Abstract
Hepatoma-derived growth factor (HDGF) is a novel mitogenic growth factor that has been implicated in many different carcinomas. Its role in keloid biology has not yet been investigated. The present study is aimed at examining the role of HDGF in keloid pathogenesis. Immunohistochemical staining and Western blot analyses were used to examine in vivo localization and expression of HDGF in keloid and normal skin tissue. This was followed by the detection of HDGF expression in fibroblasts cultured in vitro and fibroblasts exposed to serum. To investigate the effect of epithelial–mesenchymal interactions, a two-chamber system was employed in which keratinocytes on membrane inserts were co-cultured with the fibroblasts. HDGF expression levels in all cell extracts and conditioned media were assayed through Western blot analysis. In another set of experiments, the effect of exogenous recombinant HDGF on keloid fibroblasts (KF) and normal fibroblasts (NF) was examined. Cell proliferation was assessed by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and by quantifying proliferating cell nuclear antigen (PCNA) expression. Downstream targets of HDGF were identified by detecting their expression through Western blot analysis. Our results indicate that there was an increase in HDGF expression in the dermis of keloid compared with normal skin tissue. The application of serum and epithelial–mesenchymal interactions did not seem to have any effect on intracellular HDGF expression levels. However, co-culturing keloid keratinocytes with KFs resulted in increased HDGF secretion when compared with monoculture or normal controls. Furthermore, treatment with exogenous recombinant HDGF was found to increase the proliferation of KFs, activate the extracellular signal-regulated kinase (ERK) pathway and up-regulate the secretion of vascular endothelial growth factor (VEGF).
Keywords: hepatoma-derived growth factor, keloid fibroblast, proliferation, ERK, epithelial–mesenchymal interaction, wound healing, vascular endothelial growth factor
Introduction
Hepatoma-derived growth factor (HDGF) is a novel heparin-binding protein that was originally purified from the conditioned media of HuH-7 hepatoma cells [1, 2]. This growth factor is the first member of the HDGF family of proteins that was discovered to contain a well-conserved N-terminal amino acid sequence, which is called the HATH (homologous to amino terminus of HDGF) region [2, 3]. HDGF was found to have mitogenic activity for fibroblasts, endothelial cells, renal and lung epithelial cells, vascular smooth muscle cells and foetal hepatocytes [1, 2, 4–7]. Although the mitogenic effect of HDGF has been proven, the pathway by which it exerts this proliferative activity is still unclear. Two different pathways have been proposed. Despite lacking the secretory sequence present in most secretory proteins [2], it has been shown that exogenous HDGF could possibly act by binding to an as yet unknown cell surface receptor, triggering signalling events downstream that result in increased proliferation [6]. Others have shown that nuclear localization is required for the mitogenic activity of HDGF [4, 8]. Because of its role as a mitogenic growth factor, HDGF has been implicated in the development of different types of cancers, such as oesophageal cancer [9, 10], pancreatic cancer [11], hepatocellular carcinoma [12], melanoma [13], lung cancer [14] and gastric carcinoma [15, 16]. In addition, HDGF has also been speculated to play a role in renal [17], liver [5], lung [18], brain [19], gut [20] and heart [21] development. There is also some evidence linking the down-regulation of HDGF to apoptosis [22–24]. In the area of wound repair, HDGF has been found to be expressed after vascular injury [25] and during retinal pigment epithelial wound repair [26].
A keloid represents a derailment in the normal process of wound healing and is characterized by an overgrowth of dense fibrous tissue coupled with excessive deposition of extracellular matrix (ECM) components [27]. This causes a prominent elevation of scar tissue above the skin, extending beyond the original wound margins and may develop even after the most minor of skin wounds, such as insect bites or acne [28]. Keloids are frequently associated with itchiness, pain and, when involving the skin overlying a joint, restricted range of motion [29]. They are found to uniquely affect humans and will often recur following attempted resection, prompting some researchers to consider them as benign tumours [30]. Although many different treatment modalities have been proposed [31], none have proven to be optimal, and the exact cause and the clinical behaviour of keloids remain an enigma. Recent research has shown that many different cytokines and growth factors are involved in the formation of keloids [32–38]. Because of HDGF’s status as a novel mitogenic growth factor that has been implicated in the aberrant growth of tumours, we speculate that it could also play some role in the formation of keloids.
In this study, we investigated the expression and localization of HDGF in vivo by performing immunohistochemical staining (IHC) and Western blot analysis on keloid and normal skin tissue. We further studied the expression of HDGF using in vitro models of normal fibroblasts (NF) and keloid fibroblasts (KF) subjected to serum stimulation. To examine the effect of epithelial–mesenchymal interactions on the expression of HDGF, we employed a two-chamber serum-free system in which keratinocytes on membrane inserts were co-cultured with fibroblasts. This system has previously been successfully utilized by our group [32–35].
In another set of experiments, we examined the effect of exogenous recombinant HDGF on KFs and NFs. Cells treated with recombinant HDGF were assessed for increased proliferation by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and by quantifying proliferating cell nuclear antigen (PCNA) expression. Western blotting was also performed to identify some of the downstream signalling targets of HDGF.
