Abstract
Technology platforms originally developed for tissue engineering applications produce valuable models that mimic three-dimensional (3D) tissue organization and function to enhance the understanding of cell/tissue function under normal and pathological situations. These models show that when replicating physiological and pathological conditions as closely as possible investigators are allowed to probe the basic mechanisms of morphogenesis, differentiation and cancer. Significant efforts investigating angiogenetic processes and factors in tumorigenesis are currently undertaken to establish ways of targeting angiogenesis in tumours. Anti-angiogenic agents have been accepted for clinical application as attractive targeted therapeutics for the treatment of cancer. Combining the areas of tumour angiogenesis, combination therapies and drug delivery systems is therefore closely related to the understanding of the basic principles that are applied in tissue engineering models. Studies with 3D model systems have repeatedly identified complex interacting roles of matrix stiffness and composition, integrins, growth factor receptors and signalling in development and cancer. These insights suggest that plasticity, regulation and suppression of these processes can provide strategies and therapeutic targets for future cancer therapies. The historical perspective of the fields of tissue engineering and controlled release of therapeutics, including inhibitors of angiogenesis in tumours is becoming clearly evident as a major future advance in merging these fields. New delivery systems are expected to greatly enhance the ability to deliver drugs locally and in therapeutic concentrations to relevant sites in living organisms. Investigating the phenomena of angiogenesis and anti-angiogenesis in 3D in vivo models such as the Arterio-Venous (AV) loop mode in a separated and isolated chamber within a living organism adds another significant horizon to this perspective and opens new modalities for translational research in this field.
Keywords: tissue engineering, cancer research, angiogenesis, translational medicine
Introduction
History of tissue engineering
Physiological and structural aspects of 2D versus 3D culture in cancer research
State of the art of 3D culture systems in cancer research
New tissue engineering-routed scaffolds for 3D culture
Endothelial progenitor cells and tumour vasculature
In vivo models
Arteriovenous loop isolation chamber for tumour angiogenesis research
Conclusion
Introduction
Tissue engineering (TE) was defined in the 1980s from a broad and general perspective as ‘the application of the principles and methods of engineering and life sciences towards the fundamental understanding of structure–function relationships in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain or improve functions’. More widespread awareness of the term appears to have followed with perhaps the single most cited and influential paper in the field, a review paper by Langer and Vacanti [1].
Fig. 1.
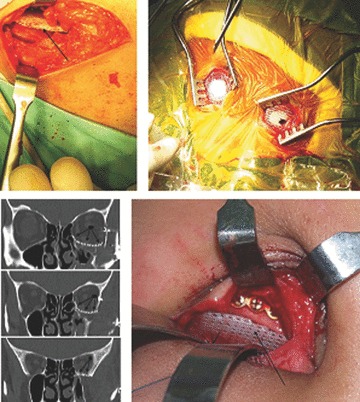
The so-called ‘first-generation scaffolds’ have been studied over the last 5 years in different clinical applications. FDA approved mPCL scaffolds (Osteopore International, Singapore) have been implanted to regenerate the iliac crest after autograft was taken for spinal fusion surgery. Burr hole plugs are used for cranioplasties and deformable but at the same time strong enough sheets for orbital floor reconstructions. Reprinted with permission from Wiley Interscience.
Three-dimensional (3D) culture has played a key role in the innovation of tissue engineering and sparked the design and development of scaffold- and matrix-based culture systems. These cell culture approaches, in contrast to conventional tissue culture plastic, provide more physiological geometries and microenvironments that more closely recapitulate the natural extracellular matrix (ECM) cells found in vivo. Accordingly, TE approaches are to become promising and influential in other biomedical research areas. For instance, TE constructs provide physiological models of human tissue that allow for studying disease pathogenesis as well as screening the effect and toxicity of drugs in vitro. As a result, the use of these tissue equivalents may significantly contribute to the development of new therapeutics.
The functional properties of cells can be explored and manipulated to an extent that is not possible in either 2D cultures or animal experiments. In particular, early events of tumour growth before effective vascularization appear to be closely reproduced. Indeed, within a short timeframe, 3D cultures of tumour cells develop hollow cores that resemble the necrotic areas of in vivo cancers; areas that are usually observed at a distance from nutrient and oxygen supplies. In the context of the development of a vascular supply, it has become apparent that 3D cultures are also better suited than 2D culture techniques to study phenomena relevant to angiogenesis itself. Although in vitro studies of angiogenesis offer limited possibilities, we and others demonstrated how properties of a 3D fibrin matrix were conductive towards growth of suspended endothelial progenitor cells in a fashion that lumen-containing blood vessel-resembling structures developed, a feature certainly not achievable in 2D culture [2, 3]. As far as tumour physiology is concerned, the proliferation of tumour cells cultured in 3D is typically slower and hence more physiological than that of monolayer cultures. Another important advantage of 3D cultures is that the interaction of different cell-types can be explored. For instance, infiltration of tumour spheroids by endothelial cells has been demonstrated and it depends not only on the production of pro-angiogenic factors by tumour cells but also on the expression of cadherins by endothelial cells [4].
Several reviews and research articles [5, 6] have accurately summarized and demonstrated that conditions and characteristics of the 3D microenvironment significantly influence and control tumorgenesis. Thus, synthetic and at the same time biomimetic matrices rooted in TE technology platforms may be utilized as 3D cell culture systems to improve in vitro and in vivo tumour modelling [7–9]. On the other hand, investigating mechanisms supporting tumour growth, e.g. in tumour angiogenesis, may be applicable to be supportive in tissue engineering applications, e.g. when it comes to the formation of a vascular network, exploiting the role of endothelial lineage cells as well as pro-angiogenic growth factors [2, 10, 11]. The development of anti-angiogenic therapies and novel drug delivery systems including growth factor or cell therapy based systems is therefore closely related in the study of angio-genetic phenomena. Therefore, an in vivo model allowing the study of developing blood vessels under isolated, well characterized and manipulatable conditions, almost like under in vitro conditions but with the benefit of integration in a living organism, would be extremely suited to study blood vessel development from a tissue engineering as well as a tumour angiogenesis background.
