Abstract
Interstitial cells of Cajal (ICC)-like cells (ICC-LCs) have been identified in many regions of the urinary tract and male genital organs by immunohistochemical studies and electron microscopy. ICC-LCs are characterized by their spontaneous electrical and Ca2+ signalling and the cellular mechanisms of their generation have been extensively investigated. Spontaneous activity in ICC-LCs rises from the release of internally stored Ca2+ and the opening of Ca2+-activated Cl− channels to generate spontaneous transient depolarizations (STDs) in a manner not fundamentally dependent on Ca2+ influx through L-type voltage-dependent Ca2+ channels. Since urogenital ICC-LCs have been identified by their immunoreactivity to Kit (CD117) antibodies, the often-used specific marker for ICC in the gastrointestinal tract, their functions have been thought likely to be similar. Thus ICC-LCs in the urogenital tract might be expected to act as either electrical pacemaker cells to drive the smooth muscle wall or as intermediaries in neuromuscular transmission. However, present knowledge of the functions of ICC-LCs suggests that their functions are not so predetermined, that their functions may be very region specific, particularly under pathological conditions. In this review, we summarize recent advances in our understanding of the location and function of ICC-LCs in various organs of the urogenital system. We also discuss several unsolved issues regarding the identification, properties and functions of ICC-LCs in various urogenital regions in health and disease.
Keywords: interstitial cells of Cajal-like cells, urogenital tract, bladder, urethra, prostate, renal pelvis, corpus cavernosum
Overview
The muscle wall of the urinary tract and male genital organs develops spontaneous contractile activity that is modulated by both efferent and afferent nerves as well as signals from the urothelium and circulating hormones. Originally, such spontaneous contractile activity was considered to originate from the smooth muscle cells (SMCs) themselves, and thus often referred to as ‘myogenic activity’. However, with the establishment that interstitial cells of Cajal (ICC) are the primary pacemakers driving slow wave generation in the gastrointestinal (GI) tract and that they can be ‘selectively’ identification with antibodies raised against the Kit receptor (cluster of differentiation [CD]117) of the receptor tyrosine kinase [1], many regions of the urogenital tract have been recently examined for the presence of similar cells. It is becoming evident that Kit+ cells with morphologies consistent with being ICC-like, particularly when examined using transmission electron microscopy, are ubiquitously distributed throughout the upper and lower urinary tract and male genital organs [2]. Although these ICC-like cells (ICC-LCs) may form close associations with their neighbours, be they other ICC-LCs or SMCs within the muscle wall, they do not form the extensive three-dimensional networks similar to the network of ICCs in the GI tract. There is also considerable species and region specific variation in the distribution, morphology and sensitivity of ICC-LCs to Kit antibodies. Transgenic mice, which have a mutation of the dominant white spotting (W) locus (Kit receptor) (W/Wv mice) or its natural ligand stem cell factor (SCF; Sl/Sld mice) and associated reductions in Kit-dependent differentiation of ICC in the GI tract, are little affected in terms of their contractility and Ca2+ signalling in ICC-LCs of the bladder [3] or renal pelvis (see below).
Since many ICC-LCs have been initially identified by their Kit (CD117)-immuno-positivity, studies into their function have been predicated on existing knowledge of the cellular mechanisms driving GI ICC. Indeed, ICC-LCs in situ and after enzymatic isolation generate spontaneous Ca2+ transients relying on Ca2+ release from the endoplasmic reticulum [4–6]. The maintenance of these spontaneous signals requires the presence of extracellular Ca2+, however this Ca2+ influx is critically not via the opening of L-type voltage-operated Ca2+ channels (VOCCs) [4–7], the opening of these channels being fundamental for action potential generation and contraction in the SMC wall [8, 9].
Spontaneous Ca2+ transients in ICC-LCs exhibit many common properties between organs and species. These Ca2+ transients are consistently recorded at lower frequencies and have a longer duration than the Ca2+ transients recorded in neighbouring smooth muscle bundles. In addition, spontaneous Ca2+ transients recorded in ICC-LCs of bladder [7] and renal pelvis [6] have little temporal relationship with the Ca2+ signals in adjacent SMCs (Fig. 1A and B). In the urethra, less than 30% of ICC-LCs in situ have a close temporal correlation with Ca2+ signals of the SMCs [5] (Fig. 1C).
Fig 1.
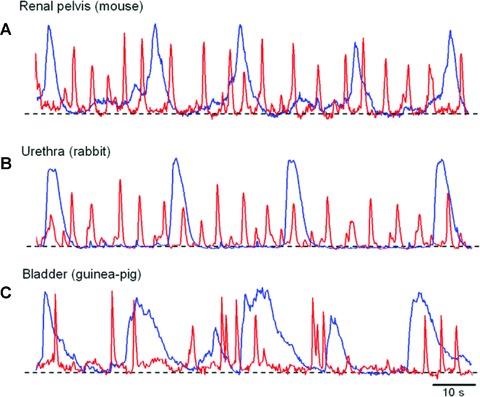
Commonality of spontaneous Ca2+ transients recorded from ICC-LCs in the urinary tract (A) simultaneous recording of spontaneous Ca2+ transients in ICC-LCs (blue) and typical smooth muscle cells (red) of the mouse renal pelvis failed to show any temporal correlation. (B) In the rabbit urethra, the spontaneous Ca2+ transients in ICC-LCs (blue) also do not have any close temporal relationship with circular SMCs (red). (C) A simultaneous recording of spontaneous Ca2+ transients from ICC-LCs (blue) and detrusor SMCs (red) in the guinea pig bladder. Note that Ca2+ transients in ICC-LCs exhibit similar temporal characteristics among tissues as well as species.
Our understanding of ICC-LCs function is complicated by the presence of other cells capable of generating spontaneous electrical activity. Single SMCs isolated from the bladder and corpus cavernosum are capable of generating spontaneous electrical signals [10–12], and thus may not require distinct pacemaker cells to drive muscle contractility. In the bladder, myofibroblasts in the suburothelial layer (also referred as suburothelial ICC-LCs) have a morphology similar to ICC-LCs and can also generate spontaneous electrical and Ca2+ activity [13]. In the renal pelvis, atypical SMCs have the morphological characteristics, distribution and Ca2+ and electrical signalling consistent with having a pacemaker role in pyeloureteric peristalsis [6, 14]. Interestingly, although the spontaneous Ca2+ transients in atypical SMCs also depend on Ca2+ release from the endoplasmic reticulum involving both InsP3 and ryanodine receptors, this released Ca2+ appears to open Ca2+ activated cation-selective channels, rather than Cl− channels, to generate their spontaneous transient depolarizations (STDs) [6].
Both three-dimensional immunohistology and electron microscopy reveal a close apposition between ICC-LCs and nerves [15–17]. They also respond to applied neurotransmitters, including acetylcholine, noradrenalin and ATP depending on their distribution [13, 18, 19]. Therefore, ICC-LCs have been proposed by some to act as intermediaries in neuromuscular transmission in the urogenital tract and that this role changes during pathological conditions. However, the smooth muscle wall in most urogenital organs also receive a reasonably dense innervation [20, 21] and respond to neurotransmitter mimetics acting on the same receptor subtypes as those on ICC-LCs [13, 18, 19]. Responses to electrical nerve stimulation is also very region specific. For example, electrical nerve stimulation has little effect on the contractility of the renal pelvis and ureter except at very high frequencies [22, 23], while the bladder and urethra appears to be more tightly controlled by parasympathetic and sympathetic innervations.
In this review, we summarize recent advances in our understanding of the location and function of ICC-LCs in various organs of the urogenital tract, as well as describe the variability in the mechanisms by which they generate their Ca2+ and electrical signals and what influence they may have on the SMC wall contractility.