Materials and methods
Immunohistochemistry
Paraffin sections were dewaxed and antigens were retrieved by immersing the slides in 0.01 M citrate buffer, pH 6.0, heating in a microwave oven (high for 2.5 min., low for 5 min.), cooling at 4°C for 20 min. and washing in water for 5 min. Endogenous peroxidase was blocked in 3% H2O2 and non-specific binding was blocked for 1 hr (CAS block; Zymed Laboratories, South San Francisco, CA, USA). The sections were incubated with antibodies specific for HDGF, diluted 1:1000 for 1 hr. After washing, the slides were incubated in antimouse IgG-peroxidase (Zymed) or anti-rabbit IgG-peroxidase (Zymed), diluted 1:500 for 2 hrs, for HDGF primary antibodies, respectively. The slides were washed in Tris-buffered NaCl (TBS) or 0.05% Tween-20, pH 7.5, and then with MilliQ, Millipore Corp, Billerica, MA, H2O. The reaction product was developed with 3,3′-diaminobenzidine tetrahydrochloride substrate kit (Zymed), and the sections were counterstained with haematoxylin. All wash steps were carried out in TBS/0.05% Tween-20. The antibodies were diluted in 1% bovine serum albumin (BSA)/TBS. Non-immune mouse/rabbit antibody of the appropriate immunoglobulin isotype was used for negative controls.
Keloid keratinocyte and fibroblast database
Keratinocytes and fibroblasts were randomly selected from a specimen bank of keratinocyte/fibroblast strains derived from excised keloid specimens. All patients had received no previous treatment for the keloids before surgical excision. A full history was taken and an examination was performed, complete with coloured slide photographic documentation, before taking informed consent prior to excision. Approval by the National University of Singapore (NUS) Institutional Review Board (NUS-IRB) was sought before excision of human tissue and collection of cells.
Keratinocyte culture from earlobe keloids and normal skin
Normal keratinocytes (NK) and keloid keratinocytes (KK) were derived from excision specimens as previously described [32]. Only cells from the second or third passage were used in all experiments.
Fibroblast culture from keloid scars and normal skin
Remnant dermis from keloid or normal skin was minced and incubated in a solution of collagenase type I (0.5 mg/ml) and trypsin (0.2 mg/ml) for 6 hrs at 37°C. The cells were pelleted and grown in tissue culture flasks. The cell strains were maintained and stored in liquid nitrogen until use. NFs and KFs from the second passage were used for all experiments.
In vitro serum stimulation of monocultured normal and keloid fibroblasts
Fibroblasts were seeded in six-well plates at a density of 1 × 104 cells/ml in 10% foetal calf serum (FCS) for 24 hrs and subsequently starved in a serum-free medium for another 48 hrs. After 48 hrs, the fibroblasts were stimulated by exposure to either 10% FCS or Dulbecco’s modified Eagle’s medium (DMEM) for 5 days before being harvested.
Keratinocyte–fibroblast co-culture
KKs and NKs obtained from randomly selected keloid and normal strains were seeded at a density of 1 × 105 cells/cm2 on Transwell clear polyester membrane inserts with 0.4-μm pore size and 4.5 cm2 (Corning Incorporated Life Sciences, Acton, MA, USA). The cells were maintained for 4 days in EpiLife medium (Cascade Biologics, OR, USA) until 100% confluent. The medium was then changed to EpiLife supplemented with increased calcium concentration and the cells were exposed to the air–liquid interface for another 3 days, allowing the keratinocytes to stratify and reach terminal differentiation. Fibroblast cell strains were seeded in six-well plates at a density of 1 × 105 cells/well in DMEM/10% FCS for 48 hrs to 80% confluency. Keratinocytes on membrane inserts and plated fibroblasts were washed twice with PBS before the inserts were placed into the six-well plates containing fibroblast cultures to initiate KK/KF or NK/NF co-cultures in fresh serum-free DMEM. Whole-cell extracts and conditioned media were harvested and analysed separately.
Treatment of normal and keloid fibroblasts with exogenous recombinant HDGF
KF and NF cultures were established as above. The cells were subsequently treated with 250 ng/ml of recombinant HDGF. Cells without treatment were used as controls. Whole-cell extracts and conditioned media were harvested and subjected to Western blot analysis for the different molecular targets.
Western blot
Frozen tissue specimens or cultured fibroblasts under different experimental conditions were lysed in cell lysis buffer containing 20 mM Tris-HCl (pH 7.5), 1% Triton X-100, 100 mM NaCl, 0.5% Nonidet P-40 and 1 mg/ml protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany). Proteins were then subjected to Western blot analysis. In total, 50 μg of whole-cell extract was separated by 14% or 8% SDS-PAGE under reducing conditions and electroblotted onto a nitrocellulose membrane. The blots were incubated with test antibodies, including mouse and rabbit anti-HDGF, mouse anti-PCNA (Santa Cruz, Biotechnology, Inc., CA, USA), mouse anti-vascular endothelial growth factor (VEGF), rabbit anti-p44/p42 mitogen-activated protein kinase (MAPK) and mouse anti-phospho p44/p42 MAPK (Cell Signaling Technology, Inc., MA, USA). The blots were visualized with a chemiluminescence-based photoblot system (Amersham Biosciences, Buckinghamshire, UK). For the analysis involving conditioned media, 4 ml of the conditioned media was concentrated using a Centricon centrifuge (Millipore Corp., MA, USA) and then subjected to Western blotting.
Cell proliferation assay
The MTT assay is a colorimetric assay that tests the metabolic activity of viable cells and is used for indirect cell quantification [39]. It was used to assess fibroblast proliferative response to exogenous HDGF at varying concentrations.