In this context, the arteriovenous loop model in an isolation chamber allows 3D vessel ingrowth into matrices of different origin and appears to be a very suitable solution to the above mentioned questions. This offers many-fold opportunities to not only study the process of angiogenesis but also modulate this process with either pro-angiogenic agents such as growth factors or endothelial progenitor cells or anti-angiogenic agents. By appropriate alterations of the conditions and contents of the AV loop, one may also be able to create a standardized, isolated environment for tumour growth were angiogenic phenomena could be studied at the same time.
In this article, we will review the current literature as well as present examples based on our work on prostate cancer; in particular, how TE technology platforms, originally developed for tissue regenerative applications, may be employed in cancer research. Specifically, we will describe how synthetic bioinspired hydrogel systems may be useful as 3D cell culture models to study specific biological questions related to prostate cancer cells. In a second example, we will show how bone tissue engineering platforms [12] can be applied to study the underlying causes of prostate cancer and its progression to bone metastases. From a translational research point of view, a novel 3D in vitro and an in vivo system based on tissue engineered human bone is proposed to further understanding of prostate cancer mechanisms, utilizing the role of prostate-specific antigen as a biomarker of proteolytic bone interactions in the bone metastasis process.
Fig. 3.
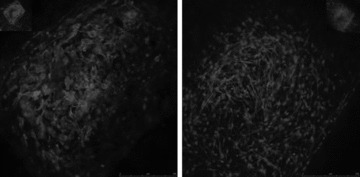
Left panel: OB scaffold + PC3-N (>40 days of culture). Right panel: OB scaffold + LNCap (>40 days of culture).
Finally, the AV loop isolation chamber angiogenesis will be proposed as an interface model that was originally developed as a platform technology to fabricate pre-vascularized grafts following a classical tissue engineering strategy. However, we now plan to use this highly reproducible model as an environment that could be altered towards a standardized isolated vascularized tumour bed.
History of tissue engineering
Today, the term regenerative medicine is often used synonymously with tissue engineering and recently the Society of Tissue Engineering was renamed ‘Tissue Engineering and Regenerative Medicine Society International’ (http://www.termis.org).
In 2003, the National Science Foundation (NSF) published a comprehensive report entitled ‘The Emergence of Tissue Engineering as a Research Field’, which gives a thorough description of the history of this field. Widespread awareness of the term TE appears to have occurred for the first time with perhaps the single most cited and influential paper in the field, a review paper by Langer and Vacanti in 1993 [1]. Today's scaffold- and matrix-based TE concepts involve the combination of a scaffold with cells and/or biomolecules and promotion of the repair and/or regeneration of tissues (Fig. 2).
Fig. 2.
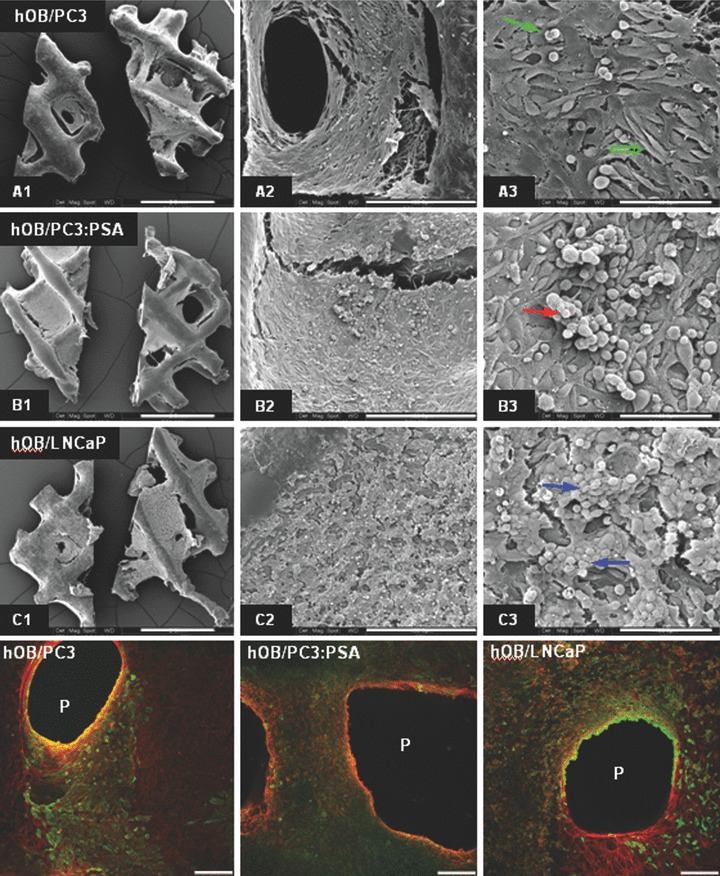
Images of the human osteoblast construct (hOB) 2 days post seeding with prostate cancer cell lines. (A) SEM images at different maginification (40×, 300× and 1000×, from top to bottom) showing morphology and distribution of the cancer cells on hOB constructs. (B) CLSM images of the co-cultures stained with anti-pan Cytokeratin (cancer cells, green labelling) and Phalloidin (osteoblasts, red labelling).