ICC-LCs in the bladder
Electrophysiological investigations have demonstrated that isolated detrusor SMCs of the bladder are capable of generating spontaneous action potentials which are almost identical to those recorded in intact preparations [10]. Moreover, detrusor smooth muscles taken from idiopathic overactive bladder have been shown to exhibit aberrant spontaneous activity, suggesting that the increased excitability of detrusor smooth muscles during this pathological condition may be attributed to the altered properties of the SMCs themselves [24]. Thus, unlike GI tissues, where the electrical activity is primarily generated by ICC, the bladder may not require distinct electrical pacemaker cells. Nevertheless, ICC-LCs in the bladder are preferentially located along the boundary of SMC bundles where many spontaneous SMC Ca2+ transients originate suggesting that they may play some role in generating spontaneous activity. Isolated single ICC-LCs are capable of generating spontaneous Ca2+ transients and responded to muscarinic receptor stimulation with Ca2+ transients that persist in the presence of nifedipine [19]. Although L-type VOCCs have been recorded from isolated ICC-LCs, existing evidence suggests that these VOCCs are not involved in the generation of their spontaneous activity. Ca2+ transients recorded from ICC-LCs in situ occur independently of those in neighbouring SMCs even when synchronous Ca2+ waves sweep across the muscle bundles [8, 19]. Therefore, although the presence of boundary ICC-LCs seems to be in the ideal location to drive the bulk of SMCs in the bladder, they are unlikely to be acting as pacemaker cells.
In human beings, the finding that Kit+ cells are increased in samples taken from patients with overactive bladder suggests a role for ICC-LCs in intercellular signal transmission [17, 25]. Imatinib mesylate (Glivecx, Novartis Pharma, Switzerland), an inhibitor of Kit receptor tyrosine kinase, is more potent at inhibiting evoked and spontaneous contractions in overactive bladder than in normal bladder [17]. Although the inhibitory action of imatinib in the normal balder, particularly on the amplitude of contractions, is most likely attributable to its non-specific action on both L-type VOCCs and Ca2+ release from intracellular stores [26], Kit-signalling pathways may be well up-regulated in the overactive bladder. In guinea pig, bladder outlet obstruction dramatically increases the number of Kit+ and vimentin+ ICC-LCs (Fig. 2) [17], indicating that ICC-LCs in the bladder must have different regulatory mechanisms than those determining the development of ICC in the GI tract [27], or ICC-LCs in the renal pelvis [28], where decreases in cell numbers have been observed in regions oral of the site of obstruction. Although the reduction of Kit signalling in W/Wv transgenic mice leads to site-specific decreases in ICC numbers and a dysregulation of GI motility [29], neither the distribution of Kit+ ICC-LCs nor the spontaneous contractile activity in the bladder is affected [3]. Similarly, bladders of Sl/Sld mice, in which the production of SCF the native ligand for the Kit receptor is diminished, do not show a reduction in the number of Kit+ ICC-LCs or any alteration in their spontaneous contractility (H. Hashitani, unpublished observations). These results suggest that the SCF/ Kit pathway may not be an exclusive trigger for the differentiation and development of ICC-LCs in the urogenital tract, despite their often common expression of Kit immunoreactivity. Interestingly, ICC-LCs have been identified in the human bladder which are (CD117) Kit–, but CD34 immunoreactive, and which have a stellate morphology that envelops and intermingles with individual muscle fascicles [30].
Fig 2.
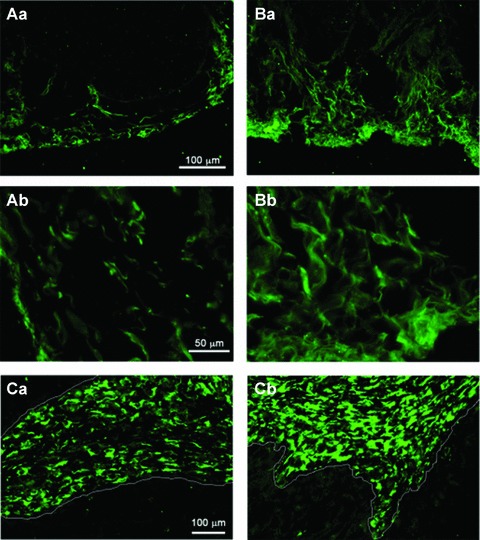
Increased population of Kit+ and vimentin+ ICC-LCs in the obstructed bladder of the guinea pig (Aa) In normal bladder Kit-immunoreactive ICC-LCs are scattered in the subserosal and detrusor muscle layers. (Ab) Kit+ cells are spindle or stellate shaped when viewed at higher magnification. (Ba) In obstructed bladders, the number of Kit+ ICC-LCs is dramatically increased. (Bb) Kit+ ICC-LCs form close contacts with each other. (Ca) In the normal bladder vimentin+ ICC-LCs are distributed in the suburothelial layer. The white line indicates the border between the urothelium and the suburothelial layer. (Cb) In obstructed bladders the number of vimentin+ ICC-LCs is increased and their distribution expanded. Modified from [17] with permission.
ICC-LCs may play an important role in the nerve-mediated modulation of detrusor smooth muscle excitability. Following stimulation with sodium nitroprusside, ICC-LCs in the bladder develop an intense induction of cGMP immunoreactivity, while the detrusor SMCs remain uniformly negative [20]. In addition, a close apposition of ICC-LCs with cholinergic nerves has also been identified by immunohistochemical studies [15]. This is consistent with the finding that isolated ICC-LCs readily respond to muscarinic receptor stimulation by firing Ca2+ transients [19]. Therefore, ICC-LCs in the bladder may be involved in the neuromuscular transmission, and thus changes in ICC-LC numbers may account for the increased excitability of detrusor smooth muscle in overactive bladders. However, it should be noted that SMCs also respond to muscarinic receptor stimulation through the same receptor subtype and at the same concentration range as do ICC-LCs [19]. Sildenafil, a PDE5 inhibitor, suppresses spontaneous contractions in detrusor smooth muscle preparations, while having little effect on the spontaneous activity in single detrusor SMC bundles [31]. Interestingly, sodium nitroprusside, a nitric oxide donor, enhances spontaneous excitations in detrusor smooth muscle in a cGMP-independent manner, while 8-Br-cGMP invariably suppresses spontaneous contractions of the intact bladder. Thus accumulation of endogenous cGMP in ICC-LCs by sildenafil may diminish communications between muscle bundles to suppress the spontaneous contractions in the bladder.
The suburothelium region of the human bladder contains a network of vimentin+ myofibroblasts (suburothelial ICC-LCs) that appear to be interconnected via the gap junction protein, connexin 43 [32]. Unlike ICC-LCs in the detrusor layer of the guinea pig bladder, these cells were first described as not expressing Kit immunoreactivity, although others have recently demonstrated the presence of Kit+, stellate-shaped ICC-LCs in this region [15]. In addition, myofibroblasts are contractile, unlike ICC-LCs in the detrusor muscle layer which do not contract either spontaneously or upon stimulation. Myofibroblasts form close associations with suburothelial afferents [33] and may also be the target of efferent nitrergic nerves [20]. Isolated myofibroblasts are spontaneously active, respond to ATP upon P2Y receptor activation, but not to muscarinic receptor activation, to generate inward currents and transient increases of intracellular Ca2+[13]. These cells are ideally located to play a modulatory role in the process of bladder sensation and may play an intermediate and variable gain stage in the process of bladder filling. Therefore, pathological changes in suburothelial myofibroblast may also account for the sensation of urgency in the overactive bladder. In obstructed bladders of guinea pig, the distribution of Kit+ ICC-LCs extends towards the detrusor muscle layer [17], suggesting that the altered distribution of ICC-LCs may play a role in the signalling pathway from the urothelium to afferent nerves or underlying detrusor smooth muscles.
