Abstract
MicroRNAs are negative regulators of gene expression that play a key role in cell-type specific differentiation and modulation of cell function and have been proposed to be involved in neovascularization. Previously, using an extensive cloning and sequencing approach, we identified miR-126 to be specifically and highly expressed in human endothelial cells (EC). Here, we demonstrate EC-specific expression of miR-126 in capillaries and the larger vessels in vivo. We therefore explored the potential role of miR-126 in arteriogenesis and angiogenesis. Using miR-reporter constructs, we show that miR-126 is functionally active in EC in vitro and that it could be specifically repressed using antagomirs specifically targeting miR-126. To study the consequences of miR-126 silencing on vascular regeneration, mice were injected with a single dose of antagomir-126 or a control ‘scramblemir’ and exposed to ischemia of the left hindlimb by ligation of the femoral artery. Although miR-126 was effectively silenced in mice treated with a single, high dose (HD) of antagomir-126, laser Doppler perfusion imaging did not show effects on blood flow recovery. In contrast, quantification of the capillary density in the gastrocnemius muscle revealed that mice treated with a HD of antagomir-126 had a markedly reduced angiogenic response. Aortic explant cultures of the mice confirmed the role of miR-126 in angiogenesis. Our data demonstrate a facilitary function for miR-126 in ischemia-induced angiogenesis and show the efficacy and specificity of antagomir-induced silencing of EC-specific microRNAs in vivo.
Keywords: angiogenesis, microRNA, ischemia, vascular biology, endothelial function
Introduction
Endothelial cells (EC) play an essential regulatory role in the capacity of the vasculature to adequately respond to injury or hypoxia. In arteriogenesis, EC react to elevated shear stress by recruiting and activating leucocytes that mediate remodelling of small collateral arterioles. In tissue ischemia, novel capillaries are generated by proliferation and migration of EC that sprout from pre-existing capillaries. The molecular mechanisms underlying this directive role of EC in vascular plasticity have been extensively studied and involve numerous environmental cues that elicit complex, but tightly coordinated responses in the expression of genes controlling proliferation, migration and cell-differentiation [1].
MicroRNAs constitute a recently recognized class of short, non-coding RNA molecules (∼21 nt) that could potentially regulate the activity of 30% of all genes at the post-transcriptional level [2]. The ability of microRNAs to regulate multiple targets provides a means for coordinated control of gene expression, while also making them especially attractive candidates for regulating both cell-type specific differentiation and modulation of cell function [3].
Recently, evidence supporting a role for endothelial microRNAs in the control of neovascularization has been provided [4], with in vitro studies demonstrating both pro-angiogenic microRNAs (let 7b, [5] miR-27b, [5] miR-130a, [6] miR-210 [7]) as well as microRNAs with anti-angiogenic actions (miR-221/222) [8, 9]. To study the role of endothelial microRNAs in neovascularization, we recently generated an inventory of known and novel microRNAs expressed by human microvascular EC and late outgrowth EC using extensive cloning and sequencing [10]. By comparing the microRNA expression profiles of various cultured EC with other tissues and cell types, we confirmed miR-126 to be highly abundant and specific for EC. MiR-126 is located in an intron of the epidermal growth factor-like-domain 7 gene (EGFL7) of man, mouse and zebrafish [11]. Because EGFL7 expression is augmented in adult angiogenesis and vascular injury, [12] we suggested that co-expression of miR-126 may also play a role in neovascularization. Recently, two papers have described a role for miR-126 in vascular development in mice [13] and zebrafish [14]. Targeted deletion of miR-126 resulted in vascular leakage, haemorrhaging and embryonic lethality in a subset of the mutant mice and abnormal vessel morphology was observed in the zebrafish. In this study, we assessed the role of miR-126 in neovascularization in the adult mouse and explored the effects of conditional silencing of this microRNA on arteriogenesis and angiogenesis in vivo. Previous research has demonstrated that intravenous injection of chemically modified and cholesterol-conjugated RNA analogues (antagomirs), specifically silences complementary microRNAs in mice for up to 23 days [15, 16] Our studies using a mouse ischemic hindlimb model demonstrate, for the first time, that antagomir-induced silencing of miR-126 impairs ischemia-induced angiogenesis in vivo.