Two decades later, one can conclude that the major outcome of TE were awareness of the key role of three-dimensionality, and the consequent development of biomaterials-based strategies that facilitated cell culture in this new dimension. This aspect has dramatically advanced the field of TE allowing development of constructs in vitro that histologically and functionally mimicked native tissue (e.g. skin, bone). Specifically, advances in TE have led to the design of scaffold- and matrix-based culture systems that better represent the geometry, chemistry and signalling environment of the natural ECM. Although less heralded today than the direct clinical applications, TE may offer a potentially powerful tool box in other biomedical research areas. For instance, it provides physiologically relevant in vitro models of human tissue that can be employed to explore disease pathogenesis in cancer and/or to screen drug effects for the development of molecular therapeutics [13].
Hence, the objective of this article is to present how technology platforms and specifically 3D culture systems based on tissue engineered constructs (TECs) can be applied to cancer research based on the current literature and our own work in the area of prostate and ovarian cancer. We review the literature as well as present our approaches accompanied with some preliminary data sets demonstrating how tissue engineering technology platforms allow for enhanced in vitro and in vivo tumour modelling which will greatly enhance future cancer research. As an example, we will demonstrate how bone engineering platforms can be applied in studying bone metastasis related to prostate cancer in vitro and in vivo. In addition, it will be illustrated how a synthetic biomimetic hydrogel system is applied to study biological questions related to ovarian cancer.
Physiological and structural aspects of 2D versus 3D culture in cancer research
All cells are embedded in a 3D microenvironment in the body. However, for many decades nearly all tissue cells including most tumour cells have been studied in two-dimensional (2D) Petri dishes, 2D multi-well plates or 2D glass slides coated with various substrata [14]. However, cells in tissues reside within multicellular 3D environment consisting of a range of ECM macromolecules (including many types of collagens, proteoglycans, laminin and other matrix proteins) depending on the type of tissue. Specifically, the native ECM is a heterogeneous collection of covalent and non-covalent molecular interactions comprised primarily of proteins and glycosaminoglycans (GACs). Covalent bonds connect chondroitin sulphate, heparan sulphate, and other sulphated GAGs to core proteins to give proteooglycans (PGs). Non-covalent interactions include electrostatic associations with ions, hydration of the polysaccharide chains, binding of link modules of PGs to hyaluronan and triple helix formation to generate collagen fibrils. They allow both attachment between cells and the basal membrane and access to oxygen, hormones and nutrients as well as removal of waste products and other cell types associated in tissues [3, 14, 15].
Hence, there are several key drawbacks to 2D cell cultures. First, the movements of cells in the 3D environment of a whole organism typically follow a chemical signal or molecular gradient. Molecular gradients play a vital role in biological differentiation, determination of cell fate, organ development, signal transduction, neural information transmission and countless other biological processes. However, it is nearly impossible to establish a physiological 3D gradient in 2D culture [16]. Furthermore, cell motility can be greatly influenced by chemical and physical properties of a 3D matrix, a feature that cannot be satisfyingly mimicked in 2D cultures.
Secondly, cells isolated directly from higher organisms frequently alter their metabolism and gene expression patterns in 2D culture. It is clear that cellular structure plays a major role in determining cellular activity through spatial and temporal ECM protein and cell receptor interactions that naturally exist in tissues and organs. The cellular membrane structure, ECM and basement membrane significantly influence cellular metabolism via the protein–protein interactions. The adaptation of cells to a 2D Petri dish requires significant adjustment of the surviving cell population not only to changes in oxygen, nutrients and ECM interactions, but also to alter waste disposal [14, 16].
The third reason entails cells growing in a 2D environment significantly altering production of their own ECM proteins, often undergoing morphological and phenotypic changes. It is not unlikely that the receptors on the cell surface could preferentially cluster on parts of the cell that directly expose to culture media rich in nutrients, growth factors and other extracellular ligands, whereas the receptors on the cells attached to the tissue culture plate surface may have less opportunity for clustering. Thus, the receptors might not be presented in correct orientation and clustering and this may presumably also affect the autocrine and/or paracrine signals between cells [3, 14].
State of the art of 3D culture systems in cancer research
Awareness of in vitro 3D cell cultures to more closely mimic tumour cell growth and responses in vivo (e.g. to anticancer treatments), compared to cell monolayer, is dated back to the 1970s when Sutherland et al. generated multicellular spheroids to explore cancer cell behaviour and their resistance to antitumour treatments [17]. Similarly, less than a decade later, Miller et al. found that tumour cells grown as spheroids within collagen gels, exhibit greater anticancer drug resistance, compared to cancer cells grown on tissue culture plastic [18]. From an anatomical and physiological point of view, cancer cells cultured in 3D via spheroid or hydrogel cultures mimic in vivo tumours to a significantly higher extent compared to monolayer cultures. In particular, early events of tumour growth before effective vascularization appear to be closely reproduced in those 3D culture systems. Usually, 3D cultures of tumour cells develop hollow cores that resemble the necrotic areas of in vivo cancers: areas that are usually observed at a distance from nutrient and oxygen supplies. In addition, the proliferation of tumour cells cultured in three dimensions is typically slower and hence more physiological than that of monolayer cultures [14].
Despites early landmark research outcome outlining the key role of three-dimensionality in cell culture for in vivo like responses, it is quite surprising that over 85% of the cancer research groups (internal data Medline search) still routinely use monolayer cultures in their research projects, and therefore fail to realize that they apply only a suboptimal culture system to answer their raised biological questions.