Computerized gel densitometry
A Bio-Rad gel scanner and densitometer program (Gel-Pro Analyzer version 4.5; MediaCybernetics, Bethesda, MD, USA) was utilized to assess the concentrations of the bands obtained by Western blots. These were measured as total density units.
Statistical analysis
The paired Student’s t-test or the Welch’s t-test was used for all analyses where appropriate. A value of P < 0.05 was considered to be statistically significant. The error bars denote the standard error of the mean (S.E.M.). All statistical analyses were done using Microsoft Excel 2003 (Redmond, WA, USA).
Results
HDGF expression is increased in keloid scar dermis compared with normal skin dermis
Immunohistochemical labelling showed that HDGF was present in both the epidermis (Fig. 1A) and the dermis (Fig. 1B) of normal and keloid tissue. Epidermal staining intensity was irregular and sample-dependent, with some keloid samples exhibiting stronger staining, while others exhibiting equal or weaker staining when compared with their normal counterparts. However, HDGF expression in the dermis was found to be higher in all keloid samples. This can be more clearly seen at a lower magnification (Fig. 1C). In the keloid tissues, almost the whole dermis was stained brown compared with a significantly smaller area in normal skin. Western blot results from the keloid and normal whole-tissue extracts reconfirmed these observations. The keloid tissue samples had a significantly higher expression of HDGF compared with the normal tissue samples (P < 0.05; Fig. 1D).
Fig 1.
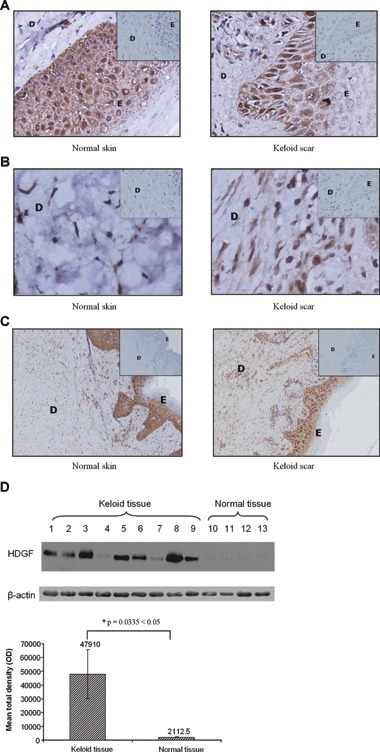
Expression and localization of HDGF in keloid and normal tissue. Paraffin sections of normal and keloid tissue were prepared and stained with antibodies against HDGF. Pictures were taken with magnification at 40× (A, B) and 10× (C). The dermis and the epidermis are represented by (D) and (E), respectively. In each panel, the inset shows the same tissue labelled with a non-immune mouse antibody of the appropriate immunoglobulin isotype as a negative control. HDGF was detected in both the epidermis (A) and the dermis (B) of normal and keloid tissue. Increased expression was observed in the dermis of keloid tissue compared with the dermis of normal tissue (B, C). (D) In total, 50 μg of tissue extracts from nine keloid tissue specimens and four normal skin specimens was subjected to Western blot analysis with antibodies against HDGF. The whole-tissue extracts include both the epidermis and the dermis. The blots were probed with anti-β-actin antibody to confirm equal loading. The bar graph represents the mean ± S.E.M. of HDGF levels in the normal and keloid samples, as quantified by gel densitometry. *indicates statistical significance as assessed by Welch’s t-test.
Serum stimulation and epithelial–mesenchymal interactions had no effect on intracellular HDGF expression in both normal and keloid fibroblast cells
The Western blot analysis indicated that treatment with serum had no significant effect on intracellular HDGF expression in both NFs and KFs (Fig. 2A and B). In addition, NFs co-cultured with NKs and KFs co-cultured with KKs did not show any significant difference in HDGF expression levels compared with the monocultured controls or when compared with each other (Fig. 2A and B).
Fig 2.
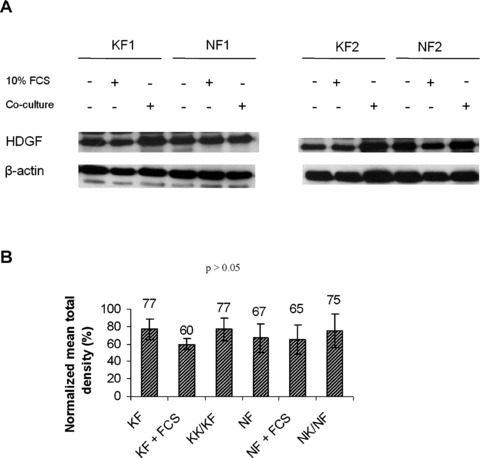
Effect of serum and epithelial–mesenchymal interactions on intracellular HDGF expression. Six different strains of keloid/normal fibroblasts were cultured with DMEM, 10% FCS or co-cultured with keloid/normal keratinocytes for 5 days. In total, 50 μg of total protein extracts was subjected to Western blot analysis with HDGF antibodies. Two representative strains are shown in (A). The bar graphs in (B) represent the normalized mean ± S.E.M. of HDGF levels in the different conditions. All blots were probed and normalized with β-actin.