Material and methods
Cells and cell culture, quantification of microRNA levels, in situ hybridization, miR-126 reporter assays, design of antagomirs, electroporation of EC-RF24 cells, hindlimb ischemia model, immunohistochemistry, quantification of angiogenic response, aortic explant cultures, scratch/wound assay, in vitro angiogenesis assay and statistical analysis are described in the ‘Material and methods’ section in Appendix S1.
Results
MiR-126 is specifically expressed in EC in vitro and in vivo
To validate the expression of miR-126 in EC, we first quantified the presence of the mature form of miR-126 in endothelial and non-EC types using a TaqMan real-time PCR assay (Fig. 1A). After normalization of the signals to U6 small nuclear RNA, we observed that human CD14-derived dendritic cells, human macrophages (Mφ), HeLa cells and embryonic kidney cells [human embryonic kidney (HEK)-293T] expressed only background levels of miR-126. As expected, both human [EC-RF24, human fore-skin derived microvascular endothelial cell (MVEC), human umbilical vein endothial cells (HUVEC)] and murine (bEnd3) EC displayed over two orders of magnitude more miR-126, confirming that miR-126 is highly expressed in microvascular and macrovascular EC in vitro.
Fig. 1.
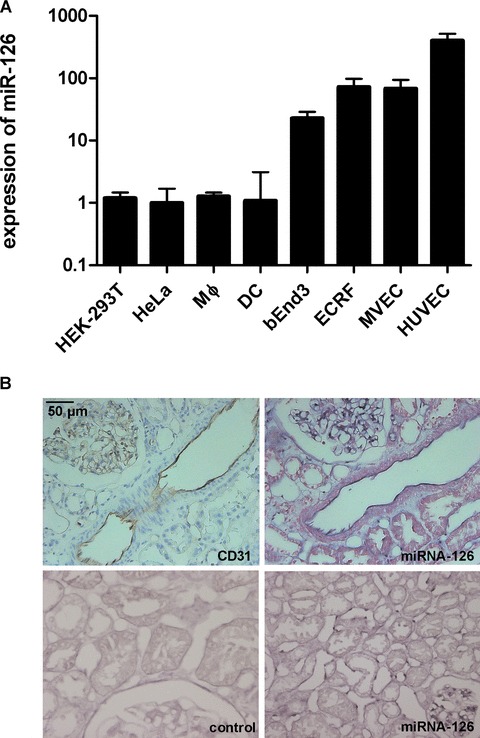
MiR-126 is specifically expressed in EC. (A) Total RNA was harvested from different cell types and analyzed by qRT-PCR on miR-126. Obtained values were normalized by qRT-PCR on U6 snRNA. (B) Representative microscopic images of immunohistochemistry of CD31 on human renal sections and in situ hybridization of miR-126 with locked nucleic acid (LNA)-probe or a control LNA-probe. Upper two pictures represent consecutive sections.
In contrast to the expression of miR-126 in cultured EC, [5, 10, 17] little is known about the tissue-specific expression of miR-126 in vivo. Therefore, we performed in situ hybridization analysis on human renal sections using digoxigenin-labelled locked nucleic acid (LNA) probes [18]. To localize the endothelium in these sections, we co-stained for the endothelial marker CD31 (Fig. 1B). In situ hybridization of consecutive human renal sections with the LNA probe for miR-126 demonstrated expression of miR-126 in the CD31+ endothelium of the glomerular and peritubular capillaries as well as the endothelium of the larger vessels. These findings confirm the EC-specificity of miR-126 expression in vivo.