More recently, however, there have been a growing number of research groups that have become increasingly aware of the limitations of conventional 2D monolayer cultures and have adopted 3D cell culture systems. Currently, multicellular spheroids are still one of the most commonly employed 3D cell culture models to study the cancer cell in vitro and assess antitumour drugs [19–21]. Despite their pivotal role in exploring different aspects of cancer cell biology (e.g. multicellular resistance to anticancer drugs [22], multicellular spheroids have some limitations, mostly because these 3D cell aggregates lack the important interaction with the extracellular microenvironment [23, 24]. In this regard, matrix-embedded cancer cells, probably the other most frequently employed 3D cell culture approach, may more intimately mimic conditions and extracellular microenvironments cells reside in vivo. Naturally derived reconstituted ECM protein-based hydrogels, Matrigel™ (a laminin-rich matrix purified from animal tumours [25, 26] and collagen gels [18, 27]) represent to date the gold standard matrices in 3D cancer cell research. During the last two decades, the pioneering work by Bissell, Brugge and coworkers has definitely contributed significantly to pave the way towards a paradigm shift that cancer cells cultured in 3D within a matrix, compared to monolayer, may more accurately express in vivo like conditions [28–32]. Using normal and cancer epithelial breast cells cultured within the gold standard naturally derived matrices, they have unequivocally demonstrated the importance of 3D cell-ECM interactions in influencing cell behaviour [28, 31, 32]. For instance, cell culture in 3D is a prerequisite in order to phenotypically discern normal and malignant cells [33]. Interestingly, they also discovered that abnormally growing and proliferating human breast cancer cells (i.e. that formed irregular cell colonies) could be reverted to a normal phenotype (i.e. changed multicellular arrangement to polarized acini) by altering their interaction with the extracellular environment in 3D through blocking of overex-pressed β-integrin receptors [6]. Additionally and most importantly, these outcome could also be confirmed in in vivo animal models [6, 34, 35].
Essentially, these landmark studies have significantly contributed to further outline the importance of the contextual conditions cells are exposed to, to understand their behaviour, and in particular, how 3D cell culture models, in contrast to cell culture plastic, offer a more comprehensive and in vivo like option to study cells in vitro. Accordingly, as Bissell stated in one of her review articles, in addition to the cell genotypic characteristics, the other ‘half of the secret of the cell lies outside the cell’[28]. Besides three-dimensionality and the key role of cell-matrix interactions, the interplay between tumour and tissue-specific cells represents another environmental condition that may significantly influence tumour formation and growth.
The use of naturally derived matrices has considerably advanced the understanding of fundamental interplay between cells and their extracellular microenvironment. However, there is wide consensus that these matrices display some limitations and drawbacks [30]. In particular, their composition varies from batch to batch which may affect experimental reproducibility [36, 37], and their characteristics (e.g. biological, biochemical and biophysical) are not easily accessible to modification because of the intrinsic features of their precursors. In order to overcome these limitations, the cancer biology community is increasingly seeking alternative matrices to naturally derived gels to better mimic the tumour environment [30]. In the next section we describe some pivotal examples of 3D cell culture matrices in cancer research that were adopted from material technology platforms originally developed for tissue regeneration applications. In addition, we also expand on these works and describe a new approach to study development and metastasis formation in bone originating from prostate cancer.
New tissue engineering-routed scaffolds for 3D culture
As outlined in the previous section microenvironmental conditions play an important role in tumorigenesis. Accordingly, controlling the extracellular milieu in which cancer cells are cultured may significantly contribute to elucidating mechanisms of cancer formation and growth, as well as sensitivity to antitumour drugs. In this context, currently used naturally derived gold standard matrices for 3D cancer cell cultures show some limitations mainly concerning their reproducibility and the flexibility of the design and modification of their characteristics.
Emerging biomaterials-based approaches in regenerative medicine and tissue engineering have pioneered the production of scaffolding matrices with malleable characteristics, thereby enabling cell culture in more controllable 3D microenvironments. In this section, we focus on examples of materials originally conceived for in vivo tissue regeneration that have been recently applied for cancer research as 3D cell culture models. Biologically passive, porous and rigid scaffolds made from hydrolytically degradable poly (lactide-co-glycolide) acid polymers were used as 3D structures to culture human oral squamous carcinoma cells. Cancer cells cultured in these 3D structures gave rise to tumour-like masses with characteristics (e.g. growth, expression of tumour specific markers, etc.) that, in contrast to monolayer and – to some extent – matrigel-cultured cells, expressed a very similar behaviour compared to animal models [38]. Hydrogels are also being increasingly used as 3D cell culture models as, compared to rigid polymeric scaffolding materials, they may more closely mimic the actual physiological environment in which cells reside in vivo[39]. Recent advances in bioengineering and biomaterials science have enabled functionalization of (semi)-synthetic hydrogels to include features found in the natural ECM [7–9, 40] and allow systematic studying their involvement in cancer development.
For instance RGD-functionalized alginate gels were used as 3D cell culture models to specifically explore the implication of the engagement of tumour cell integrins in angiogenic signalling in vitro and in vivo[41]. Commercially available Extragel, consisting of chemically modified hyaluronan and gelatin cross-linked with polyethylene glycol (PEG), has potential as a tunable 3D cell culture matrix in cancer cell research [42, 43]. These matrices have been already utilized as delivery vehicle for tumour cells for the creation of orthotopic human tumour xenografts in animal models [44–47]. Zhang, Stupp and coworkers independently reported the discovery of a self-assembling peptide system that can undergo spontaneous physical cross-linking into nanofibre scaffold hydrogels by alteration of salt concentration at physiological pH. Structurally, these peptide-based synthetic hydrogels resemble the natural ECM, and, if desired, can also incorporate bioactive peptides to incentivize cellular responses [48, 49]. These matrices have been applied in a range of in vitro and in vivo studies [50, 51], and the commercially available Puramatrix, originally developed in Zhang laboratories (Boston, MA, USA), have been also used as 3D cell culture matrices in cancer research [52, 53].