Epithelial–mesenchymal interactions in keloidco-culture increased secretion of HDGF into the conditioned media
Conditioned media obtained when KFs were co-cultured with KKs showed a significant increase in HDGF compared with conditioned media obtained from monocultured KFs from day 1 to 5 (P < 0.05 for asterisks; Fig. 3A). In contrast, HDGF was undetected from day 1 to 3, and a weak increase was only detected when NFs were co-cultured with NKs on day 5 (P < 0.05; Fig. 3B). Monocultured KFs showed a higher secretion compared with monocultured NFs and keloid co-cultures showed a higher secretion compared with normal skin cell co-cultures. Monocultured keratinocytes show a moderately high secretion of HDGF but no significant difference was seen between NKs and KKs harvested at day 5 (Fig. 3C).
Fig 3.
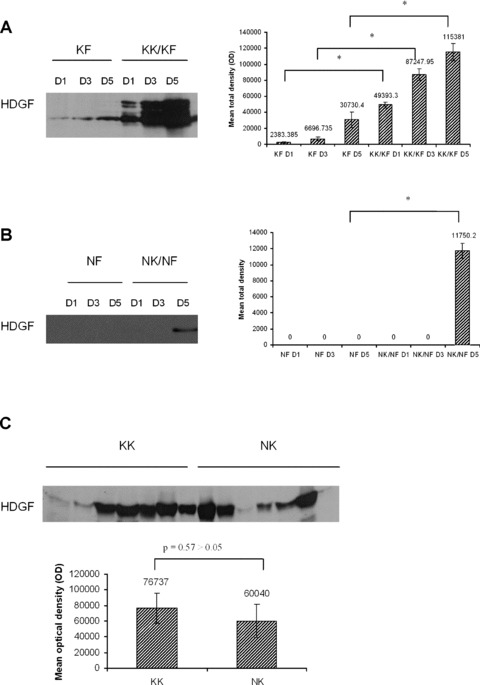
Expression of HDGF in conditioned media of monocultured and co-cultured cells. (A) Conditioned media of keloid fibroblasts monoculture (KF) and keloid fibroblasts co-cultured with keloid keratinocytes (KK/KF) were collected at days 1, 3 and 5. (B) Conditioned media of normal fibroblast monoculture (NF) and normal fibroblast co-cultured with normal keratinocytes (NK/NF) were collected at days 1, 3 and 5. Experiments were performed in duplicates. (C) Conditioned media of seven samples of singly cultured keloid keratinocytes (KK) and normal keratinocytes (NK) were collected at day 5. Four millilitres of the conditioned media from (A), (B) and (C) was then concentrated and subjected to Western blot analysis with anti-HDGF antibody. Representative figures are shown. The bar graphs represent the mean ± S.E.M. of HDGF levels. * indicates statistical significance as determined by the paired t-test.
Increased proliferation of keloid fibroblasts when stimulated with exogenous recombinant HDGF
There was no significant difference in NF proliferation when treated for 72 hrs with various doses of HDGF (Fig. 4A). However, there was a significant dose-dependent increase of up to ∼13% in the proliferation of KFs (P < 0.05 for asterisks; Fig. 4B). In addition, Western blot analysis after 48 hrs showed a significant increase of proliferating cell nuclear antigen (PCNA) expression in treated KFs compared with untreated keloid controls, but this effect was not seen in treated NFs compared with untreated normal controls (P < 0.05; Fig. 5A). In total, 250 ng/ml of recombinant HDGF was used for treatment of both KFs and NFs.
Fig 4.
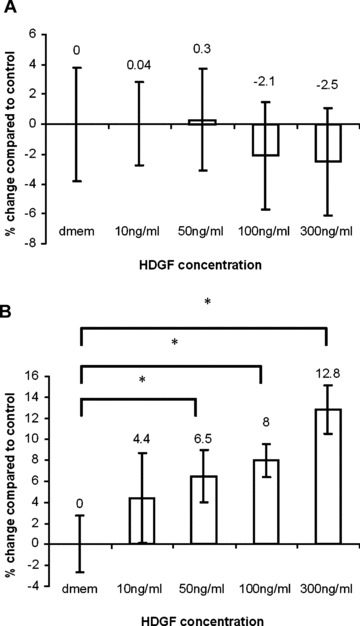
Increased proliferation of keloid fibroblasts treated with recombinant HDGF. Cultures of keloid or normal fibroblasts were grown until 50% confluence and then serum starved for 48 hrs. The fibroblasts were then treated with HDGF (10, 50, 100 and 300 ng/ml) for 72 hrs and then subjected to the MTT proliferation assay. Untreated samples were used as control. The bar graph in (A) represents the mean proliferative response of treated normal fibroblasts as a percentage of the control. The bar graph in (B) represents the mean proliferative response of treated keloid fibroblasts as a percentage of the control. * indicates statistical significance compared with DMEM control as assessed by Student’s t-test.
Fig 5.