MiR-126 is functionally active in EC
Next, we sought to investigate whether miR-126 is functionally active in EC. For this, we constructed reporter plasmids containing the firefly luciferase gene under the control of the constitutive cytomegalovirus (CMV) promoter and no (pMIR), one (pMIR-126 M) or four (pMIR-126Q) perfect miR-126 target sites in the 3’ untranslated region of the luciferase reporter gene. Twenty four hours after electroporation of the reporter plasmids into the target cells, firefly luciferase activity was measured and normalized for electroporation efficiency using renilla luciferase activity derived from a co-electro-porated expression plasmid. In HEK-293T cells, which lack miR-126 expression, luciferase expression was identical for all three reporters, indicating that the incorporation of the miR-126 target sites did not impact the luciferase transcript translation efficiency (Fig. 2A). In contrast, EC-RF24 cells displayed markedly reduced luciferase activity, with the presence of one or four miR-126 target sites reducing luciferase expression to 13% (P< 0.01) and less than 1% (P < 0.01), respectively. These data clearly demonstrate a potent negative regulatory role of miR-126 in cultured EC.
Fig. 2.
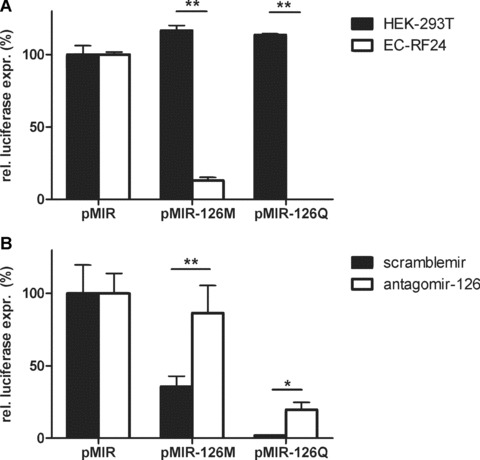
MiR-126 is functionally active in vitro and can be specifically repressed by antagomir-126 in vitro and in vivo. (A) Relative firefly luciferase expression of HEK-293T and EC-RF24 transfected with a luciferase reporter plasmid harbouring 0 (pMIR), 1 (pMIR-126 M) or 4 (pMIR-126Q) perfect match target sites for miR-126 in the 3’UTR of the luciferase transcript (**=P< 0.01). (B) Relative firefly luciferase expression of transfected EC-RF24 incubated for 16 hrs with 5 μg/ml scram-blemir or antagomir-126 (*=P< 0.05 and **=P< 0.01).
Antagomir silencing of MiR-126 in EC
Antagomirs have been used to specifically silence microRNAs [15], which prompted us to determine whether antagomirs can also be used to silence miR-126 in EC. Therefore, we designed both a cholesterol-conjugated modified 21 nucleotide RNA, complementary to mature miR-126 (antagomir-126) as well as a control RNA analogue of identical composition and length, but with a random sequence (scramblemir). Using a bioinformatic approach, this sequence was chosen because it did not match any known miR or mRNA. Addition of 5 μg/ml scramblemir to the culture medium of EC-RF24 cells, 16 hrs before electroporation with the miR-126 reporter plasmids (Fig. 2B), had little effect on the miR-126-repressed luciferase levels of pMiR-126M (35%) and pMiR-126Q (2%). In contrast, pre-culturing the cells with 5 μg/ml antagomir-126 restored luciferase levels to 86% (P < 0.01) and 20% (P < 0.05) for pMiR-126 M and pMiR-126Q, respectively. This supports the notion that antagomirs can be efficiently taken up by EC and that antagomir-126 can be used to specifically counteract the negative regulation of target gene expression by miR-126.
Antagomir silencing of MiR-126 has no effect on in vitro angiogenesis, migration and proliferation
To assess whether miR-126 plays a role in the capacity of EC to form capillary-like structures on matrigel, HUVEC were incubated overnight with 5 μg/ml antagomir-126 or scramblemir. Eight hours after cell seeding, tube formation was not affected by silencing of miR-126 (Fig. 3A and B). Next, similarly treated HUVEC were cultured to confluence and their capacity to migrate and proliferate was assessed by measuring the degree to which a 800-μm scratch was repopulated by the cells in 24 hrs. Again, we observed no effects of miR-126 silencing on proliferation and migration of the cells (Fig. 3C and D).