Another synthetic hydrogel system, arguably one of the most versatile in term of modularly design biological, biochemical and mechanical properties, has been pioneered by Hubbell, Lutolf and coworkers [9, 54, 55]. These biomimetic PEG-based hydrogels have shown in vivo performance comparable to naturally derived matrices [56] and high design flexibility of their characteristics enabling to systematically study mechanisms governing cell migration in 3D [54, 57]. We have adopted these hydrogel systems in our group to explore the behaviour of prostate cancer cells cultured in 3D. In particular, we are employing biomimetic hydrogels that are formed from peptide functionalized multi-arm PEG via the FXIII-catalysed cross-linking mechanism [55, 58]. By means of the same reaction during material formation bioactive molecules (e.g. RGD [58] and growth factors [55] can be stably incorporated in the hydrogels. In addition, sensitivity of these matrices to proteolytic degradation can be precisely controlled through design of specific matrix metallo-proteinase substrates within the hydrogel network [58].
Although the molecular composition of the ECM is a well-known regulator of cellular responses, physical properties of the matrix in 3D models can also play surprisingly important roles. In particular, recent evidence points to direct roles for the stiffness (compliance) of the ECM in regulating multiple cellular functions. Also described as rigidity, elasticity or pliability, this property is sensed by cells through bidirectional interaction with the surrounding ECM. Cell surface integrin receptors and the contractile cytoskeleton pull against the ECM to sense the stiffness of the microenvironment. Biologically, cells need to sense and respond appropriately to their local microenvironment. The stiffness of microenvironments is variable; examples include loose versus dense connective tissue, soft (skin, lung, etc.) versus hard tissues (such as bones) and early versus late stages of wound healing. Hence, the capability to control the mechanical properties of our PEG-based hydrogel system allow us to investigate whether different cancer populations of tumour cells in 3D structures might favour a soft or harder environment.
Endothelial progenitor cells and tumour vasculature
Angiogenesis and vascularization of tissues have been the focus of research wherever blood vessel formation was either desirable – such as in ischemic or bioartificial tissues – or unwanted – such as in tumours. Endothelial progenitor cells (EPCs) are precursor cells capable of differentiation into mature endothelial cells and have been shown to play an important role in angiogenesis as well as vasculogenesis in a multitude of disease states. Recent research has also highlighted their impact on angiogenetic phenomena in tissue engineering, particularly in 3D in vitro cultures. In this context, we (Fig. 4) and others demonstrated how properties of a 3D matrix were conducive towards growth of suspended endothelial progenitor cells in a fashion that lumen-containing blood vessel-resembling structures developed, a feature certainly not achievable in 2D culture [2, 3].
Fig. 4.
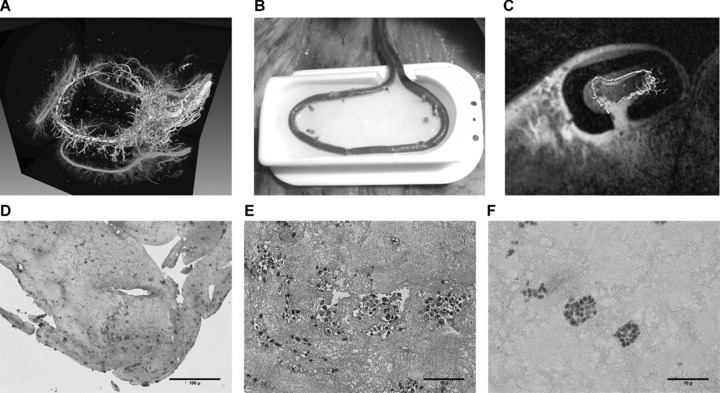
(A) Isosurface rendering of micro-CT scan from a rat AV-loop following Microfil®– perfusion and explanation, demonstrating dense vascular sprouting originating from the AV-loop. (B) First application of the AV-loop sheep model using fibrin as a matrix: intra-operative aspect of micro-anastomosed AV-loop in the sheep's groin placed into a custom made isolation chamber, which is then filled with biocompatible fibrin matrix. (C) Intra-vital imaging of AV-loop in the sheep model: by super-imposing serial MRI – scans and segmented angio-CT – scans the increase of vascular sprouting from the sheep AV-loop in concordance with increased perfusion within the chamber can be visualized intravitally. (D) Murine embryonal EPCs were suspended in a 3D fibrin matrix and constructs were subjected to histological analysis after 8 days. Cell proliferation in numerous multicellular clusters, some of them forming lumen-like structures, can be appreciated. Magnification 25-fold. (E) A detailed view confirms presence of multicellular clusters of EPCs. Magnification 200-fold. (F) Morphologic observations are confirmed by the presence of Ki-67+ EPCs, identified by their pink staining, indicating cell proliferation within the fibrin matrix. Magnification 200-fold.
EPCs are chemotactically recruited to the site of ischemia, differentiate and contribute to new blood vessel growth which can either occur by angiogenesis, i.e. proliferation and sprouting of existing blood vessels, or vasculogenesis, i.e. de novoclonal formation of blood vessels from the aforementioned cells. EPCs, however, are not only crucial for neo-vascularization, but also exert a significant influence on existing blood vessels due to their highly pro-angiogenic features including angioinductive growth factors [59].
This role in blood vessel formation is not only applicable to disease states such as myocardial infarction or lower extremity ischemia, but also to angiogenetic phenomena in tumour growth. Therefore, research into endothelial progenitor cells has focused on their angiogenic properties, on the one hand to enhance blood vessel formation in disease states such as tissue ischemia, e.g. myocardial infarction and lower extremity or tissue engineering, and, on the other hand, on their prominent role in tumour blood vessel growth.
Angiogenesis and – as a therapeutic strategy – inhibition of angiogenesis (anti-angiogenesis) are intensively investigated in the context of tumour growth [60]. Due to their affinity to newly forming blood vessels, these cells could be employed as a means to serve as drug delivery systems and transport pro- or anti-angiogenic substances to the area of vascularization. Gene transfer to endothelial progenitor cells has become a valid method to change the angiogenic properties of endothelial progenitor cells in tissue engineering as well as tumour research [61, 62].
