standard error of the mean represent quintiplicate cultures. The differences in cell numbers among the different co-cultures are not statistically significant (two-way analysis of variance).
Fig. S4. A representative flow cytometric analysis that underlies the data shown in Fig. 1c,d is shown. The magenta-coloured values represent the frequency of the specific cell populations as a percentage of the parental flow cytometric gate.
Fig. S5. The isotype controls used to establish the acquisition gates for the flow cytometric analysis of the co-cultures described in Fig. 2a are shown.
Fig. S6. Confirmation that Aldefluor+ cells reside inside the CD11c+ cell population in control dendritic cells (cDC) and immunosuppressive DC (iDC) generated from peripheral blood mononuclear cells (PBMC) of two unrelated healthy adult individuals. The magenta coloured values represent the frequency of Aldefluor+ cells inside the CD11c+ gate. Aldefluor mea fluorescence intensity (MFI) is shown in magenta colour at the bottom of the specific histograms.
References
- 1.Marino E, Grey ST. B cells as effectors and regulators of autoimmunity. Autoimmunity. 2012;45:377–387. doi: 10.3109/08916934.2012.665527. [DOI] [PubMed] [Google Scholar]
- 2.Salinas GF, Braza F, Brouard S, Tak PP, Baeten D. The role of B lymphocytes in the progression from autoimmunity to autoimmune disease. Clin Immunol. 2012;146:34–45. doi: 10.1016/j.clim.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Dorner T, Jacobi AM, Lee J, Lipsky PE. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J Immunol Methods. 2011;363:187–197. doi: 10.1016/j.jim.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Radic M, Herrmann M, van der Vlag J, Rekvig OP. Regulatory and pathogenetic mechanisms of autoantibodies in SLE. Autoimmunity. 2011;44:349–356. doi: 10.3109/08916934.2010.536794. [DOI] [PubMed] [Google Scholar]
- 5.Aringer M, Burkhardt H, Burmester GR, et al. Current state of evidence on ‘off-label’ therapeutic options for systemic lupus erythematosus, including biological immunosuppressive agents, in Germany, Austria and Switzerland – a consensus report. Lupus. 2012;21:386–401. doi: 10.1177/0961203311426569. [DOI] [PubMed] [Google Scholar]
- 6.Calero I, Sanz I. Targeting B cells for the treatment of SLE: the beginning of the end or the end of the beginning? Discov Med. 2010;10:416–424. [PMC free article] [PubMed] [Google Scholar]
- 7.Lo MS, Tsokos GC. Treatment of systemic lupus erythematosus: new advances in targeted therapy. Ann NY Acad Sci. 2012;1247:138–152. doi: 10.1111/j.1749-6632.2011.06263.x. [DOI] [PubMed] [Google Scholar]
- 8.Looney RJ, Anolik J, Sanz I. A perspective on B cell-targeting therapy for SLE. Mod Rheumatol. 2010;20:1–10. doi: 10.1007/s10165-009-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes. 1997;46:941–946. doi: 10.2337/diab.46.6.941. [DOI] [PubMed] [Google Scholar]
- 10.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new ‘speed congenic’ stock of NOD.Ig mu null mice. J Exp Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–1168. [PubMed] [Google Scholar]
- 12.Marino E, Batten M, Groom J, et al. Marginal-zone B cells of nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T cells. Diabetes. 2008;57:395–404. doi: 10.2337/db07-0589. [DOI] [PubMed] [Google Scholar]
- 13.Noorchashm H, Lieu YK, Noorchashm N, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163:743–750. [PubMed] [Google Scholar]
- 14.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 15.Silveira PA, Johnson E, Chapman HD, Bui T, Tisch RM, Serreze DV. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur J Immunol. 2002;32:3657–3666. doi: 10.1002/1521-4141(200212)32:12<3657::AID-IMMU3657>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Tian J, Zekzer D, Lu Y, Dang H, Kaufman DL. B cells are crucial for determinant spreading of T cell autoimmunity among beta cell antigens in diabetes-prone nonobese diabetic mice. J Immunol. 2006;176:2654–2661. doi: 10.4049/jimmunol.176.4.2654. [DOI] [PubMed] [Google Scholar]
- 17.Greeley SA, Katsumata M, Yu L, et al. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat Med. 2002;8:399–402. doi: 10.1038/nm0402-399. [DOI] [PubMed] [Google Scholar]