ICC-LCs in the urethra
Smooth muscle strips taken from the urethra develop spontaneous tone maintained by the firing of slow waves generated from the summation of STDs [34, 35]. As in the gut, the generation of STDs occurs in ICC-LCs, while isolated single SMCs are electrically quiescent, indicating that ICC-LCs in the urethra may have a genuine pacemaker role [36]. However, the sustained mechanical tone of the urethral smooth muscle strips, is clearly different from the slow wave-associated phasic contractions in the gut suggesting that the signal transmission from ICC-LCs to SMCs in the urethra may well be quite different from that in the gut.
Spontaneous Ca2+ transients recorded in ICC-LCs of the rabbit urethra in situ occur at a frequency of 1–10/min., and have a much longer duration (5–30 sec.) than the Ca2+ transients in the urethral SMCs (1–3 sec. duration) measured in same preparation (Fig. 1B) [5]. Urethral ICC-LCs are distributed either separately and sparsely or as small clusters – they do not form an extensive network [16]. Nicardipine, which either abolishes or greatly reduces the amplitude of Ca2+ transients in SMCs has little effect on Ca2+ transients of ICC-LCs; however, the generation Ca2+ transients in ICC-LCs is prevented by omitting extracellular Ca2+[5]. Spontaneous Ca2+ transients of ICC-LCs are readily abolished by CPA, ryanodine or caffeine, while their amplitude was greatly suppressed by 2-APB, suggesting that their generation results from the Ca2+ release from intracellular Ca2+ stores through both ryanodine-sensitive and InsP3-sensitive Ca2+ release channels. These properties are maintained in single urethral ICC-LCs after enzymatic isolation [37] confirming that spontaneous activity of ICC-LCs relies on Ca2+ mobilization through intracellular Ca2+ stores. In addition, the essential role of mitochondria in generating spontaneous Ca2+ transients in ICC-LCs has also recently been demonstrated [38].
Despite ICC-LCs ability of generating spontaneous Ca2+ and electrical activity, they often fail to show a close temporal relationship with neighbouring SMCs (Figs 1 and 3), suggesting they are not necessarily strongly coupled to their neighbours, in contrast to GI ICC [5]. Fluo-4 Ca2+ imaging shows that spontaneous Ca2+ transients occur randomly in individual SMC bundles. Even within a muscle bundle, Ca2+ transients seldom form intercellular Ca2+ waves. In the circular muscle of the rabbit urethra, STDs and slow waves recorded in intact preparations are very similar to the electrical signals generated by isolated ICC-LCs [34] suggesting that the electrical transients generated in ICC-LCs faithfully propagate into neighbouring SMCs, which appear to have relatively high input resistances. However, the electrical coupling between urethral SMCs seems relatively poor, and this lack of synchronicity between and within muscle bundles may also contribute to the poor temporal relationship between ICC-LCs and SMCs. The situation may be different in the longitudinal muscle of the urethra where bursting action potentials are regularly recorded [39, 40].
Fig 3.
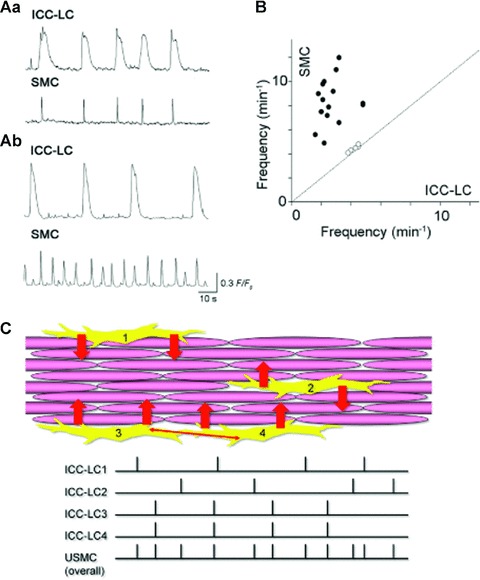
ICC-LCs in the urethra act as ‘less secure’ pacemaker cells (Aa) In circular smooth muscle layer of the rabbit urethra, about 30% of ICC-LCs generated spontaneous Ca2+ transients that had a close temporal relationship with adjacent SMCs. (Ab) In the remaining 70% of cells, ICC-LCs generated spontaneous Ca2+ transients that were independent of the smooth muscle activity. (B) The frequency of ICC-LCs Ca2+ transients was invariably lower than the Ca2+ transients in the smooth muscle. (C) Model of ICC-LC pacemaking in the urethra. Individual or coupled ICC-LCs send depolarizing inputs (STDs) to adjacent SMCs. Because of low cell-to-cell electrical coupling between urethral SMCs, pacemaker signals from ICC-LCs seldom cause intercellular Ca2+ waves within muscle bundles. However, summed signal inputs from ICC-LCs to smooth muscle increase the urethras overall excitability.
Spontaneous electrical and Ca2+ signals generated by ICC-LCs in the urethra are accelerated by α-adrenoceptor stimulation and suppressed by nitric oxide [5, 18, 41]. Thus ICC-LCs may well act as mediators of the effects of both excitatory and inhibitory neurotransmission, particularly if ICC-LCs electrically drive the urethral smooth muscle. However, urethral SMCs are also capable of responding to noradrenaline and nitric oxide [40, 42]. Thus a parallel innervation appears to present in which both ICC-LCs and SMCs are effectors of efferent nerves in the urethra. Because of the ‘less secure’ pacemaking activity of ICC-LCs as well as the relatively low cell-to-cell coupling between urethral smooth muscle, neural inputs that facilitate spontaneous contractility may be required to promote the synchronous contractions within and across muscle bundles and play an important role in maintaining urethral muscle tone.
ICC-LCs in the penis
Spontaneous contractions have been recorded in corporal smooth muscle preparations taken from various mammals, including man, and are considered to contribute to the sustained smooth muscle tone in the flaccid penis. These contractions result from the spontaneous generation of action potentials and associated transient increases in internal Ca2+ that are readily prevented by L-type VOCCs blockers [43].
Spontaneous Ca2+ transients recorded from corpus spongiosum smooth muscle of the guinea pig are blocked by CPA, ryanodine or 2-APB, suggesting that Ca2+ release from intracellular Ca2+ stores via both InsP3- and ryanodine-receptors contributes to their generation [44]. Spontaneous transient inward currents have been recorded in single SMCs of rat and human corpus cavernosum smooth muscle (CCSM) [11]. In single rabbit CCSM, the generation of spontaneous transient inward currents relies on the opening of Ca2+-activated Cl− channels upon the release of Ca2+ from intracellular stores [12]. Consistently, spontaneous action potentials recorded form rabbit CCSM in situ are prevented by either CPA or niflumic acid, a blocker of Ca2+-activated Cl− channels [45]. Therefore, spontaneous activity of corporal smooth muscle appears to be primarily initiated by Ca2+ release from intracellular stores that opens Ca2+-activated Cl− channels to depolarize the membrane. Since the resting membrane potential in CCSM (about −45 mV) is close to the activation threshold for L-type Ca2+ channels, the resulting oscillatory depolarizations would ‘securely’ trigger action potential discharge.