In vivo models
Microenvironmental conditions regulate tumour genesis and biomimetic in vivo model systems are necessary to study how cancer and metastatic spread is dependent on these conditions. Tumour aggressiveness is enhanced by altered 3D cell-cell and cell–ECM interactions in connection with the development of central hypoxia and signalling between cells residing within spatially distinct niches. These conditions are not fully reflected by several currently applied in vivo model systems [63]. These facts highlight the demand to develop a new generation of improved models amenable to detailed cellular and molecular biology studies.
However, tissue engineers also need to keep in mind that using a permissive matrix that promotes tissue remodelling might be preferred to over engineering the final form of a complex tissue. Similarly, exact mimicry of the complexity of the native ECM may be unnecessary and a pragmatic biomimetic approach may be sufficient. In other words, it may be adequate to provide a TEC as simple as possible and then utilize the patient's own body as a bioreactor. However, it is important to assemble the correct components inside a TEC, namely scaffold, exogenous cells and/or growth factors [64]. Nonetheless, the limiting step is angiogenesis, and both microvascularization and macrovascularization are required to provide nutrients and oxygen in 3D to the TEC. The sequential release of multiple growth factors is one way to achieve this outcome and this concept is studied by several TE groups around the world. One of the leading groups in the field reported the application of their originally developed technology platform for the in vivo engineering of human 3D tumours [13]. They used biodegradable scaffolds fabricated in combination with carcinoma cells recreated microenvironmental characteristics representative of tumours in vivo. Remarkably, the angiogenic characteristics of tumour cells were dramatically altered upon 3D culture within this system, and corresponded much more closely to tumours formed in vivo. The group could also show that cells in this model were also less sensitive to chemotherapy and yielded tumours with enhanced malignant potential.
The Rosenblatt/Kaplan group was the first to report the application of a bone tissue engineering platform into an animal model to study the mechanism of bone metastases [65]. Silk scaffolds were coupled with bone morphogenetic protein-2 (BMP-2), seeded with bone marrow stromal cells (BMSC) and maintained in culture for 7 weeks, 4 weeks and 1 day before implantation in a mouse model of human breast cancer metastasis from the orthotopic site. Following injection of SUM1315 cells into mouse mammary fat pads, tumour burden of implanted tissues was observed only in 1-day scaffolds. Scaffold development and implantation was then reinitiated to identify the elements of the engineered bone that contribute to metastatic spread. Migration of SUM1315 cells was detected in four of four mice bearing scaffolds with BMP-2 treatment and with BMSC treatment, respectively, whereas only one of six mice of the BMP-2/BMSC combination showed evidence of metastatic spread. Histology confirmed active matrix modelling and stromal cell/fibroblast infiltration in scaffolds as positive for the presence of metastasis. These results show the first successful integration of engineered bone in a model system of human breast cancer metastasis.
CaP is the most common cancer and the second leading cause of male cancer deaths. Despite its common occurrence, the underlying cause of this cancer and its progression to bone metastases remains poorly characterized. An ideal in vivo model would reproduce the genetic and phenotypic changes that occur with human cancer cells seeding in human bone as close as possible. Recently developed mouse models indicate that CaP cells have a preference for human bone. While mouse tibia invasion models provide important data on bone-CaP cell interactions in vivo, they do not allow ‘homing’ of CaP cells and are not considered a direct metastatic model [66]. Human foetal long bone chips implanted subcutaneously into the flanks of SCID (severe combined immunodeficient) mice provide a more appropriate human-specific bone microenvironment with intact anatomic and hematopoietic features. However, this model also has major limitations; firstly, the implanted human bone chips often get vascularized poorly and hence the dead bone does not reflect the real clinical situation; secondly in case of poor bone quality it is very difficult to control size and shape of the bone core implants which makes it difficult to establish a reproducible model; thirdly is it more appropriate to use cancellous or cortical bone.
Hence, our interdisciplinary research programme is in the process of creating a novel ‘all human’ model in which tissue engineered human bone is transplanted into immuno-deficient non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice and compared to the standard bone chip model. By using this model, we will test the hypothesis that distinct ‘tool kits’ are used by CaP metastasizing to human tissue engineered bone. In addition, we are identifying components within bone stroma that are essential for metastasis and osteotropism genes expressed by bone in response to the presence of CaP.
Arteriovenous loop isolation chamber for tumour angiogenesis research
Among various techniques that have been investigated to overcome the problem of early angiogenesis in TE products the microsurgical implantation of small calibre vessels in different models is one of the possible means to overcome current limitations of applied tissue engineering. Numerous experiments with this type of approach have gained insights into basic principles of angiogenesis and consequently methods of anti-angiogenesis [10, 11, 67–69]. As a result, the rat arteriovenous loop model provides a unique standardized, isolated, well characterized and manipulatable environment that is vascularized over time by a defined main axis of blood vessels. We and others have [10, 11, 67–71] demonstrated the potential of this system to provide blood vessel ingrowth into a clinically approved fibrin matrix as well as hard matrices of different composition. We also demonstrated that this isolated defined environment is accessible to manipulation and responds to pro-angiogenic stimuli such as recombinant growth factors. These features make the presented model a very attractive tool not only for tissue engineering purposes, but also to dissect mechanisms of tumour angiogenesis by establishing a tumour within the chamber followed by analysis of the newly forming vascular network associated with it. In this context, anti-angiogenetic treatments and their impact on blood vessel formation from the AV loop and/or tumour growth may also be investigated in the future.
Taken together, this model, initially established as a tool to enhance the multitude of tissue engineering techniques in research and in therapeutic applications, may be transformed towards a platform to investigate mechanisms of tumour growth as far as they relate to tumour angiogenesis and thereby offer new insights towards therapies in an effort to improve treatment options for cancer patients.
