Abstract
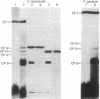
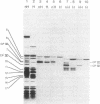
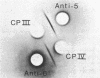
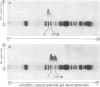
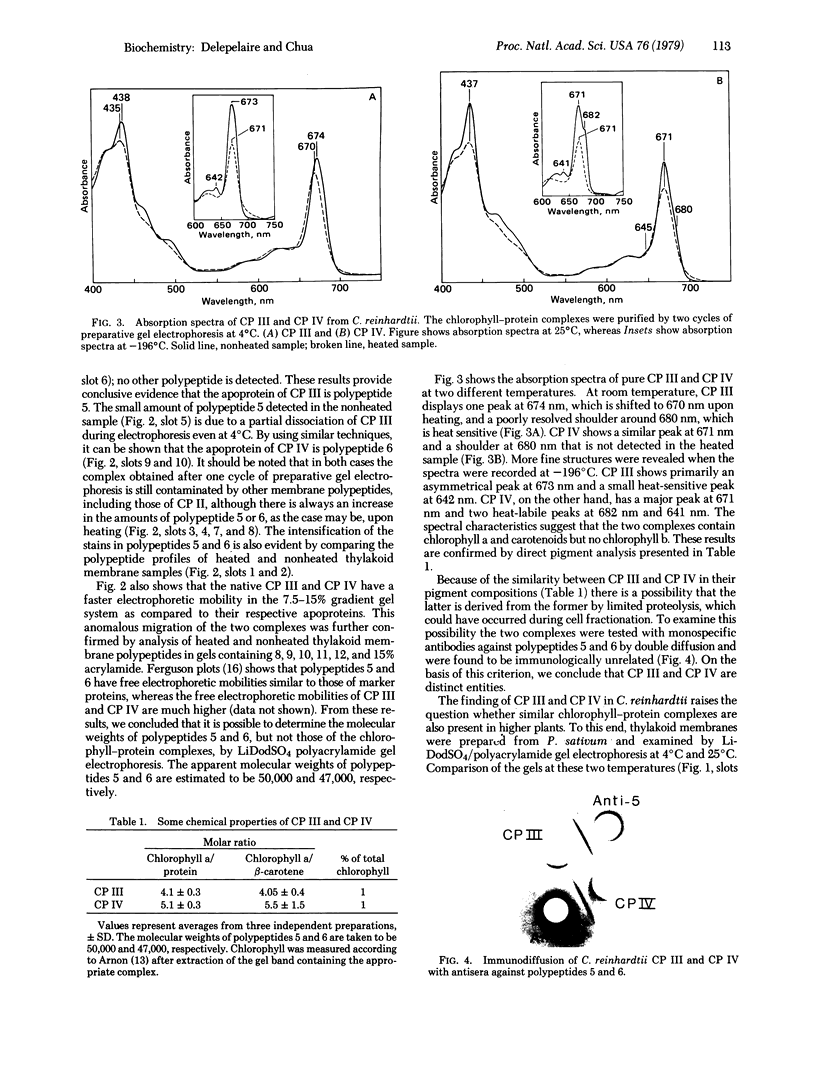
Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of Chlamydomonas reinhardtii thylakoid membranes at room temperature gave two chlorophyll-protein complexes, CP I and CP II, as had been reported previously. However, when the electrophoresis was performed at 4°C, there was an increase in the amount of chlorophyll associated with CP I and CP II, and in addition, three other chlorophyll-protein complexes appeared. Two of these complexes, designated CP III and CP IV, were characterized and found to be similar in their compositions. Each complex contains four to five molecules of chlorophyll a, one molecule of β-carotene, and one polypeptide chain. The apoprotein of CP III is polypeptide 5 (Mr 50,000) and that of CP IV is polypeptide 6 (Mr 47,000); the two polypeptides are structurally unrelated. Chlorophyll-protein complexes similar to C. reinhardtii CP III and CP IV were also detected in higher plants (e.g., Pisum sativum). The apoproteins of the higher plant complexes are immunochemically related to those of the C. reinhardtii complexes, as shown by crossed immunoelectrophoresis. Absorption spectra of CP III and CP IV at -196°C revealed a component at 682 nm. This observation, together with the previous results on photosystem II mutants [Chua, N.-H. & Bennoun, P. (1975) Proc. Natl. Acad. Sci. USA 72, 2175-2179], provides indirect evidence that CP III and CP IV may be involved in the primary photochemistry of photosystem II.
Keywords: Chlamydomonas reinhardtii, Pisum sativum, photosystem II reaction centers, absorption spectra, immunochemical techniques
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker R., Helenius A., Simons K. Solubilization of the Semliki Forest virus membrane with sodium dodecyl sulfate. Biochemistry. 1975 May 6;14(9):1835–1841. doi: 10.1021/bi00680a005. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Matlin K., Bennoun P. A chlorophyll-protein complex lacking in photosystem I mutants of Chlamydomonas reinhardtii. J Cell Biol. 1975 Nov;67(2PT1):361–377. doi: 10.1083/jcb.67.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse C. A., Papermaster D. S. Membrane protein analysis by two-dimensional immunoelectrophoresis. Science. 1975 Aug 8;189(4201):469–472. doi: 10.1126/science.1154021. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Characterization of three new chlorophyll-protein complexes. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1113–1118. doi: 10.1016/0006-291x(78)91251-2. [DOI] [PubMed] [Google Scholar]
- Kan K. S., Thornber J. P. The Light-harvesting Chlorophyll a/b-Protein Complex of Chlamydomonas reinhardii. Plant Physiol. 1976 Jan;57(1):47–52. doi: 10.1104/pp.57.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Obata F., Shibata K. Two pigment proteins in spinach chloroplasts. Biochim Biophys Acta. 1966 Feb 7;112(2):223–234. doi: 10.1016/0926-6585(66)90323-2. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Gregory R. P., Smith C. A., Bailey J. L. Studies on the nature of the chloroplast lamella. I. Preparation and some properties of two chlorophyll-protein complexes. Biochemistry. 1967 Feb;6(2):391–396. doi: 10.1021/bi00854a004. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]