Abstract
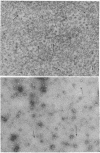
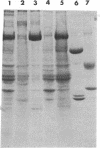
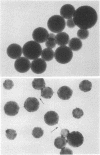
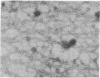
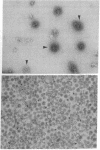
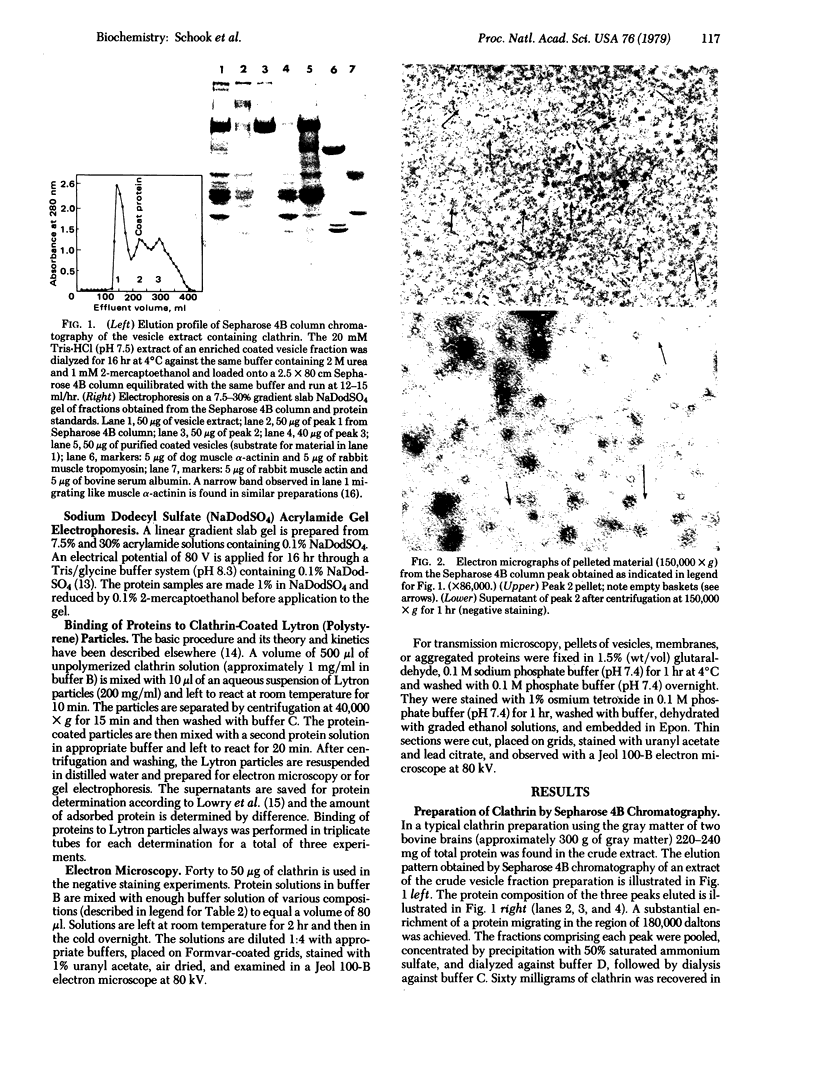
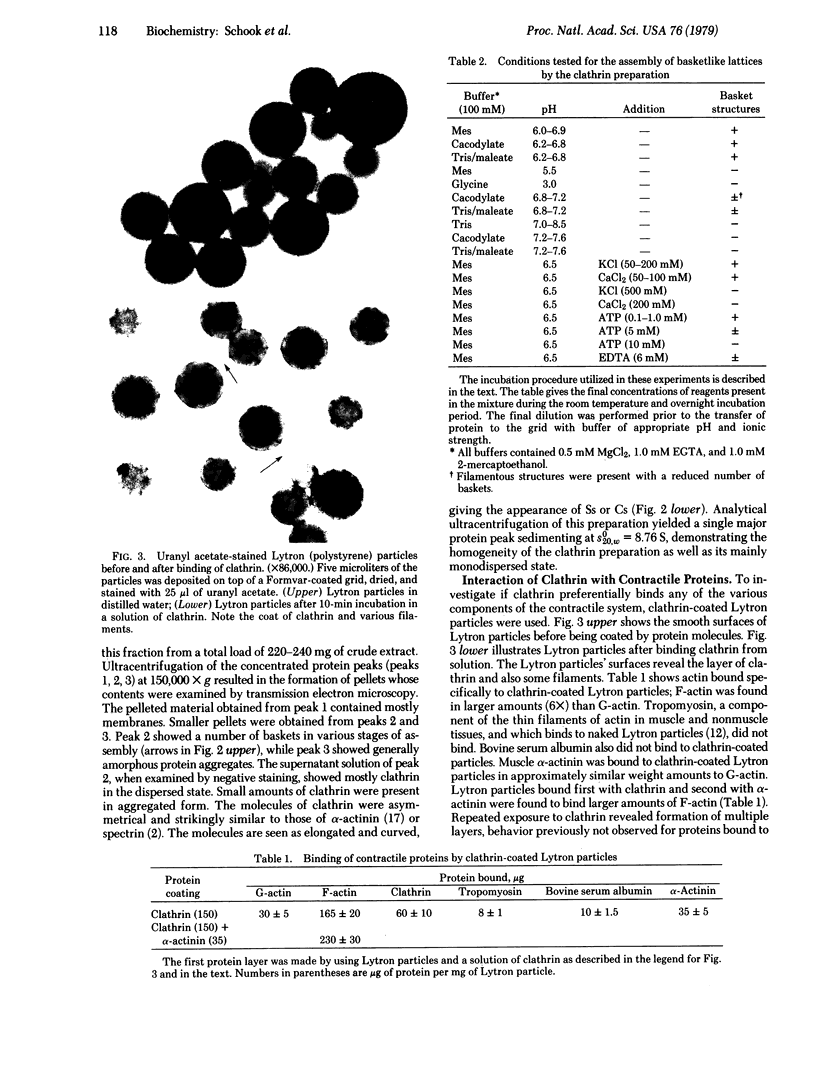
Two molar urea (pH 7.5) and column chromatography on Sepharose 4B were used to separate clathrin (coat protein) from the membrane of coated vesicles from bovine brain. Lytron (polystyrene) particles were used for study of the interaction of clathrin with contractile proteins. Muscle G-actin, F-actin, and alpha-actinin were bound by clathrin-coated Lytron particles, while no interaction was found when muscle tropomyosin and serum albumin were tested. Clathrin molecules dispersed in a solution of 20 mM Tris-HCl (pH 7.5) were found to be elongated. When the pH was adjusted from 7.5 to 6.5, clathrin molecules associated into basketlike or cage structures similar in size and shape to those observed in enriched preparations of coated vesicles. Below pH 6.0, cages or baskets became amorphous aggregates. Raising the pH from 6.5 to 8.0, addition of 5-10 mM ATP or EDTA, or addition of 200 mM KCl resulted in the dissassembly of baskets and the formation of filamentous arrays of various widths. Because of clathrin's biochemical and biophysical properties, its interaction with contractile proteins, and its presence in the membrane of vesicles of various cell types, we classified clathrin in the group of mechanochemical proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blitz A. L., Fine R. E., Toselli P. A. Evidence that coated vesicles isolated from brain are calcium-sequestering organelles resembling sarcoplasmic reticulum. J Cell Biol. 1977 Oct;75(1):135–147. doi: 10.1083/jcb.75.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Stossel T. P. Interactions of actin, myosin, and an actin-binding protein of chronic myelogenous leukemia leukocytes. J Clin Invest. 