Conclusion
Technology platforms originally developed for tissue engineering applications produce valuable models that mimic 3D tissue organization and function to enhance the understanding of cell/tissue function under normal and pathological situations. These models show that when replicating physiological and pathological conditions as closely as possible investigators are allowed to probe the basic mechanisms of morphogenesis, differentiation and cancer. Significant efforts investigating angiogenetic processes and factors in tumorigenesis are currently undertaken to establish ways of targeting angiogenesis in tumours. Anti-angiogenic agents have been accepted for clinical application as attractive targeted therapeutics for the treatment of cancer. Combining the areas of tumour angiogenesis, combination therapies, and drug delivery systems is therefore closely related to the understanding of the basic principles that are applied in tissue engineering models. Studies with 3D model systems have repeatedly identified complex interacting roles of matrix stiffness and composition, integrins, growth factor receptors and signalling in development and cancer. These insights suggest that plasticity, regulation and suppression of these processes can provide strategies and therapeutic targets for future cancer therapies. The historical perspective of the fields of tissue engineering and controlled release of therapeutics, including inhibitors of angiogenesis in tumours is becoming clearly evident as a major future advance in merging these fields. New delivery systems are expected to greatly enhance the ability to deliver drugs locally and in therapeutic concentrations to relevant sites in living organisms. Targeted therapies of cancer may become more efficient by these possible achievements.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Bleiziffer O, Horch RE, Hammon M, et al. T17b murine embryonal endothelial progenitor cells can be induced towards both proliferation and differentiation in a fibrin matrix. J Cell Mol Med. 2009;13:926–35. doi: 10.1111/j.1582-4934.2008.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, Rabkin-Aikawa E, Guleserian KJ, et al. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287:H480–7. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 4.Feder-Mengus C, Ghosh S, Reschner A, Martin I, et al. New dimensions in tumor immunology: what does 3D culture reveal? Trends Mol Med. 2008;14:333–40. doi: 10.1016/j.molmed.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 8.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–92. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 9.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering, Nature. Biotechnology. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 10.Arkudas A, Tjiawi J, Saumweber A, et al. Evaluation of blood vessel ingrowth in fibrin gel subject to type and concentration of growth factors. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00410.x. DOI: 10:1111/j.1582-4934.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arkudas A, Pryymachuk G, Hoereth T, et al. Dose-finding study of fibrin gelimmobilized vascular endothelial growth factor 165 and basic fibroblast growth factor in the arteriovenous loop rat model. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2008.0477. DOI: 10.1089/ten.tea.2008.0477. [DOI] [PubMed] [Google Scholar]
- 12.Hutmacher DW, Cool S. Concepts of scaffold-based tissue engineering–the rationale to use solid free-form fabrication techniques. J Cell Mol Med. 2007;11:654–69. doi: 10.1111/j.1582-4934.2007.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischbach C, Chen R, Matsumoto T, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–60. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 14.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. 2005;15:365–77. doi: 10.1016/j.semcancer.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Wang ZZ. Mechanisms that mediate stem cell self-renewal and differentiation. J Cell Biochem. 2008;103:709–18. doi: 10.1002/jcb.21460. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland RM, Eddy H, Bareham B, et al. Resistance to adriamycin in multicellular spheroids. Int J Radiat Oncol Biol Phys. 1979;5:1225–30. doi: 10.1016/0360-3016(79)90643-6. [DOI] [PubMed] [Google Scholar]
- 18.Miller BE, Miller FR, Heppner GH. Factors affecting growth and drug sensitivity of mouse mammary tumor lines in collagen gel cultures. Cancer Res. 1985;45:4200–5. [PubMed] [Google Scholar]
- 19.Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;240:177–84. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 20.Kunz-Schughart LA, Freyer JP, Hofstaedter F, et al. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen. 2004;9:273–85. doi: 10.1177/1087057104265040. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich J, Ebner R, Kunz-Schughart LA. Experimental anti-tumor therapy in 3-D: spheroids–old hat or new challenge? Int J Radiat Biol. 2007;83:849–71. doi: 10.1080/09553000701727531. [DOI] [PubMed] [Google Scholar]
- 22.Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance. Crit Rev Oncol Hematol. 2000;36:193–207. doi: 10.1016/s1040-8428(00)00086-x. [DOI] [PubMed] [Google Scholar]
- 23.Helmlinger G, Netti PA, Lichtenfeld HC, et al. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778–83. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 24.Santini MT, Rainaldi G, Indovina PL. Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Crit Rev Oncol Hematol. 2000;36:75–87. doi: 10.1016/s1040-8428(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 25.Kleinman HK, McGarvey ML, Liotta LA, et al. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–93. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 26.Lee GY, Kenny PA, Lee EH, et al. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–65. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wozniak MA, Keely PJ. Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cells. Biol Proced Online. 2005;7:144–61. doi: 10.1251/bpo112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bissell MJ. Modelling molecular mechanisms of breast cancer and invasion: lessons from the normal gland. Biochem Soc Trans. 2007;35:18–22. doi: 10.1042/BST0350018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–88. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 30.Abbott A. Cell culture: biology's new dimension. Nature. 2003;424:870–2. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 31.Bissell MJ, Radisky D. Putting tumours in context. Nature Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bissell MJ, Weaver VM, Lelièvre SA, et al. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757–63. [PubMed] [Google Scholar]