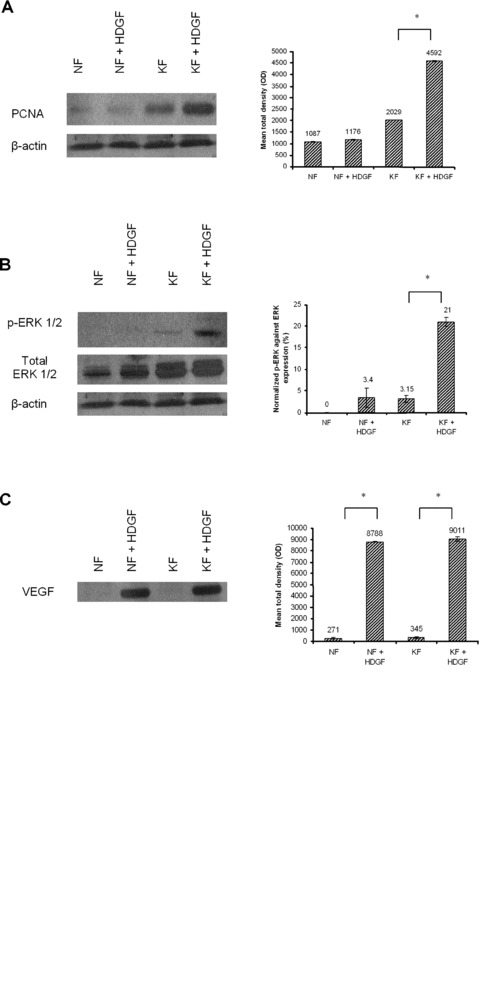
Effect of HDGF on the expression of downstream targets. Normal fibroblasts and keloid fibroblasts were treated with either DMEM or 250 ng/ml of recombinant HDGF, harvested after 48 hrs and lysed for Western blot analysis, as described under experimental procedures. Blots were incubated with anti-PCNA (A), anti-phospho-ERK 1/2 and total ERK 1/2 (B) antibodies. The blots were also incubated with anti-β-actin antibody to confirm equal loading. Four millilitres of conditioned media from the same set of experiments was concentrated and subjected to Western blot analysis. Blots were then incubated with anti-VEGF (C) antibodies. In another set of experiments, normal fibroblasts and keloid fibroblasts were treated with either DMEM or HDGF and harvested after 1 hr, 6 hrs and 24 hrs for Western blot analysis. Blots were incubated with anti-phospho-ERK 1/2 and total ERK 1/2 (D) antibodies. All experiments were performed in duplicates. Representative figures are shown. The bar graphs represent the mean ± S.E.M. of HDGF levels. Phospho-ERK 1/2 was normalized against total ERK1/2 expression. * indicates statistical significance as determined by the paired t-test.
Treatment of fibroblasts with HDGF activated the ERK pathway and increased secretion of VEGF
KFs treated with HDGF after 48 hrs showed a significant increase in the expression of intracellular phospho-extracellular signal-regulated kinase (ERK) 1/2 compared with untreated keloid controls, but this increase was not seen in treated NFs compared with untreated normal controls (P < 0.05; Fig. 5B). Secretion of VEGF into the conditioned media from both KFs and NFs was also increased upon treatment with HDGF for 48 hrs (P < 0.05; Fig. 5C). At earlier time points, no significant increase in phospho-ERK was detected (Fig. 5D). In total, 250 ng/ml of recombinant HDGF was used for treatment of both KFs and NFs.
Discussion
HDGF has not been as well studied as other growth factors and cytokines but there is a growing wealth of information that links it to cancer [9–16]. Furthermore, HDGF gene expression has been found to be up-regulated in human dermal fibroblasts subjected to mechanical stimulation from stressed collagen lattices [40]. Abnormal scarring has been correlated to regions of the body with higher mechanical force than others [41, 42], thus making HDGF an interesting growth factor to study.
Immunohistochemical results from this study indicate that HDGF is quite highly expressed in the epidermis of both keloid and normal skin tissue. However, in the dermal layer, there is a higher expression of HDGF in keloid tissue compared with normal skin. This result led us to focus our efforts on the fibroblasts, which are the major cell type in the dermis [43]. Preliminary in vitro results demonstrated a significant expression of both intracellular and extracellular HDGF in monocultured epidermal keratinocytes. We were unable to detect any significant difference in HDGF expression between KK and NK samples grown in vitro. However, the very presence of HDGF in these cells suggested that this growth factor played some as yet unknown role in epidermal biology that is worth investigating.
The process of cutaneous wound healing can be arbitrarily divided into three phases-inflammation, scar tissue formation and scar remodelling [44]. The serum stimulation model is an in vitro model that can be used to determine the involvement of growth factors or cytokines in the early stages of the wound healing phase. Fibroblasts interpret the presence of serum as a physiological wounding signal and would respond to it as if it had occurred in vivo[45]. Our data show no difference in HDGF expression levels between serum-stimulated and non-serum-stimulated fibroblasts, suggesting the lack of involvement of HDGF in the early phases of wound healing.
Recent studies from our group and others have shown the importance of paracrine signalling via epithelial–mesenchymal interactions in keloid pathogenesis [32–36]. The co-culture experiments were used to assess the effect of this paracrine signalling on HDGF expression. We found no significant difference in intracellular HDGF expression in whole-cell extracts of co-cultured fibroblasts compared with the singly cultured fibroblasts. However, conditioned media collected from the keloid co-culture configuration had significantly higher levels of HDGF compared with those collected from the singly cultured KFs. Furthermore, secretion of HDGF from the keloid co-culture configuration was also higher than from normal skin cell co-culture configuration. When singly cultured, the keratinocytes secreted fairly high amounts of HDGF but there was no significant difference between KKs and NKs. This suggests that the secretion of HDGF is somehow modulated by epithelial–mesenchymal interactions.
HDGF has been reported to act as a potent exogenous mitogen for a wide variety of cells, including 3T3 fibroblasts [1, 6] and mice dermal fibroblasts [46]. The susceptibility of KFs to other mitogenic stimuli has also been previously established [32, 33, 39]. Therefore, it was not surprising that both our MTT and PCNA results showed KFs having a better proliferative response to HDGF stimuli compared with their normal skin counterparts.