ICC-like cells have been identified in the corporal tissue by their immunoreactivity to antibodies raised against the Kit receptor [44, 45]. However, corporal SMCs themselves are capable of generating Ca2+ store-dependent depolarizations to trigger action potentials, and thus ICC-LCs may play other roles in addition to being pacemaker potential generators. Immunohistochemical and biochemical findings show that cyclooxygenase-2 (COX-2) is highly expressed in ICC-LCs in the corpus cavernosum and the corpus spongiosum of the rabbit [45] (Fig. 4A). Haematoxylin and eosin staining reveals that cells with COX-2 immunoreactivity are spindle or stellate shaped and are often interconnected. Furthermore, COX-2 immunoreactive cells are also strongly immunoreactivity to Kit and vimentin antibodies, confirming them as ICC-LCs [39] (Fig. 4A). Therefore, unlike ICC in the GI tract, ICC-LCs in CCSM may modulate spontaneous activity originating in the SMCs themselves, by releasing prostaglandins produced by constitutively active COX-2. This paracrine role of ICC-LCs in corporal tissues is supported by the findings that spontaneous electrical and contractile activities in the rabbit CCSM are largely attenuated by COX-2 inhibitors [45]. Moreover, inhibition of COX-2 activity also suppresses CCSM contraction evoked by nerve stimulation and the activation of α-adrenoceptors.
Fig 4.
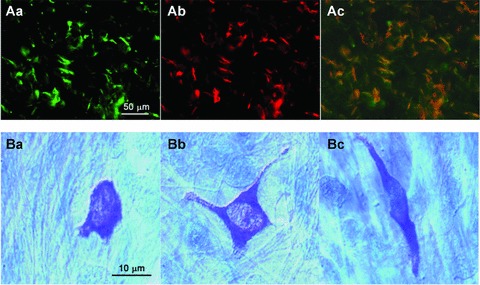
COX-2 expression in Kit+ ICC-LCs and distribution of mast cells in corporal tissues (Aa, b) Scattered distribution of Kit+ ICC-LCs (Aa) and COX-2 immunoreactive cells (Ab) in rabbit corpus cavernosum. The merged picture in Ac shows Kit+ and COX-2+ cells (orange). Note that background SMCs are uniformly negative to Kit or COX-2 antibodies. (B) In the guinea pig corpus spongiosum, toluidine blue staining revealed the presence of many mast cells of various forms (round Ba, stellate Bb and spindle-shaped Bc). Modified from [45] with permission.
In guinea pig CSSM, numerous mast cells have also been identified by toluidine blue staining (Fig. 4B). Mast cells, although typically round in shape, also displayed other morphologies, including spindle and stellate shapes. Therefore, a proportion of the Kit+ cells could well be mast cells. This is a particular interest as given their changing paracrine function in health and disease. Discrimination between ICC-LCs and mast cells requires further examination.
ICC-LCs in the upper urinary tract
Spindle- and stellate-shaped cells that are Kit+ have been described within the urothelial layer, the lamina propria and the muscle layer of the renal pelvis and proximal ureter of mouse (Fig. 5) [6, 46–49], rat [49] and pig [50] and human [51, 52]. However, many of these studies have exclusively used sectioned material and not been verified using electron microscopy, nor have all have been extensive in excluding other Kit+ haematopoietic cells, or macrophages and melanocytes, even nerve bundles (Fig. 6) and glial cells [53]. In unfixed whole mount preparations of the mouse renal pelvis, relatively few spindle- and stellate-shaped Kit+ cells accumulate FITC-dextran FD-70S consistent with these cells being ICC-LCs and not macrophages (Fig. 5) [6, 54]. These ICC-LCs display a sparse distribution similar to the fusiform interstitial cells firing nifedipine-insensitive Ca2+ signals of low frequency and long duration (Figs 1A and 6) that distinguishes them from the high-frequency Ca2+ responses in typical or atypical SMCs. ICC-LCs in the renal pelvis of mouse (Fig. 5B) and rat [55], when viewed with electron microscopy, fulfil many of the ultrastructural criteria for ICC or ICC-LCs (see below), are sparsely distributed in the lamina propria and muscle layer and do not appear to make an interconnecting network. We have also not been able to demonstrate that ICC-LCs pre-labelled with a Kit antibody that selectively binds at an extracellular site are in fact the interstitial cells displaying low-frequency Ca2+ signals. Moreover, as in the bladder [3], the renal pelvis obtained from adult wild-type and W/Wv mice, do not display any noticeable differences in their Ca2+ signalling, contractility (Fig. 6) or responses to electrical nerve stimulation [6, 14]. However, Kit antibody application to mouse embryonic kidneys in organ culture can markedly alter ureteric development and the initiation of peristalsis without affecting SMC differentiation [48], perhaps suggesting that the SCF/ Kit transduction pathway is involved in the initial development of the kidney but that other mechanisms establish the development of pyeloureteric peristalsis in older W/Wv mice.
Fig 5.
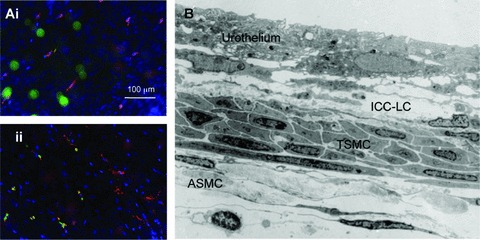
Presence of Kit+ cells and ICC-lCs in the mouse renal pelvis (A) fluorescence micrographs of the lamina propria (Ai) and serosal (Aii) regions of an unfixed whole mount preparation of mouse renal pelvis exposed to FITC FD70S (green) to label macrophages and then the extraceullarly binding Kit antibody H300 (red) and followed by the nuclear stain, Hoechest blue. Less than 20% of Kit+ cells also displayed the FITC-dextran fluorescence. (B) Low magnification electron micrograph through the mid region of the mouse renal pelvis illustrating the urothelium, ICC-LCs within the lamina propria and both typical (TSMC) and atypical (ASMC) SMCs.
Fig 6.
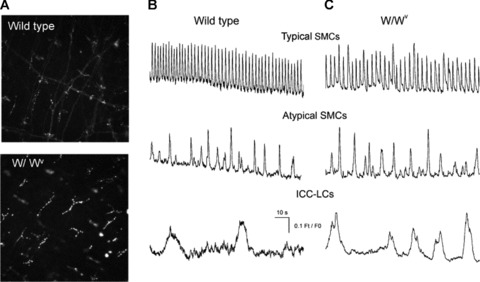
Comparison of Kit+ cells and Ca2+ signalling in the renal pelvis of wild-type and W//Wv mice (A) fluorescence micrographs of unfixed whole mount preparations of mouse renal pelvis obtained from wild-type and W/Wv transgenic mice and exposed to the Kit antibody AK2. Ca2+ waves in typical SMCs in the absence of nifedipine and Ca2+ transients recorded in atypical SMCs and ICC-LCs recorded in the presence of 1 μM nifedipine were indistinguishable in wild-type (B) and W/Wv mice (C).
In the guinea pig renal pelvis, ICC-LCs form a distinct network within the lamina propria, displaying close associations with neighbouring like cells and with both typical and atypical SMCs [56]. Indeed, injections of a fluorescent tag into an individual ICC-LC has been observed to travel into several neighbouring ICC-LCs [54]. However, ICC-LCs in the guinea pig renal pelvis are clearly not immuno-positive for Kit, only rounded Kit+ mast cells are evidence after staining with Kit antibodies. We have previously suggested that ICC-LCs in the guinea pig renal pelvis could well be acting as integrators of the atypical SMC pacemaker drive [24, 56] so that the long processes of ICC-LCs rapidly distribute a pacemaker signal over a relatively wide area of the renal pelvis. This may well be critical in the proximal regions of the renal pelvis where the distribution of both atypical and typical SMCs is relatively sparse and not as well organized as in the more distal regions. The lack of such an extensive network in the rat and mouse renal pelvis perhaps suggests that ICC-LCs have a more paracrine role.