Fig. 3.
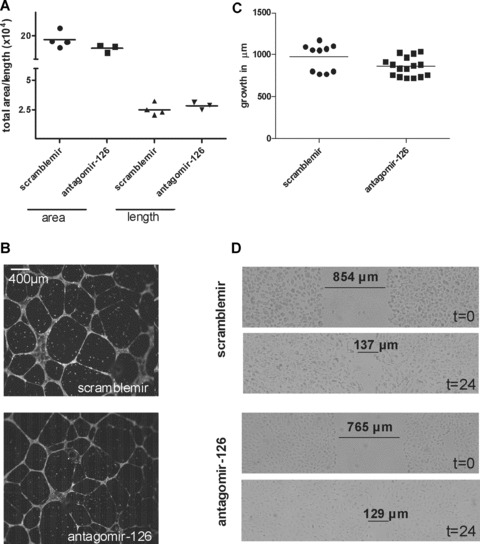
Repression of miR-126 in vitro does not influence the formation of capillary structures, or EC proliferation and migration. (A) Quantification of area and length of capillary structures after 8 hrs of culture on matrigel (data expressed in arbitrary units). (B) Representative microscopic images of the formation of capillary-like structures by HUVEC. (C) Quantification of the degree of EC regrowth in a scratch/wound assay (expressed in μm from scratch border). (D) Representative microscopic images of HUVEC incubated with antagomir-126 or scramblemir before and 24 hrs after the scratch/wound assay.
Antagomir silencing of MiR-126 does not affect arteriogenesis
The murine ischemic hindlimb model makes it possible to assess the consequences of interventions on collateral formation around the ligated femoral artery as well as on the hypoxia-induced angiogenic response in the distal calf muscle [19]. To investigate the role of miR-126 in neovascularization and the use of antagomirs to silence endothelial microRNAs in vivo, we injected four groups (n= 6) of male C57Bl/6 WT mice in the tail vein with either a low dose (0.1 mg, LD) or a high dose (1.0 mg, HD) of antagomir-126 or scramblemir. After 24 hrs, unilateral hindlimb ischemia was induced by electrocoagulation of the left common femoral artery and blood flow recovery was measured over 10 days using laser Doppler perfusion imaging. As shown in Fig. 4C, the progression of blood flow recovery was similar for all treated groups, reaching normal levels between days 7 and 10.
Fig. 4.
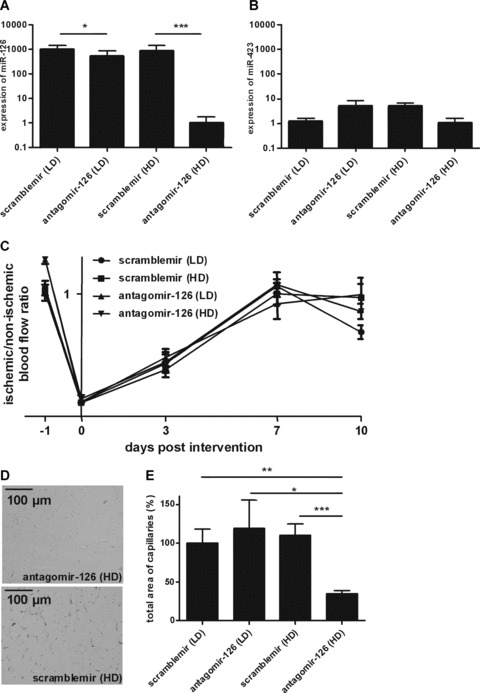
MiR-126 silencing has no effect on collateral formation, but miR-126 facilitates ischemia-induced angiogenesis. (A and B) Total RNA was harvested from lungs of mice after 11 day treatment with high and low doses of antagomir-126 or scramblemir (HD or LD). Samples were analyzed by qRT-PCR for A, miR-126 and (B) miR-423 expression. Obtained values were normalized by qRT-PCR on U6 snRNA (*=P< 0.05 and ***=P< 0.001). (C) Quantitative evaluation of blood flow recovery measured before and after induction of ischemia by laser Doppler perfusion imaging and expressed as a ratio of the left (ischemic) and right (non-ischemic) limb. (D) Representative microscopic images of CD31 staining in ischemic murine gastrocnemius muscles. (E) Quantification of the total area of capillaries in sections of the gastrocnemius muscle 10 days after induction of ischemia (*=P< 0.05, **=P< 0.01 and ***=P< 0.001).