Downstream molecular targets of HDGF that were found to be up-regulated after 48 hrs include ERK and VEGF, while those found to be unaffected include alpha-smooth muscle actin (α-SMA), fibronectin and connective tissue growth factor (CTGF; data not shown). We were however unable to detect any increase in ERK phosphorylation at the earlier time points of 1 hr, 6 hrs and 24 hrs. HDGF has previously been found to induce the phosphorylation of ERK in human pulmonary endothelial cells [47] and gastric epithelial cells [48]. However, in 3T3 fibroblasts, it has been reported that extracellular HDGF does not enter the cell but instead binds to the cell membrane. Furthermore, it stimulates proliferation but does not activate the ERK signalling pathway [6]. Our findings that there is no increase in intracellular HDGF and that extracellular HDGF has mitogenic activity without activating ERK at early time points suggest that this may also be the case in KFs. However, while the ERK pathway may not be directly activated by HDGF, it does seem to be somehow involved downstream of HDGF stimulation. In addition to the ERK pathway, we also tested the Akt pathway but were unable to detect any phosphorylation of Akt (data not shown). Thus, the pathway by which HDGF exerts its proliferative activity remains as elusive as ever.
The induction of VEGF by HDGF has also been shown previously in the process of tumourigenesis [49]. In the study conducted by Okuda et al., NIH3T3 fibroblasts overexpressing HDGF were found to induce sarcomatous tumours after injection into nude mice, and the tumour formation was induced mainly by angiogenesis due to induction of VEGF. We report a similar induction of VEGF by HDGF in both keloid and normal primary skin fibroblasts, suggesting that HDGF could play some role in the normal angiogenic process during wound healing. However, in our in vitro co-culture models, we observed very little secretion of HDGF in the normal co-culture experiments. Therefore, while HDGF might induce production of VEGF in both NFs and KFs, the absence of HDGF in the normal condition limits the production of VEGF through this mechanism. This result tallies with previous findings from our group. In the study conducted by Ong et al., keloid tissue was shown to have a higher expression of VEGF compared with normal tissue, and there was also a significant increase in VEGF from keloid co-culture compared with normal co-culture conditions [35]. These results suggest that the production of VEGF could be tied to the presence or absence of HDGF.
Figure 6 summarizes the main findings of our study. First, we have shown that in the keloid condition, HDGF acts mainly as a secreted growth factor that is modulated by epithelial–mesenchymal interactions. Second, exogenous HDGF exerts a proliferative effect on KFs and is likely to be indirectly involved in ERK signalling. Finally, the presence of HDGF increases the production of VEGF and indirectly contributes to the process of angiogenesis.
Fig 6.
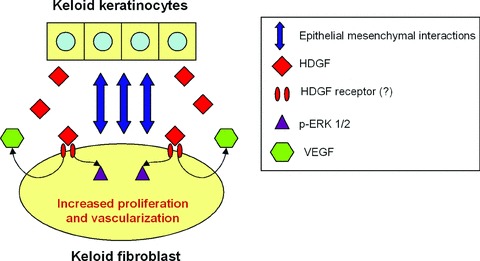
Schematic representation of the role of HDGF in keloid pathogenesis. Epithelial–mesenchymal interactions result in an increased secretion of HDGF in keloids. Overproduction of extracellular HDGF leads to the phosphorylation of ERK 1/2 and increased proliferation of keloid fibroblasts, most likely through a receptor-mediated pathway. HDGF also stimulates the fibroblasts to produce VEGF.
Acknowledgments
This work was supported by grants from the Biomedical Research Council, Singapore (03/1/21/19/251, 04/1/21/19/338, 05/1/21/19/390 and 06/1/21/19/442).
References
- 1.Nakamura H, Kambe H, Egawa T, et al. Partial purification and characterization of human hepatoma-derived growth factor. Clin Chim Acta. 1989;183:273–84. doi: 10.1016/0009-8981(89)90361-6. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura H, Izumoto Y, Kambe H, et al. Molecular cloning of complementary DNA for a novel human hepatoma-derived growth factor. Its homology with high mobility group-1 protein. J Biol Chem. 1994;269:25143–9. [PubMed] [Google Scholar]
- 3.Izumoto Y, Kuroda T, Harada H, et al. Hepatoma-derived growth factor belongs to a gene family in mice showing significant homology in the amino terminus. Biochem Biophys Res Commun. 1997;238:26–32. doi: 10.1006/bbrc.1997.7233. [DOI] [PubMed] [Google Scholar]
- 4.Everett AD, Stoops T, McNamara CA. Nuclear targeting is required for hepatoma-derived growth factor-stimulated mitogenesis in vascular smooth muscle cells. J Biol Chem. 2001;276:37564–8. doi: 10.1074/jbc.M105109200. [DOI] [PubMed] [Google Scholar]
- 5.Enomoto H, Yoshida K, Kishima Y, et al. Hepatoma-derived growth factor is highly expressed in developing liver and promotes fetal hepatocyte proliferation. Hepatology. 2002;36:1519–27. doi: 10.1053/jhep.2002.36935. [DOI] [PubMed] [Google Scholar]