- 18.Inoue Y, Kaifu T, Sugahara-Tobinai A, Nakamura A, Miyazaki J, Takai T. Activating Fc gamma receptors participate in the development of autoimmune diabetes in NOD mice. J Immunol. 2007;179:764–774. doi: 10.4049/jimmunol.179.2.764. [DOI] [PubMed] [Google Scholar]
- 19.Ryan GA, Wang CJ, Chamberlain JL, et al. B1 cells promote pancreas infiltration by autoreactive T cells. J Immunol. 2010;185:2800–2807. doi: 10.4049/jimmunol.1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiu Y, Wong CP, Bouaziz JD, et al. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 22.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6:636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- 24.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann NY Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 25.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussain S, Delovitch TL. Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol. 2007;179:7225–7232. doi: 10.4049/jimmunol.179.11.7225. [DOI] [PubMed] [Google Scholar]
- 29.Bleesing JJ, Fleisher TA. Human B cells express a CD45 isoform that is similar to murine B220 and is downregulated with acquisition of the memory B cell marker CD27. Cytometry B Clin Cytom. 2003;51:1–8. doi: 10.1002/cyto.b.10007. [DOI] [PubMed] [Google Scholar]
- 30.Bleesing JJ, Morrow MR, Uzel G, Fleisher TA. Human T cell activation induces the expression of a novel CD45 isoform that is analogous to murine B220 and is associated with altered O-glycan synthesis and onset of apoptosis. Cell Immunol. 2001;213:72–81. doi: 10.1006/cimm.2001.1865. [DOI] [PubMed] [Google Scholar]
- 31.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blair PA, Norena LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Evans JG, Chavez-Rueda KA, Eddaoudi A, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 34.Moreb JS, Ucar D, Han S, et al. The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact. 2012;195:52–60. doi: 10.1016/j.cbi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreb JS, Zucali JR, Ostmark B, Benson NA. Heterogeneity of aldehyde dehydrogenase expression in lung cancer cell lines is revealed by Aldefluor flow cytometry-based assay. Cytometry B Clin Cytom. 2007;72:281–289. doi: 10.1002/cyto.b.20161. [DOI] [PubMed] [Google Scholar]
- 36.Bonhoeffer S, Mohri H, Ho D, Perelson AS. Quantification of cell turnover kinetics using 5-bromo-2′-deoxyuridine. J Immunol. 2000;164:5049–5054. doi: 10.4049/jimmunol.164.10.5049. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen XD, Eichler H, Dugrillon A, Piechaczek C, Braun M, Kluter H. Flow cytometric analysis of T cell proliferation in a mixed lymphocyte reaction with dendritic cells. J Immunol Methods. 2003;275:57–68. doi: 10.1016/s0022-1759(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 38.Tough DF, Sprent J, Stephens GL. Measurement of T and B cell turnover with bromodeoxyuridine. Curr Protoc Immunol. 2007;Chapter 4:Unit 4 7. doi: 10.1002/0471142735.im0407s77. [DOI] [PubMed] [Google Scholar]
- 39.Ballow M, Wang X, Xiang S, Allen C. Expression and regulation of nuclear retinoic acid receptors in human lymphoid cells. J Clin Immunol. 2003;23:46–54. doi: 10.1023/a:1021900331580. [DOI] [PubMed] [Google Scholar]
- 40.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 41.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced Treg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins CB, Aherne CM, Kominsky D, et al. Retinoic acid attenuates ileitis by restoring the balance between T-helper 17 and T regulatory cells. Gastroenterology. 2011;141:1821–1831. doi: 10.1053/j.gastro.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golovina TN, Mikheeva T, Brusko TM, Blazar BR, Bluestone JA, Riley JL. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLoS ONE. 2011;6:e15868. doi: 10.1371/journal.pone.0015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawazoe Y, Sugita S, Keino H, et al. Retinoic acid from retinal pigment epithelium induces T regulatory cells. Exp Eye Res. 2012;94:32–40. doi: 10.1016/j.exer.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PLoS ONE. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang G, Zhong A, Wang S, Dong N, Sun Z, Xia J. Retinoic acid attenuates acute heart rejection by increasing regulatory T cell and repressing differentiation of Th17 cell in the presence of TGF-beta. Transpl Int. 2010;23:986–997. doi: 10.1111/j.1432-2277.2010.01080.x. [DOI] [PubMed] [Google Scholar]