To verify that the injected antagomir-126 had indeed silenced miR-126 in the endothelium of the mice at the end of the experiment, the lungs of the mice were harvested and the expression levels of miR-126 and a control EC-enriched microRNA miR-423, were quantified by real-time PCR (Fig. 4A and B). Whereas the mice treated with antagomir-126 (LD) show only a marginal reduction in miR-126 expression (1.9 fold, P< 0.05 versus scramblemir [LD]), mice treated with antagomir-126 (HD) displayed an over 1000 fold reduction in miR-126 expression compared to the scramblemir-treated mice (P< 0.001). In contrast, no significant differences were observed for the miR-423 levels in all groups. Our data demonstrate that in vivo silencing of the endothelial miR-126 remains readily detectable ten days after administration of a single dose of 1.0 mg of antagomir-126. Moreover, we conclude that miR-126 is not directly involved in arteriogenesis.
Antagomir silencing of MiR-126 impairs ischemia-induced angiogenesis
To assess the effect of miR-126 silencing on the ischemia-induced angiogenic response, we performed a detailed quantitative analysis of CD31 stained capillaries in sections of gastrocnemius muscle of all treated mice (Fig. 4D and E). Mice treated with a single dose antagomir-126 (HD) showed a markedly lower density of capillary vessels compared to antagomir-126 (LD) or both control groups (35%versus 118% (n= 6, P< 0.05), 109% (n= 6, P < 0.001) and 100% (n= 6, P< 0.01)). These studies demonstrate that silencing of miR-126 impairs the angiogenic response to ischemia.
Antagomir silencing of MiR-126 impairs EC outgrowth in aortic explant cultures
The degree of EC outgrowth from freshly dissected pieces of abdominal aorta has been used as an assay for the potency of compounds to induce angiogenic sprouting of the aortic EC. Therefore, we cultured aortic explants on fibronectin-coated plates in angiogenic medium and assessed the endothelial outgrowth after 11 days from mice of all four treatment groups. As shown in Fig. 5, endothelial outgrowth was strongly impaired only in aortic explant cultures derived from mice treated with antagomir-126 (HD). These data support our finding that the endothelial miR-126 is required for an appropriate angiogenic response.
Fig. 5.
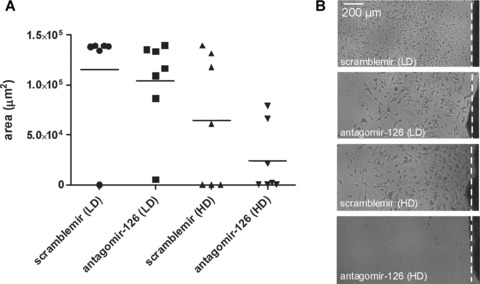
Repression of miR-126 impairs endothelial outgrowth or aortic explants. (A) Quantification of total surface (in μm2) covered by endothelial outgrowth of aortic explants. (B) Representative microscopic images of endothelial outgrowth from aortic explants.