The agents described above that interrupt store uptake and IP3-depedent release of Ca2+ in the bladder and urethra are equally effective at blocking Ca2+ signalling in ICC-LCs in the mouse [9] and guinea pig [57] renal pelvis. In comparison to the relatively modest effect on Ca2+ transients in ICC-LCs in the prostate [58, 59], but similar to the urethra [5], ryanodine completely blocks Ca2+ transient discharge in ICC-LCs in the renal pelvis. Selective blockade of COX-1 has little effect on the frequency or amplitude of the spontaneous contractions of the guinea pig renal pelvis but decreases contraction amplitudes in the rat. In contrast, COX-2 inhibition reduces contractility only in the guinea pig [60]. This suggests that prostaglandin or prostacyclin production is essential in maintaining the spontaneous contractility in the upper urinary tract in a COX- and species-related manner. In contrast to corporal tissues, the effects of nerve stimulation are little affected by indomethacin, the non-selective blocker of COX [60, 61].
Although Ca2+ transients of ICC-LCs in the mouse renal pelvis, in the presence or absence of nifedipine, appear as large and long-lasting signals at the cell body (Fig. 1A), we have not yet been able to detect Ca2+ transients in their long projections, nor any synchronicity between neighbouring ICC-LCs [6]. Nor have we been able to observe Ca2+ transients generated in ICC-LCs travelling into any of neighbouring typical SMC bundles. Thus it appears that, as in the bladder, ICC-LCs under physiological conditions are relatively uncoupled from the SMCs in the muscle wall. Together with their intrinsically low frequency of discharge, it is unlikely that ICC-LCs are acting as a primary pacemaker. However, it is tempting to suggest that ICC-LCs could provide a secondary pacemaker drive which can take over pacemaking during conditions that dislocate the ureter from the proximal atypical SMC pacemaker drive, e.g. during ureteric obstruction or after kidney transplantation.
ICC-LCs in the prostate
The male prostate in the guinea pig [62, 63], mouse [64] and human [65, 66] is also endowed with a population of Kit+ cells, while the dog prostate displays a population of vimentin+ cells that could well be ICC-LCs [67]. In the guinea pig, an electron microscopic investigation has established that these Kit+ cells have the necessary gross morphology, intracellular apparatus and close appositions with their neighbours to establish them as ICC-LCs (Fig. 7). These prostatic ICC-LCs form a network within the lamina propria border between the epithelium and stroma – they display projections that lay between and within the smooth muscles bundles and form close associations with nerve bundles [62] (Fig. 7). In W/Wv mice, the prostate is significantly smaller after 4 weeks of age, after 8 weeks the difference in size does not appear to be so marked. Kit antibodies also reduce the size and the number of branching points of 4-day-old wild-type prostates placed in organ culture for a further 4 days [64]. These prostates have an increased basal/luminal cell ratio but do not have any apparent defects in terms of SMCs recruitment into the stroma or vasculature and survival of the prostate epithelium suggesting that Kit signalling plays a key role in luminal cell differentiation [64].
Fig 7.
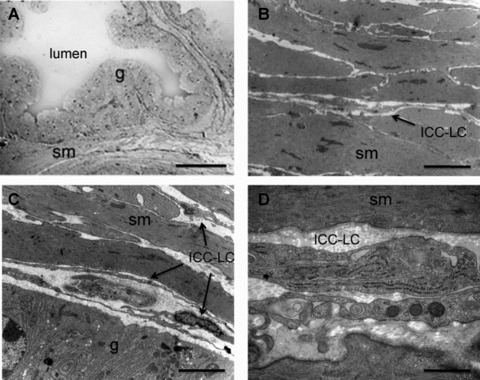
ICC-like cells in the guinea pig prostate (A) Low magnification light micrograph of the junction between 3 prostatic acini within the ventral lobe of the guinea pig prostate illustrating that the lumen is surrounded by a glandular secretory epithelial cells (g) enveloped by a stroma consisting mainly of SMCs (sm). Scale bar represents 50 μm. Higher magnification electron microscopy demonstrates that the stroma is tightly packed with SMCs (B) and that ICC-LC cells are found within the space between the lamina propria and the epithelial cells (C). ICC-LC processes were also found between the SMCs (B); scale bars represent 2 μm. (D) higher magnification of an ICC-like cell illustrating internal organelles such as the rough endoplasmic reticulum and cavaeloe, scale bar 0.5 μm.
Prostatic preparations of the guinea pig impaled with intracellular microelectrodes filled with a fluorescent tag reveals that slow waves are recorded in the SMCs within the stromal wall, and that they are driven by a population of nifedipine-resistant pacemaker potentials [62]. These slow waves are envisaged as being made up of a depolarizing transient that triggers a number of nifedipine-sensitive Ca2+-dependent spikes. The amplitude of these depolarizing transients is not constant, but appears to vary as a consequence of the varying electrical distance between their ICC-LC pacemaker cell and the point of recording of the prostatic slow wave. However, this has proven difficult to demonstrate in the prostate with its complex multi-acini geometry. Thus, ICC-LCs and slow waves in the guinea pig prostate appear to have properties most similar to ICC-generated slow waves in the GI tract. The frequency of pacemakers potentials and slow waves are accelerated by histamine or the α-adrenoceptor agonist, phenylepherine, in the absence or presence of nifedipne, and readily blocked by inhibitors (CCCP) of mitochondria function and IP3-dependent Ca2+ mobilization; ryanodine causes only a transient increase in pacemaker frequency [58, 59]. This blockade is accompanied by a membrane depolarization that sometimes triggers high frequency, brief, nifedipine-sensitive action potentials. Prostatic slow waves are also blocked by the Cl− channel blockers, 9-AC and niflumic acid [68]. Interestingly, blockade of slow wave activity by niflumic acid is readily reversed upon the addition of phenylepherine. However, phenylepherine could not reverse the inhibitory actions of blocking Ca2+ uptake into the internal stores or mitochondria [59].
Mircoelectrode impalements of the guinea pig prostate also reveal a proportion of cells firing spontaneous, large, brief nifedipine-sensitive action potentials. These cells are recorded in cells with depolarized membrane potentials compared to cells displaying slow waves or pacemaker potentials and resemble the action potentials recorded in CPA- or CCCP-arrested preparations [63], perhaps suggesting that these spike potentials reflect the spontaneous activity generated in the SMC bundles themselves. Electrical characterization of single SMCs from the guinea pig prostatic stroma reveals that these cells are indeed capable of generating a simple spike potential as they express a L-type Ca2+ current, a 4-aminopyridine sensitive ‘A type’ K+ current, as well as iberiotoxin-sensitive Ca-activated large conductance K+ channels that were capable of generating spontaneous transient outward currents at positive potentials [69, 70].
Future issues of ICC-LC research in the urogenital organs
Kit (CD117) is a member of the heterogeneous family of CD cell surface molecules, which perform a number of essential roles in the development of the immune system and physiology of various cells, including GI ICCs. Kit (CD117) is also a proto-oncogene so that its mutation or overexpression can lead to the development of cancers in a number of organs including the ovaries, testes, skin, GI tract, blood and the kidney. Kit(CD117)-immunoreactivity is also used to identify bone marrow haematopoietic progenitors such as haematopoietic stem cells, myeloid progenitors and multipotent progenitors, as well as mast cells, melanocytes, even nerve bundles [53]. A subpopulation of Kit (CD117)+ cells in the mouse prostate has also recently been identified as stem cells and shown to generate a prostate after transplantation [64].