- 47.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 48.Somenzi G, Sala G, Rossetti S, Ren M, Ghidoni R, Sacchi N. Disruption of retinoic acid receptor alpha reveals the growth promoter face of retinoic acid. PLoS ONE. 2007;2:e836. doi: 10.1371/journal.pone.0000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Amine M, Melo M, Kang Y, Nguyen H, Qian J, Scott DW. Mechanisms of tolerance induction by a gene-transferred peptide-IgG fusion protein expressed in B lineage cells. J Immunol. 2000;165:5631–5636. doi: 10.4049/jimmunol.165.10.5631. [DOI] [PubMed] [Google Scholar]
- 50.Maseda D, Smith SH, DiLillo DJ, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188:1036–1048. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berger TG, Schulze-Koops H, Schafer M, Muller E, Lutz MB. Immature and maturation-resistant human dendritic cells generated from bone marrow require two stimulations to induce T cell anergy in vitro. PLoS ONE. 2009;4:e6645. doi: 10.1371/journal.pone.0006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiurbe G, Matuschek A, Kammerer U, et al. Inhibitory effects of rat bone marrow-derived dendritic cells on naive and alloantigen-specific CD4+ T cells: a comparison between dendritic cells generated with GM-CSF plus IL-4 and dendritic cells generated with GM-CSF plus IL-10. BMC Res Notes. 2009;2:12. doi: 10.1186/1756-0500-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokota A, Takeuchi H, Maeda N, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21:361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai A, Lu N, Guo Y, Liu Z, Chen J, Peng Z. All-trans retinoic acid down-regulates inflammatory responses by shifting the Treg/Th17 profile in human ulcerative and murine colitis. J Leukoc Biol. 2009;86:959–969. doi: 10.1189/jlb.0109006. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z, Lin F, Gao Y, et al. FOXP3 and RORgammat: transcriptional regulation of Treg and Th17. Int Immunopharmacol. 2011;11:536–542. doi: 10.1016/j.intimp.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Volchenkov R, Karlsen M, Jonsson R, Appel S. Type 1 regulatory T cells and regulatory B cells induced by tolerogenic dendritic cells. Scand J Immunol. 2013;77:246–254. doi: 10.1111/sji.12039. [DOI] [PubMed] [Google Scholar]
- 57.Giannoukakis N, Trucco M. A role for tolerogenic dendritic cell-induced B-regulatory cells in type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2012;19:279–287. doi: 10.1097/MED.0b013e328355461b. [DOI] [PubMed] [Google Scholar]
- 58.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 60.Song L, Wang J, Wang R, et al. Retroviral delivery of GAD-IgG fusion construct induces tolerance and modulates diabetes: a role for CD4+ regulatory T cells and TGF-beta? Gene Ther. 2004;11:1487–1496. doi: 10.1038/sj.gt.3302327. [DOI] [PubMed] [Google Scholar]
- 61.Soukhareva N, Jiang Y, Scott DW. Treatment of diabetes in NOD mice by gene transfer of Ig-fusion proteins into B cells: role of T regulatory cells. Cell Immunol. 2006;240:41–46. doi: 10.1016/j.cellimm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Tu W, Lau YL, Zheng J, et al. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008;112:2554–2562. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng J, Liu Y, Qin G, et al. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J Immunol. 2009;183:3742–3750. doi: 10.4049/jimmunol.0901329. [DOI] [PubMed] [Google Scholar]
- 64.Blair PA, Chavez-Rueda KA, Evans JG, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N. Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol. 2004;173:4331–4341. doi: 10.4049/jimmunol.173.7.4331. [DOI] [PubMed] [Google Scholar]
- 66.Yoshizaki A, Miyagaki T, DiLillo DJ, et al. Regulatory B cells control T cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.