- 33.Petersen OW, Ronnov-Jessen L, Howlett AR, et al. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–8. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Hansen RK, Radisky D, et al. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park CC, Zhang H, Pallavicini M, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–35. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–45. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 37.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18:311–21. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischbach C, Chen R, Matsumoto T, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–60. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 39.Cushing MC, Anseth KS. Materials science. Hydrogel cell cultures. Science. 2007;316:1133–4. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 40.Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:149–65. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischbach C, Kong HJ, Hsiong SX, et al. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci USA. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serban MA, Scott A, Prestwich GD. Use of hyaluronan-derived hydrogels for three-dimensional cell culture and tumor xenografts. Curr Protoc Cell Biol. 2008;10:10–14. doi: 10.1002/0471143030.cb1014s40. Unit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prestwich GD. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res. 2008;41:139–48. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Shu XZ, Prestwich GD. Tumor engineering: orthotopic cancer models in mice using cell-loaded, injectable, cross-linked hyaluronan-derived hydrogels. Tissue Eng. 2007;13:1091–101. doi: 10.1089/ten.2006.0297. [DOI] [PubMed] [Google Scholar]
- 45.Scaife CL, Shea JE, Dai Q, et al. Synthetic extracellular matrix enhances tumor growth and metastasis in an orthotopic mouse model of pancreatic adenocarcinoma. J Gastrointest Surg. 2008;12:1074–80. doi: 10.1007/s11605-007-0425-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S, Gelain F, Zhao X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin Cancer Biol. 2005;15:413–20. doi: 10.1016/j.semcancer.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Beniash E, Zubarev ER, et al. Assembling a lasing hybrid material with supramolecular polymers and nanocrystals. Nat Mater. 2003;2:689–94. doi: 10.1038/nmat983. [DOI] [PubMed] [Google Scholar]
- 48.Silva GA, Czeisler C, Niece KL, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–5. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 49.Gelain F, Bottai D, Vescovi A, et al. Designer self-assembling Peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE. 2006;1:1–12. doi: 10.1371/journal.pone.0000119. e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sargeant TD, Oppenheimer SM, Dunand DC, et al. Titanium foam-bioactive nanofiber hybrids for bone regeneration. J Tissue Eng Regen Med. 2008;2:455–62. doi: 10.1002/term.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang S. Designer self-assembling Peptide nanofiber scaffolds for study of 3-d cell biology and beyond. Adv Cancer Res. 2008;99:335–62. doi: 10.1016/S0065-230X(07)99005-3. [DOI] [PubMed] [Google Scholar]
- 52.Kim MS, Yeon JH, Park JK. A microfluidic platform for 3-dimensional cell culture and cell-based assays. Biomed Microdevices. 2007;9:25–34. doi: 10.1007/s10544-006-9016-4. [DOI] [PubMed] [Google Scholar]
- 53.Birgersdotter A, Baumforth KR, Porwit A, et al. Three-dimensional culturing of the Hodgkin lymphoma cell-line L1236 induces a HL tissue-like gene expression pattern. Leuk Lymphoma. 2007;48:2042–53. doi: 10.1080/10428190701573190. [DOI] [PubMed] [Google Scholar]
- 54.Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374–88. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehrbar M, Rizzi SC, Schoenmakers RG, et al. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials. 2007;28:3856–66. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 56.Lutolf MP, Weber FE, Schmoekel HG, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–8. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 57.Lutolf MP. Integration column: Artificial ECM: expanding the cell biology tool box in 3D. Integr Biol. 2009;1:235–41. doi: 10.1039/b902243k. [DOI] [PubMed] [Google Scholar]
- 58.Ehrbar M, Rizzi SC, Schoenmakers RG, et al. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules. 2007;8:3000–7. doi: 10.1021/bm070228f. [DOI] [PubMed] [Google Scholar]
- 59.Kupatt C, Horstkotte J, Vlastos GA, et al. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–8. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- 60.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murasawa S, Asahara T. Gene modified cell transplantation for vascular regeneration. Curr Gene Ther. 2007;7:1–6. [PubMed] [Google Scholar]
- 62.Wei J, Blum S, Unger M, et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metas-tases after intravenous delivery. Cancer Cell. 2004;5:477–88. doi: 10.1016/s1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 63.Rosol TJ, Tannehill-Gregg SH, LeRoy BE, et al. Animal models of bone metastasis. Cancer. 2003;97:748–57. doi: 10.1002/cncr.11150. [DOI] [PubMed] [Google Scholar]
- 64.Meijer GJ, de Bruijn JD, Koole R, et al. Cell-based bone tissue engineering. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040009. e119. 0260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreau JE, Anderson K, Mauney JR, et al. Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res. 2007;67:10304–8. doi: 10.1158/0008-5472.CAN-07-2483. [DOI] [PubMed] [Google Scholar]
- 66.Miller FR. Xenograft models of premalignant breast disease. J Mammary Gland Biol Neoplasia. 2000;5:379–391. doi: 10.1023/a:1009577811584. [DOI] [PubMed] [Google Scholar]
- 67.Kneser U, Polykandriotis E, Ohnolz J, et al. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006;12:1721–31. doi: 10.1089/ten.2006.12.1721. [DOI] [PubMed] [Google Scholar]
- 68.Polykandriotis E, Arkudas A, Horch RE, et al. Autonomously vascularized cellular constructs in tissue engineering: opening a new perspective for biomedical science. J Cell Mol Med. 2008;11:6–20. doi: 10.1111/j.1582-4934.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polykandriotis E, Tjiawi J, Euler S, et al. The venous graft as an effector of early angiogenesis in a fibrin matrix. Microvasc Res. 2008;75:25–33. doi: 10.1016/j.mvr.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 70.Fiegel HC, Pryymachuk G, Rath S, et al. Fetal hepatocyte transplantation in a vascularized AV-loop transplantation model in the rat. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00369.x. May 24. [Epub ahead of print]. DOI: 10:1111/j.1582-4934.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horch RE, Popescu LM, Vacanti C, et al. Ethical issues in cellular and molecular medicine and tissue engineering. J Cell Mol Med. 2008;12:1785–93. doi: 10.1111/j.1582-4934.2008.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