- 6.Abouzied MM, El-tahir HM, Prenner L, et al. Hepatoma-derived growth factor: significance of amino acid residues 81–100 in cell surface interaction and proliferative activity. J Biol Chem. 2005;280:10945–54. doi: 10.1074/jbc.M414652200. [DOI] [PubMed] [Google Scholar]
- 7.Mori M, Morishita H, Nakamura H, et al. Hepatoma-derived growth factor is involved in lung remodeling by stimulating epithelial growth. Am J Respir Cell Mol Biol. 2004;30:459–69. doi: 10.1165/rcmb.2003-0013OC. [DOI] [PubMed] [Google Scholar]
- 8.Kishima Y, Yamamoto H, Izumoto Y, et al. Hepatoma-derived growth factor stimulates cell growth after translocation to the nucleus by nuclear localization signals. J Biol Chem. 2002;277:10315–22. doi: 10.1074/jbc.M111122200. [DOI] [PubMed] [Google Scholar]
- 9.Matsuyama A, Inoue H, Shibuta K, et al. Hepatoma-derived growth factor is associated with reduced sensitivity to irradiation in esophageal cancer. Cancer Res. 2001;61:5714–7. [PubMed] [Google Scholar]
- 10.Yamamoto S, Tomita Y, Hoshida Y, et al. Expression level of hepatoma-derived growth factor correlates with tumor recurrence of esophageal carcinoma. Ann Surg Oncol. 2007;14:2141–9. doi: 10.1245/s10434-007-9369-9. [DOI] [PubMed] [Google Scholar]
- 11.Uyama H, Tomita Y, Nakamura H, et al. Hepatoma-derived growth factor is a novel prognostic factor for patients with pancreatic cancer. Clin Cancer Res. 2006;12:6043–8. doi: 10.1158/1078-0432.CCR-06-1064. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida K, Nakamura H, Okuda Y, et al. Expression of hepatoma-derived growth factor in hepatocarcinogenesis. J Gastroenterol Hepatol. 2003;18:1293–301. doi: 10.1046/j.1440-1746.2003.03191.x. [DOI] [PubMed] [Google Scholar]
- 13.Bernard K, Litman E, Fitzpatrick JL, et al. Functional proteomic analysis of melanoma progression. Cancer Res. 2003;63:6716–25. [PubMed] [Google Scholar]
- 14.Ren H, Tang X, Lee JJ, et al. Expression of hepatoma-derived growth factor is a strong prognostic predictor for patients with early-stage non-small-cell lung cancer. J Clin Oncol. 2004;22:3230–7. doi: 10.1200/JCO.2004.02.080. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto S, Tomita Y, Hoshida Y, et al. Expression of hepatoma-derived growth factor is correlated with lymph node metastasis. Clin Cancer Res. 2006;12:117–22. doi: 10.1158/1078-0432.CCR-05-1347. [DOI] [PubMed] [Google Scholar]
- 16.Chang KC, Tai MH, Lin JW, et al. Hepatoma-derived growth factor is a novel prognostic factor for gastrointestinal stromal tumors. Int J Cancer. 2007;121:1059–65. doi: 10.1002/ijc.22803. [DOI] [PubMed] [Google Scholar]
- 17.Oliver JA, Al-Awqati Q. An endothelial growth factor involved in rat renal development. J Clin Invest. 1998;102:1208–19. doi: 10.1172/JCI785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cilley RE, Zgleszewski SE, Chinoy MR. Fetal lung development: airway pressure enhances the expression of developmental genes. J Pediatr Surg. 2000;35:113–8. doi: 10.1016/s0022-3468(00)80026-3. [DOI] [PubMed] [Google Scholar]
- 19.Abouzied MM, Baader SL, Dietz F, et al. Expression patterns and different subcellular localization of the growth factors HDGF (hepatoma-derived growth factor) and HRP-3 (HDGF-related protein-3) suggest functions in addition to their mitogenic activity. J Biochem. 2004;378:169–76. doi: 10.1042/BJ20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepourcelet M, Tou L, Cai L, et al. Insights into developmental mechanisms and cancers in the mammalian intestine derived from serial analysis of gene expression and study of the hepatoma-derived growth factor (HDGF) Development. 2005;132:415–27. doi: 10.1242/dev.01579. [DOI] [PubMed] [Google Scholar]
- 21.Everett AD. Identification, cloning, and developmental expression of hepatoma-derived growth factor in the developing rat heart. Dev Dyn. 2001;222:450–8. doi: 10.1002/dvdy.1204. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Ren H, Zhang L, et al. Small interfering RNA targeting hepatoma-derived growth factor (HDGF) induces apoptosis in lung cancer cells. AACR Meeting Abstr. 2004:835. [Google Scholar]
- 23.Machuy N, Thiede B, Rajalingam K, et al. A global approach combining proteome analysis and phenotypic screening with RNA interference yields novel apoptosis regulators. Mol Cell Proteomics. 2005;4:44–55. doi: 10.1074/mcp.M400089-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Tsang TY, Tang WY, Tsang WP, et al. Downregulation of hepatoma-derived growth factor activates the Bad-mediated apoptotic pathway in human cancer cells. Apoptosis. 2008;13:1135–47. doi: 10.1007/s10495-008-0241-6. [DOI] [PubMed] [Google Scholar]
- 25.Narron JV, Stoops TD, Barringhaus K, et al. Hepatoma-derived growth factor is expressed after vascular injury in the rat and stimulates smooth muscle cell migration. Pediatr Res. 2006;59:778–83. doi: 10.1203/01.pdr.0000219299.24435.4f. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Zheng JJ, Peiper SC, et al. Gene expression profile of ARPE-19 during repair of the monolayer. Graefes Arch Clin Exp Ophthalmol. 2001;239:946–51. doi: 10.1007/s004170100371. [DOI] [PubMed] [Google Scholar]