Discussion
Previously, miR-126 was found to be expressed in the heart and blood vessels of zebrafish embryos [18]. We demonstrate here that miR-126 is specifically expressed in EC of capillaries and arterioles in vivo. To gain insight into a possible regulatory role for this microRNA in neovascularization, we aimed to obtain a specific miR-126 inhibitor for conditional silencing of miR-126 in the vascular endothelium. Recent work has resulted in the development of two potent approaches for the in vivo silencing of microRNAs, notably: (1) LNA-modified oligonucleotides for the efficient and long lasting silencing of miR-122 function in the liver of mice and non-human primates [20, 21] and (2) chemically modified and cholesterol-conjugated RNAs termed antagomirs for the rapid and specific degradation of microRNAs in multiple tissues after tail vein injection [15]. As cholesterol uptake is a salient feature shared by virtually all cells, including EC, we designed an antagomir directed to miR-126. We provide evidence that a dose of 5 μg/ml of antagomir-126 specifically silenced miR-126 function in the reporter assay in cultured EC whereas the scramblemir had little effect. Higher doses of scramblemir (>50 μg/ml) resulted in non-specific silencing of miR-126 (data not shown). This effect is likely the result of excessive cellular uptake of small single-stranded RNA analogues leading to non-specific interference with microRNA repression. These results emphasize the need for equally dosed scramblemir controls, for studies assessing microRNA silencing. This may be particularly relevant for in vivo studies where there is less control over the distribution of antagomirs over the different tissues.
In mice treated with antagomir-126, we validated the specificity of miR-126 silencing by quantifying the level of mature miR-126 in total lung tissue. This was based on previous observations that, of all organs profiled for microRNA-expression by extensive cloning and sequencing, the lung displays the highest levels of miR-126 expression [22, 13] (e.g. 3-fold higher than skin, 8-fold higher than in heart and 130-fold higher than in total brain tissue) [10]. We observed that 10 days after administration of a single, 1.0 mg injection of antagomir-126 per mouse, was sufficient to almost completely abrogate miR-126 expression in lung tissue, whereas miR-126 remained readily detectable in the LD group as well as the control scramblemir groups. As a single injection of 1.0 mg is low compared to the reported dose needed for silencing of the liver specific miR-122 (three consecutive injections of 2 mg per mouse), we conclude that EC readily take up antagomirs from the circulation and may therefore be highly useful for studying endothelial microRNA function in vivo.
Leucocyte recruitment by the endothelium also plays a critical role in arteriogenesis [1]. Recently, it has been reported that vascular cell adhesion molecule 1 (VCAM-1) is a target for miR-126 in HUVEC and that decreasing miR-126 levels increased the adherence of leucocytes in vitro[23]. However, we did not observe any differences in blood flow recovery after femoral artery ligation in either of the treated groups. Therefore, we conclude that miR-126 regulation of VCAM-1 expression is probably not a ratelimiting factor for in vivo arteriogenesis.
Our data do support, however, a role for miR-126 in the angiogenic response. The reduction of tissue miR-126 expression in the HD-treated mice is associated with a reduction in capillary density in gastrocnemius muscle compared to the scramblemir-treated mice. Likewise, we observed impaired outgrowth of EC from aortic sections of miR-126-silenced mice.
Surprisingly, in vitro experiments designed to assess the relatively short term effects of antagomir-126 silencing in HUVEC revealed no differences in the formation of capillary-like structures, or cellular migration and proliferation (scratch/wound assay). This observation is compatible with the notion that different mechanisms are required for three-dimensional sprouting of EC into a matrix compared to two-dimensional cell movement in a culture dish [24]. The effects of miR-126 on angiogenesis most likely involve mechanisms operational in EC in the in vivo context that involves the interaction with pericytes, EC matrix and the basement membrane. For instance, physical contact between EC and pericytes is thought to induce a quiescent, non-sprouting phenotype [25]. Initiation of angiogenic sprouting is preceded by the formation of so-called tip cells that lead the sprouting, while the trailing EC must maintain their connection to the patent vasculature [26]. Tip-cell formation in both mice and zebrafish is regulated by Notch signalling pathways and vascular EC growth factor receptor-3 (VEGFR-3) is involved in the generation of tip cells. In mice, it has been established that the Sprouty-related Ena/VASP homology 1 domain containing protein (Spred-1) can function as a potent repressor of VEGFR-3 [26]. Spred-1 and phosphoinositol-3 kinase regulatory subunit 2 (PIK3R2, p85-β), another protein actively involved in the negative regulation of VEGF signalling [27], are both predicted targets of miR-126 (http://www.targetscan.org). Consequently, up-regulation of miR-126 would thus facilitate angiogenesis by reducing the expression of both repressors of VEGF signalling whereas low levels of miR-126 would be associated with elevation of Spred-1 or PIK3R2 and repress angiogenic signalling. Indeed, two recent studies reported that targeted deletion of miR-126 in mice and zebrafish impairs angiogenesis likely through dysregulation of Spred-1 and PIK3R2 expression suggesting a critical role for this microRNA in angiogenic signalling events during embryogenesis [13, 14]. Here, we demonstrate that miR-126 also plays a key role in the regulation of ischemia-induced angiogenesis in adult mice. Using qRT-PCR we observed that antagomir-mediated silencing of miR-126 in murine EC (bEnd3) led to a concomitant four fold up-regulation of Spred-1 mRNA (data not shown). Therefore, it is tempting to speculate that up-regulation of Spred-1 and PIK3R2 levels also underlies the anti-angiogenic effects observed in our study.