Thus we believe that the identification of Kit+ cells in sectioned material of the urogenital tract is not sufficient to establish these cells as ICC-LCs. Immuno-staining of whole mount preparations and low-magnification electron micrographs of preparations are required to provide evidence of the general morphology and position of Kit+ cells within the urogenital wall, whether they indeed form a network of intercommunicating cells and close associations with, and therefore a possible intermediatory role between their target SMCs and nerve bundles, all essential criteria in the original definitions of ICC [71]. Higher-magnification electron micrographs are also required to provide evidence of the intracellular structures previously used to define Kit+ cells as ICC in the GI and urogenital tracts. The presence or absence of various ‘gold standard’ internal structures, such as an extensive smooth endoplasmic reticulum, bundles of intermediate filaments, large Golgi apparatus, numerous mitochondria and caveolae, a discontinuous basal lamina, etc., are essential criteria in the definition of ICC-LCs [56]. However, it should be noted that, as in the intestine where ICC can range between myoid (smooth muscle)-like to fibroblasts-like [71–74], these criteria for ICC-LCs are likely to be somewhat fluid between species, tissues and location. This is particularly true now that studies are beginning to concentrate on the presence of ICC-LCs in the urogenital tract under pathological conditions where these cells, and possibly the SMCs within the wall, may well be undergoing various stages of differentiation, de-differentiation or trans-differentiation.
Indeed there are an increasing number of reports describing that the number of Kit+ cells decreases in pathological of conditions in the upper urinary tract; conditions including ureteropelvic obstruction [28] or refluxing and obstructive megaureter in human [75–77] and ureteral obstruction induced in rats [78] or ureterovescial reflux in pigs [79, 80]. In contrast, the number of Kit+ cells is increased in bladder outlet obstruction in guinea pigs [17] and human overactive bladder [25]. It has been reported that apoptotic cell death is continuing process in the GI ICC and these cells may be continually regenerated with a relatively fast cell cycle to maintain their network [81]. Thus another area of research interest involves understanding the mechanisms controlling the proliferation and differentiation of ICC-LCs themselves.
Little is known of the time course of ICC-LC remodelling with obstruction or upon the relief of obstruction. However, obstruction in the urogenital tract is likely to result in ischemic conditions. Thus the trigger of ICC-LC remodelling could well be the diminished by the supply of substrates for mitochondrial respiration. Since the mitochondrial function is closely linked to Ca2+ oscillation in ICC-LCs [38, 82], changes in Ca2+ dynamics arising from this diminished mitochondrial function could affect many cellular pathways, including nuclear function, protein synthesis and mitochondrial function itself.
ICC-LCs have been characterized as being richly endowed with mitochondria [36, 38], and their involvement in generating spontaneous activity has been well established pharmacologically [38, 58, 82]. ICC-LCs may well sense the local metabolic supply that is critical for providing substrates of mitochondrial respiration, i.e. glucose and oxygen. Their role in producing ATP to meet the local energy demands suggests that ICC-LCs may also act as metabolic sensors. These mechanisms could well undergo considerable stress and remodelling during pathological conditions that involve urinary or seminal stasis. It is very curious that the bladder and renal pelvis or ureter do not respond in the same way in terms of the numbers of Kit+ cells present when their outlets are obstructed.
Although myofibroblasts in the suburothelial layer of the bladder have some contractile functionality, other ICC-LCs do not. So it is of interest to know how ICC-LCs consume the energy produced by their numerous mitochondria. Certainly, providing ATP for the CaATPase to refill intracellular stores after each Ca2+ transient is critical in generating spontaneous activity in ICC-LCs. This ATP production may also be essential in the synthesis and secretion of substances from ICC-LCs critical to maintaining signal transmission in surrounding SMCs or nerves. ICC-LCs in the corporal tissue and bladder express COX-2 immuno-reactivity, while COX inhibition strongly suppresses spontaneous electrical and contractile activity in corpus carvernosum [45] and renal pelvis [60, 61]. Therefore, ICC-LCs may be constitutively producing and releasing essential paracrine substances, the nature of which has yet to be elucidated.
In summary, the role of mitochondria in regulating ICC-LCs function and the consequences of their malfunction under conditions of metabolic dysfunction, e.g. during ischemia or diabetes, may well reveal the fundamental role of urogenital ICC-LCs in physiology and disease. In addition, whether ICC-LCs can transform into other cell types, e.g. SMCs or (myo)fibroblasts, should be investigated to establish if ICC-LCs have a role as progenitor or precursor cells.
Acknowledgments
This project was supported by Grant-in-Aid for Scientific Research (B) from JSPS to H.H. (No. 19390418) and the NHMRC (Australia) to R.J.L.
Notes
After manuscript acceptance, newer information since appeared. The terms “TELOCYTES” and “TELOPODES”, respectively, were proposed for IntersititIal Cajal-like Cells and their prolongations.
a. Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010; 14: 729–40.
b. Faussone-Pellegrini MS, Bani D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J Cell Mol Med. 2010; 14: 1061–3.
c. Bani D, Formigli L, Gherghiceanu M, Faussone-Pellegrini MS. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. May 2010. In press.
d. Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010. DOI: 10.1111/j.1582-4934.2010.01111.x.
e. Sanders KM. Interstitial cells in smooth muscles Review Series. J Cell Mol Med. 2010; 14: 1197–8.
For more details please visit: http://www.telocytes.com
References
- 1.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 2.Brading AF, McCloskey KD. Mechanisms of disease: specialized interstitial cells of the urinary tract–an assessment of current knowledge. Nat Clin Pract Urol. 2005;2:546–54. doi: 10.1038/ncpuro0340. [DOI] [PubMed] [Google Scholar]
- 3.McCloskey KD, Anderson UA, Davidson RA, et al. Comparison of mechanical and electrical activity and interstitial cells of Cajal in urinary bladders from wild-type and W/Wv mice. Br J Pharmacol. 2009;156:273–83. doi: 10.1111/j.1476-5381.2008.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston L, Sergeant GP, Hollywood MA, et al. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–61. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashitani H, Suzuki H. Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal-like cells of the rabbit urethra in situ. J Physiol. 2007;583:505–19. doi: 10.1113/jphysiol.2007.136697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang RJ, Hashitani H, Tonta MA, et al. Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis. J Physiol. 2007;583:1049–68. doi: 10.1113/jphysiol.2007.137034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–81. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141:183–93. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RJ, Hashitani H, Tonta MA, et al. Role of Ca2+ entry and Ca2+ stores in atypical smooth muscle cell autorhythmicity in the mouse renal pelvis. Br J Pharmacol. 2007;152:1248–59. doi: 10.1038/sj.bjp.0707535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery BS, Fry CH. The action potential and net membrane currents in isolated human detrusor smooth muscle cells. J Urol. 1992;147:176–84. doi: 10.1016/s0022-5347(17)37192-6. [DOI] [PubMed] [Google Scholar]