1976 Apr;57(4):964–976. doi: 10.1172/JCI108373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Lüder M. R., Kartenbeck J., Zerban H., Keenan T. W. Involvement of vesicle coat material in casein secretion and surface regeneration. J Cell Biol. 1976 Apr;69(1):173–195. doi: 10.1083/jcb.69.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Puszkin S., Kochwa S., Puszkin E. G., Rosenfield R. E. A solid-liquid biphasic model for characterization of properties of muscle and platelet contractile proteins. J Biol Chem. 1975 Mar 25;250(6):2085–2094. [PubMed] [Google Scholar]
- Puszkin S., Puszkin E., Maimon J., Rouault C., Schook W., Ores C., Kochwa S., Rosenfield R. alpha-Actinin and tropomyosin interactions with a hybrid complex of erythrocyte-actin and muscle-myosin. J Biol Chem. 1977 Aug 10;252(15):5529–5537. [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H. Interactions of actin, myosin, and a new actin-binding protein of rabbit pulmonary macrophages. II. Role in cytoplasmic movement and phagocytosis. J Cell Biol. 1976 Mar;68(3):602–619. doi: 10.1083/jcb.68.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Goll D. E., Singh I., Allen R. E., Robson R. M., Stromer M. H. Some properties of purified skeletal muscle alpha-actinin. J Biol Chem. 1976 Nov 10;251(21):6860–6870. [PubMed] [Google Scholar]
- Tilney L. G., Detmers P. Actin in erythrocyte ghosts and its association with spectrin. Evidence for a nonfilamentous form of these two molecules in situ. J Cell Biol. 1975 Sep;66(3):508–520. doi: 10.1083/jcb.66.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Filamin, a new high-molecular-weight protein found in smooth muscle and nonmuscle cells. Purification and properties of chicken gizzard filamin. Biochemistry. 1977 May 3;16(9):1857–1865. doi: 10.1021/bi00628a015. [DOI] [PubMed] [Google Scholar]