- 27.Urioste SS, Arndt KA, Dover JS. Keloids and hypertrophic scars: review and treatment strategies. Semin Cutan Med Surg. 1999;18:159–71. doi: 10.1016/s1085-5629(99)80040-6. [DOI] [PubMed] [Google Scholar]
- 28.Niessen FB, Spauwen PH, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435–58. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 29.Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998;4:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 30.Nemeth AJ. Keloids and hypertrophic scars. J Dermatol Surg Oncol. 1993;19:738–46. doi: 10.1111/j.1524-4725.1993.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 31.Louw L. The keloid phenomenon: progress toward a solution. Clin Anat. 2007;20:3–14. doi: 10.1002/ca.20374. [DOI] [PubMed] [Google Scholar]
- 32.Phan TT, Lim IJ, Bay BH, et al. Role of IGF system of mitogens in the induction of fibroblast proliferation by keloid-derived keratinocytes in-vitro. Am J Physiol Cell Physiol. 2003;284:C860–9. doi: 10.1152/ajpcell.00350.2002. [DOI] [PubMed] [Google Scholar]
- 33.Xia W, Phan TT, Lim IJ, et al. Complex epithelial-mesenchymal interactions modulate transforming growth factor-beta expression in keloid-derived cells. Wound Repair Regen. 2004;12:546–56. doi: 10.1111/j.1067-1927.2004.012507.x. [DOI] [PubMed] [Google Scholar]
- 34.Khoo YT, Ong CT, Mukhopadhyay A, et al. Upregulation of secretory connective tissue growth factor (CTGF) in keratinocyte-fibroblast coculture contributes to keloid pathogenesis. J Cell Physiol. 2006;208:336–43. doi: 10.1002/jcp.20668. [DOI] [PubMed] [Google Scholar]
- 35.Ong CT, Khoo YT, Tan EK, et al. Epithelial-mesenchymal interactions in keloid pathogenesis modulate vascular endothelial growth factor expression and secretion. J Pathol. 2007;211:95–108. doi: 10.1002/path.2081. [DOI] [PubMed] [Google Scholar]
- 36.Mukhopadhyay A, Chan SY, Lim IJ, et al. The role of activin system in keloid pathogenesis. Am J Physiol Cell Physiol. 2006;292:C1331–8. doi: 10.1152/ajpcell.00373.2006. [DOI] [PubMed] [Google Scholar]
- 37.McCauley RL, Chopra V, Li YY, et al. Altered cytokine production in black patients with keloids. J Clin Immunol. 1992;12:300–8. doi: 10.1007/BF00918154. [DOI] [PubMed] [Google Scholar]
- 38.Xue H, McCauley RL, Zhang W. Elevated interleukin-6 expression in keloid fibroblasts. J Surg Res. 2000;89:74–7. doi: 10.1006/jsre.1999.5805. [DOI] [PubMed] [Google Scholar]
- 39.Lim IJ, Phan TT, Song C, et al. Investigation of the influence of keloids-derived keratinocytes on fibroblast growth and proliferation in-vitro. Plast Reconstr Surg. 2001;107:797–808. doi: 10.1097/00006534-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Kessler D, Dethlefsen S, Haase I, et al. Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem. 2001;276:36575–85. doi: 10.1074/jbc.M101602200. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Fong KD, Phan TT, et al. Increased transcriptional response to mechanical strain in keloid fibroblasts due to increased focal adhesion complex formation. J Cell Physiol. 2006;206:510–7. doi: 10.1002/jcp.20486. [DOI] [PubMed] [Google Scholar]
- 42.Aarabi S, Bhatt KA, Shi Y, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250–61. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 43.Tuan TL, Keller LC, Sun D, et al. Dermal fibroblasts activate keratinocyte outgrowth on collagen gels. J Cell Sci. 1994;107:2285–9. doi: 10.1242/jcs.107.8.2285. [DOI] [PubMed] [Google Scholar]
- 44.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 45.Iyer VR, Eisen MB, Ross DT, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–7. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 46.Gallitzendoerfer R, Abouzied MM, Hartmann D, et al. Hepatoma-derived growth factor (HDGF) is dispensable for normal mouse development. Dev Dyn. 2008;237:1875–85. doi: 10.1002/dvdy.21589. [DOI] [PubMed] [Google Scholar]
- 47.Everett AD, Narron JV, Stoops T, et al. Hepatoma-derived growth factor is a pulmonary endothelial cell-expressed angiogenic factor. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1194–201. doi: 10.1152/ajplung.00427.2003. [DOI] [PubMed] [Google Scholar]
- 48.Mao J, Xu Z, Fang Y, et al. Hepatoma-derived growth factor involved in the carcinogenesis of gastric epithelial cells through promotion of cell proliferation by Erk1/2 activation. Cancer Sci. 2008;99:2120–7. doi: 10.1111/j.1349-7006.2008.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okuda Y, Nakamura H, Yoshida K, et al. Hepatoma-derived growth factor induces tumorigenesis in-vivo through both direct angiogenic activity and induction of vascular endothelial growth factor. Cancer Sci. 2003;94:1034–41. doi: 10.1111/j.1349-7006.2003.tb01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