- 11.Karkanis T, DeYoung L, Brock GB, et al. Ca2+-activated Cl− channels in corpus cavernosum smooth muscle: a novel mechanism for control of penile erection. J Appl Physiol. 2003;94:301–13. doi: 10.1152/japplphysiol.00660.2002. [DOI] [PubMed] [Google Scholar]
- 12.Craven M, Sergeant GP, Hollywood MA, et al. Modulation of spontaneous Ca2+-activated Cl− currents in the rabbit corpus cavernosum by the nitric oxide-cGMP pathway. J Physiol. 2004;556:495–506. doi: 10.1113/jphysiol.2003.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol. 2004;559:231–43. doi: 10.1113/jphysiol.2004.067934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RJ, Hashitani H, Tonta MA, et al. Spontaneous electrical and Ca signals in the mouse renal pelvis that drive pyeloureteric peristalsis. Clin Exp Pharmacol Physiol. 2010;37:509–15. doi: 10.1111/j.1440-1681.2009.05226.x. [DOI] [PubMed] [Google Scholar]
- 15.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–90. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- 16.Lyons AD, Gardiner TA, McCloskey KD. Kit-positive interstitial cells in the rabbit urethra: structural relationships with nerves and smooth muscle. BJU Int. 2007;99:687–94. doi: 10.1111/j.1464-410X.2006.06617.x. [DOI] [PubMed] [Google Scholar]
- 17.Kubota Y, Hashitani H, Shirasawa N, et al. Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction. Neurourol Urodyn. 2008;27:330–40. doi: 10.1002/nau.20502. [DOI] [PubMed] [Google Scholar]
- 18.Sergeant GP, Thornbury KD, McHale NG, et al. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol Cell Physiol. 2002;283:C885–94. doi: 10.1152/ajpcell.00085.2002. [DOI] [PubMed] [Google Scholar]
- 19.Johnston L, Carson C, Lyons AD, et al. Cholinergic-induced Ca2+ signaling in interstitial cells of Cajal from the guinea pig bladder. Am J Physiol Renal Physiol. 2008;294:F645–55. doi: 10.1152/ajprenal.00526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smet PJ, Jonavicius J, Marshall VR, et al. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–48. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- 21.Gosling JA, Dixon JS. The structure and innervation of smooth muscle in the wall of the bladder neck and proximal urethra. Br J Urol. 1975;47:549–8. doi: 10.1111/j.1464-410x.1975.tb06260.x. [DOI] [PubMed] [Google Scholar]
- 22.Exintaris B, Lang RJ. Effects of nerve stimulation on spontaneously active preparations of the guinea pig ureter. Urol Res. 1999;27:328–35. doi: 10.1007/s002400050159. [DOI] [PubMed] [Google Scholar]
- 23.Lang RJ, Exintaris B, Teele ME, et al. Electrical basis of peristalsis in the mammalian upper urinary tract. Clin Exp Pharmacol Physiol. 1998;25:310–21. doi: 10.1111/j.1440-1681.1998.tb02357.x. [DOI] [PubMed] [Google Scholar]
- 24.Sui G, Fry CH, Malone-Lee J, et al. Aberrant Ca2+ oscillations in smooth muscle cells from overactive human bladders. Cell Calcium. 2009;45:456–64. doi: 10.1016/j.ceca.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Biers SM, Reynard JM, Doore T, et al. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006;97:612–6. doi: 10.1111/j.1464-410X.2005.05988.x. [DOI] [PubMed] [Google Scholar]
- 26.Hashitani H, Hayase M, Suzuki H. Effects of imatinib mesylate on spontaneous electrical and mechanical activity in smooth muscle of the guinea-pig stomach. Br J Pharmacol. 2008;154:451–9. doi: 10.1038/bjp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang IY, Glasgow NJ, Takayama I, et al. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555–68. doi: 10.1111/j.1469-7793.2001.0555c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solari V, Piotrowska AP, Puri P. Altered expression of interstitial cells of Cajal in congenital ureteropelvic junction obstruction. J Urol. 2003;170:2420–2. doi: 10.1097/01.ju.0000097401.03293.f0. [DOI] [PubMed] [Google Scholar]
- 29.Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen H, Hansen A, Smedts F, et al. CD34-positive interstitial cells of the human detrusor. APMIS. 2007;115:1260–6. doi: 10.1111/j.1600-0643.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 31.Yanai Y, Hashitani H, Hayase M, et al. Role of nitric oxide/cyclic GMP pathway in regulating spontaneous excitations in detrusor smooth muscle of the guinea-pig bladder. Neurourol Urodyn. 2008;27:446–53. doi: 10.1002/nau.20517. [DOI] [PubMed] [Google Scholar]
- 32.Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol. 2004;171:938–43. doi: 10.1097/01.ju.0000108120.28291.eb. [DOI] [PubMed] [Google Scholar]
- 33.Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- 34.Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–32. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514:459–70. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sergeant GP, Hollywood MA, McCloskey KD, et al. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–66. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston L, Sergeant GP, Hollywood MA, et al. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–61. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sergeant GP, Bradley E, Thornbury KD, et al. Role of mitochondria in modulation of spontaneous Ca2+ waves in freshly dispersed interstitial cells of Cajal from the rabbit urethra. J Physiol. 2008;586:4631–42. doi: 10.1113/jphysiol.2008.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashitani H. Interaction between interstitial cells and smooth muscles in the lower urinary tract and penis. J Physiol. 2006;576:707–14. doi: 10.1113/jphysiol.2006.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashitani H, Yanai Y, Kohri K, et al. Heterogeneous CPA sensitivity of spontaneous excitation in smooth muscle of the rabbit urethra. Br J Pharmacol. 2006;148:340–9. doi: 10.1038/sj.bjp.0706729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sergeant GP, Johnston L, McHale NG, et al. Activation of the cGMP/PKG pathway inhibits electrical activity in rabbit urethral interstitial cells of Cajal by reducing the spatial spread of Ca2+ waves. J Physiol. 2006;574:167–81. doi: 10.1113/jphysiol.2006.108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Pascual A, Sancho M, Costa G, et al. Interstitial cells of Cajal in the urethra are cGMP-mediated targets of nitrergic neurotransmission. Am J Physiol Renal Physiol. 2008;295:F971–83. doi: 10.1152/ajprenal.90301.2008. [DOI] [PubMed] [Google Scholar]
- 43.Hashitani H, Fukuta H, Dickens EJ, et al. Cellular mechanisms of nitric oxide-induced relaxation of corporeal smooth muscle in the guinea-pig. J Physiol. 2002;538:573–81. doi: 10.1113/jphysiol.2001.013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashitani H, Suzuki H. Identification of interstitial cells of Cajal in corporal tissues of the guinea-pig penis. Br J Pharmacol. 2004;141:199–204. doi: 10.1038/sj.bjp.0705622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashitani H, Yanai Y, Shirasawa N, et al. Interaction between spontaneous and neurally mediated regulation of smooth muscle tone in the rabbit corpus cavernosum. J Physiol. 2005;569:723–35. doi: 10.1113/jphysiol.2005.099309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pezzone MA, Watkins SC, Alber SM, et al. Identification of c-kit-positive cells in the mouse ureter: the interstitial cells of Cajal of the urinary tract. Am J Physiol-Renal Physiol. 2003;284:F925–9. doi: 10.1152/ajprenal.00138.2002. [DOI] [PubMed] [Google Scholar]
- 47.Lang RJ, Klemm MF. Interstitial cell of Cajal-like cells in the upper urinary tract. J Cell Mol Med. 2005;9:543–56. doi: 10.1111/j.1582-4934.2005.tb00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David SG, Cebrian C, Vaughan ED, et al. C-kit and ureteral peristalsis. J Urol. 2005;173:292–5. doi: 10.1097/01.ju.0000141594.99139.3d. [DOI] [PubMed] [Google Scholar]
- 49.Metzger R, Schuster T, Till H, et al. Cajal-like cells in the upper urinary tract: comparative study in various species. Pediatr Surg Int. 2005;21:169–74. doi: 10.1007/s00383-004-1314-4. [DOI] [PubMed] [Google Scholar]