Taken together, we have demonstrated that functional activity of miR-126 is required for the ischemia-induced angiogenic response in vivo. In addition, our study supports the potential therapeutic use of antagomir-based approaches for conditional silencing of microRNAs in the endothelium in vivo.
Acknowledgments
C.v.S. and H.C.d.B. are supported by grants (2006B145 and 2005B106 respectively) from the Netherlands Heart Foundation, The Hague. R.B. is supported by a grant (C07.2227) from the Dutch Kidney Foundation. A.J.v.Z. is supported by a grant from the Genzyme Renal Innovations Program.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Appendix S1.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1582-4934.2008.00613.x (This link will take you to the article abstract).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–14. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 3.Xie X, Lu J, Kulbokas EJ, et al. Systematic discovery of regulatory motifs in human promoters and 3’ UTRs by comparison of several mammals. Nature. 434:338–45. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–8. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 5.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic home-obox genes GAX and HOXA5. Blood. 2008;111:1217–26. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasanaro P, D’Alessandra Y, Di Stefano V, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–83. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poliseno L, Tuccoli A, Mariani L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–71. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 9.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–73. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 10.Berezikov E, van Tetering G, Verheul M, et al. Many novel mammalian microRNA candidates identified by extensive cloning and RAKE analysis. Genome Res. 2006;16:1289–98. doi: 10.1101/gr.5159906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–10. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campagnolo L, Leahy A, Chitnis S, et al. EGFL7 is a chemoattractant for endothelial cells and is up-regulated in angiogenesis and arterial injury. Am J Pathol. 2005;167:275–84. doi: 10.1016/S0002-9440(10)62972-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fish JE, Santoro MM, Morton SU, et al. MiR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 16.Krutzfeldt J, Kuwajima S, Braich R, et al. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35:2885–92. doi: 10.1093/nar/gkm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005;579:5911–22. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 18.Wienholds E, Kloosterman WP, Miska E, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–1. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 19.Madeddu P, Emanueli C, Spillmann F, et al. Murine models of myocardial and limb ischemia: diagnostic end-points and relevance to clinical problems. Vascul Pharmacol. 2006;45:281–301. doi: 10.1016/j.vph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Elmen J, Lindow M, Schutz S, et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–9. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 21.Elmen J, Lindow M, Silahtaroglu A, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36:1153–62. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagos-Quintana M, Rauhut R, Yalcin A, et al. Identification of tissue-specific micro RNAs from mouse. Curr Biol. 2002;12:735–9. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 23.Harris TA, Yamakuchi M, Ferlito M, et al. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–21. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diehl F, Rossig L, Zeiher AM, et al. The histone methyltransferase MLL is an upstream regulator of endothelial-cell sprout formation. Blood. 2007;109:1472–8. doi: 10.1182/blood-2006-08-039651. [DOI] [PubMed] [Google Scholar]
- 25.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 26.Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. Bioessays. 2008;30:303–13. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- 27.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–53. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