- 50.Metzger R, Neugebauer A, Rolle U, et al. C-Kit receptor (CD117) in the porcine urinary tract. Pediatr Surg Int. 2008;24:67–76. doi: 10.1007/s00383-007-2043-2. [DOI] [PubMed] [Google Scholar]
- 51.Metzger R, Schuster T, Till H, et al. Cajal-like cells in the human upper urinary tract. J Urol. 2004;172:769–72. doi: 10.1097/01.ju.0000130571.15243.59. [DOI] [PubMed] [Google Scholar]
- 52.van der Aa F, Roskams T, Blyweert W, et al. Identification of kit positive cells in the human urinary tract. J Urol. 2004;171:2492–6. doi: 10.1097/01.ju.0000125097.25475.17. [DOI] [PubMed] [Google Scholar]
- 53.Zhang SC, Fedoroff S. Cellular localization of stem cell factor and c-kit receptor in the mouse nervous system. J Neurosci Res. 1997;47:1–15. [PubMed] [Google Scholar]
- 54.Lang RJ, Tonta MA, Zoltkowski BZ, et al. Pyeloureteric peristalsis: role of atypical smooth muscle cells and interstitial cells of Cajal-like cells as pacemakers. J Physiol. 2006;576:695. doi: 10.1113/jphysiol.2006.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang RJ, Takano H, Davidson ME, et al. Characterization of the spontaneous electrical and contractile activity of smooth muscle cells in the rat upper urinary tract. J Urol. 2001;166:329–34. [PubMed] [Google Scholar]
- 56.Klemm MF, Exintaris B, Lang RJ. Identification of the cells underlying pacemaker activity in the guinea- pig upper urinary tract. J Physiol. 1999;519:867–84. doi: 10.1111/j.1469-7793.1999.0867n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lang RJ, Hashitani H, Keller S, et al. Modulators of internal Ca2+ stores and the spontaneous electrical and contractile activity of the guinea-pig renal pelvis. Br J Pharmacol. 2002;135:1363–74. doi: 10.1038/sj.bjp.0704609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Exintaris B, Nguyen DT, Lam M, et al. Inositol trisphosphate-dependent Ca stores and mitochondria modulate slow wave activity arising from the smooth muscle cells of the guinea pig prostate gland. Br J Pharmacol. 2009;56:1098–106. doi: 10.1111/j.1476-5381.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen DT, Lang RJ, Exintaris B. alpha(1)-adrenoceptor modulation of spontaneous electrical waveforms in the guinea-pig prostate. Eur J Pharmacol. 2009;608:62–70. doi: 10.1016/j.ejphar.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 60.Davidson ME, Lang RJ. Effects of selective inhibitors of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) on the spontaneous myogenic contractions in the upper urinary tract of the guinea-pig and rat. Br J Pharmacol. 2000;129:661–70. doi: 10.1038/sj.bjp.0703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Lang RJ. Effects of intrinsic prostaglandins on the spontaneous contractile and electrical activity of the proximal renal pelvis of the guinea-pig. Br J Pharmacol. 1994;113:431–8. doi: 10.1111/j.1476-5381.1994.tb17007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Exintaris B, Klemm MF, Lang RJ. Spontaneous slow wave and contractile activity of the guinea pig prostate. J Urol. 2002;168:315–22. [PubMed] [Google Scholar]
- 63.Exintaris B, Nguyen DT, Dey A, et al. Spontaneous electrical activity in the prostate gland. Auton Neurosci. 2006;126:371–9. doi: 10.1016/j.autneu.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 64.Leong KG, Wang BE, Johnson L, et al. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–8. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 65.Van der Aa F, Roskams T, Blyweert W, et al. Interstitial cells in the human prostate: a new therapeutic target. Prostate. 2003;56:250–5. doi: 10.1002/pros.10264. [DOI] [PubMed] [Google Scholar]
- 66.Shafik A, Shafik I, el-Sibai O. Identification of c-kit-positive cells in the human prostate: the interstitial cells of Cajal. Arch Androl. 2005;51:345–51. doi: 10.1080/014850190944456. [DOI] [PubMed] [Google Scholar]
- 67.Aumuller G, Habenicht UF, el Etreby MF. Pharmacologically induced ultrastructural and immunohistochemical changes in the prostate of the castrated dog. Prostate. 1987;11:211–8. doi: 10.1002/pros.2990110302. [DOI] [PubMed] [Google Scholar]
- 68.Lang RJ, Nguyen DTT, Matsuyama H, et al. Characterization of spontaneous depolarizations in smooth muscle cells of the guinea pig prostate. J Urol. 2006;175:370–80. doi: 10.1016/S0022-5347(05)00003-0. [DOI] [PubMed] [Google Scholar]
- 69.Oh SJ, Kim KM, Chung YS. Ion-channel currents of smooth muscle cells isolated from the prostate of guinea-pig. BJU Int. 2003;92:1022–30. doi: 10.1111/j.1464-410x.2003.04510.x. [DOI] [PubMed] [Google Scholar]
- 70.Lang RJ, Mulholland E, Exintaris B. Characterization of the ion channel currents in single myocytes of the guinea pig prostate. J Urol. 2004;172:1179–87. doi: 10.1097/01.ju.0000135456.65892.ed. [DOI] [PubMed] [Google Scholar]
- 71.Huizinga JD, Faussone-Pellegrini MS. About the presence of interstitial cells of Cajal outside the musculature of the gastrointestinal tract. J Cell Mol Med. 2005;9:468–73. doi: 10.1111/j.1582-4934.2005.tb00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Komuro T. Comparative morphology of interstitial cells of Cajal: ultrastructural characterization. Microsc Res Tech. 1999;47:267–85. doi: 10.1002/(SICI)1097-0029(19991115)47:4<267::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 73.Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol. 1999;62:295–316. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- 74.Rasmussen H, Rumessen JJ, Hansen A, et al. Ultrastructure of Cajal-like interstitial cells in the human detrusor. Cell Tissue Res. 2009;335:517–27. doi: 10.1007/s00441-008-0736-z. [DOI] [PubMed] [Google Scholar]
- 75.Kang HJ, Lee HY, Jin MH, et al. Decreased interstitial cells of Cajal-like cells, possible cause of congenital refluxing megaureters: histopathologic differences in refluxing and obstructive megaureters. Urol. 2009;74:318–23. doi: 10.1016/j.urology.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 76.Arena E, Nicotina PA, Arena S, et al. Interstitial cells of Cajal network in primary obstructive megaureter. Pediatr Med Chir. 2007;29:28–31. [PubMed] [Google Scholar]
- 77.Arena F, Nicotina PA, Arena S, et al. C-kit positive interstitial cells of Cajal network in primary obstructive megaureter. Minerva Pediatr. 2007;59:7–11. [PubMed] [Google Scholar]
- 78.Kuzgunbay B, Doran F, Bayazit Y, et al. The effects of ureteral obstruction on Cajal-like cells in rats. J Pediatr Urol. 2009;5:269–73. doi: 10.1016/j.jpurol.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Oberritter Z, Rolle U, Juhasz Z, et al. Altered expression of c-kit-positive cells in the ureterovesical junction after surgically created vesicoureteral reflux. Pediatr Surg Int. 2009;25:1103–7. doi: 10.1007/s00383-009-2487-7. [DOI] [PubMed] [Google Scholar]
- 80.Arena S, Fazzari C, Arena F, et al. Altered ‘active’ antireflux mechanism in primary vesico-ureteric reflux: a morphological and manometric study. BJU Int. 2007;100:407–12. doi: 10.1111/j.1464-410X.2007.06921.x. [DOI] [PubMed] [Google Scholar]
- 81.Gibbons SJ, De Giorgio R, Pellegrini MS, et al. Apoptotic cell death of human interstitial cells of Cajal. Neurogastroenterol Motil. 2009;21:85–93. doi: 10.1111/j.1365-2982.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hashitani H, Lang RJ, Mitsui R, et al. Distinct effects of CGRP on typical and atypical smooth muscle cells involved in generating spontaneous contractions in the mouse renal pelvis. Br J Pharmacol. 2009;158:2030–45. doi: 10.1111/j.1476-5381.2009.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hashitani H, Yanai Y, Shirasawa N, et al. Interaction between spontaneous and neurally mediated regulation of smooth muscle tone in the rabbit corpus cavernosum. J Physiol. 2005;569:723–35. doi: 10.1113/jphysiol.2005.099309. [DOI] [PMC free article] [PubMed] [Google Scholar]


