Abstract
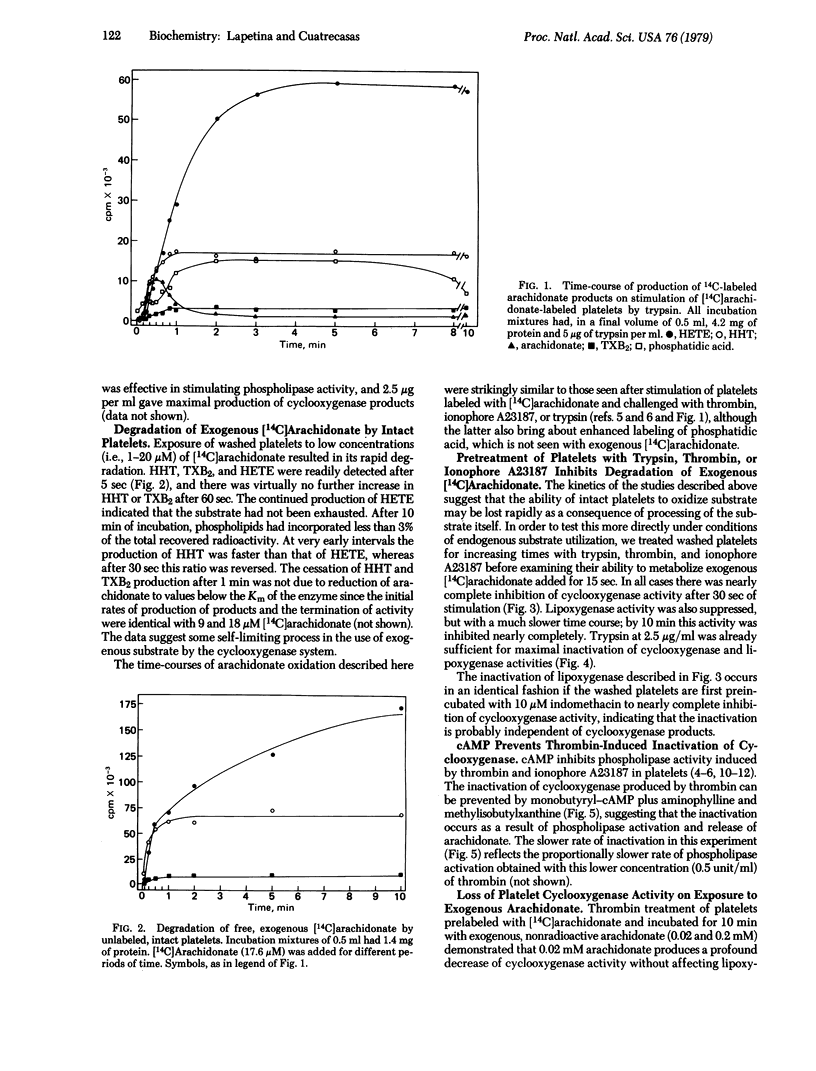
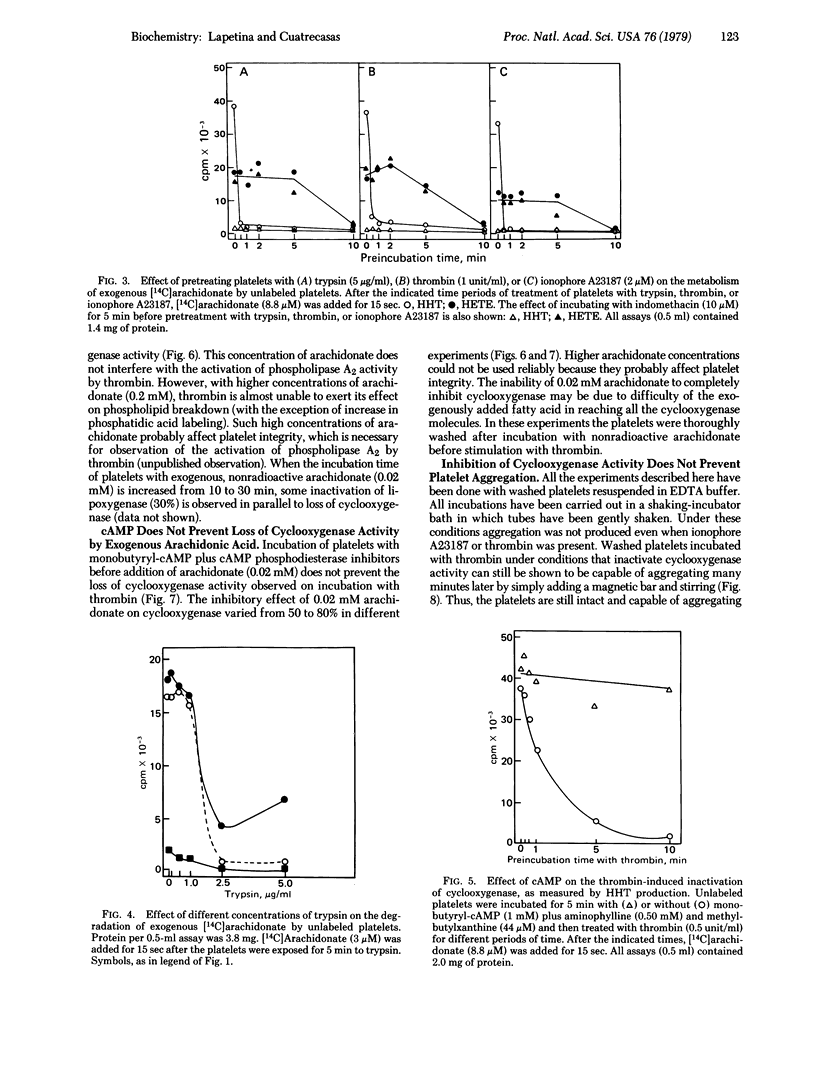
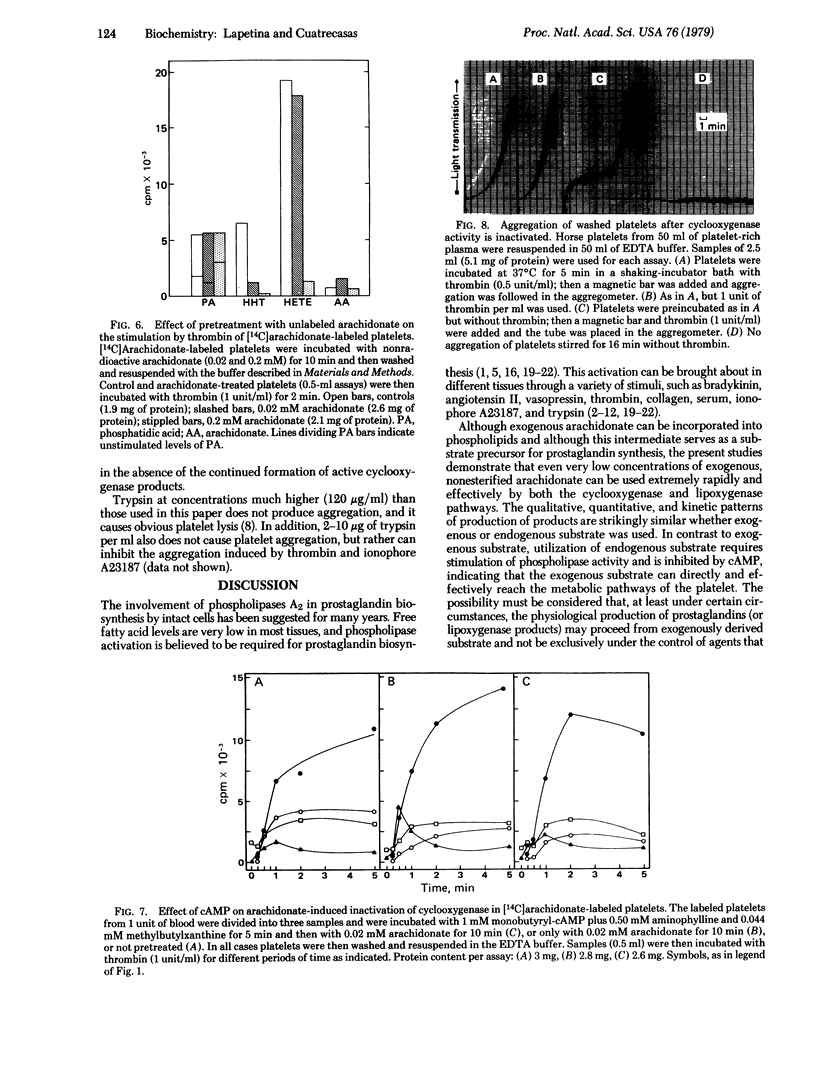
Trypsin, thrombin, and ionophore A23187 activate phospholipid breakdown of platelets that have been labeled with [14C]arachidonate, releasing their cyclooxygenase and lipoxygenase products. Intact platelets can also very effectively directly degrade low concentrations of exogenous, free [14C]arachidonate. Pretreatment of platelets with trypsin, thrombin, or ionophore A23187 for a minimum time of 30 sec leads to complete inactivation of cyclooxygenase activity, as demonstrated by subsequent exposure to [14C]arachidonate. Lipoxygenase activity is lost after 5 min. The thrombin-induced inactivation of cyclooxygenase and lipoxygenase is prevented by cyclic AMP (which inhibits the stimulated activity of phospholipase A2), although cyclic AMP does not affect the degradation of exogenous [14C]arachidonate. Exposure of platelets labeled with [14C]arachidonate to unlabeled arachidonate under conditions that lead to use of the latter also results in a similarly rapid inhibition of cyclooxygenase activity, as determined by subsequent challenge with thrombin. Under these conditions lipoxygenase activity is much less markedly inactivated. The arachidonate-induced inhibition of cyclooxygenase activity is not prevented by cyclic AMP. Trypsin does not induce platelet aggregation, and platelets whose cyclooxygenase activity has been inactivated are intact insofar as they are still able to undergo aggregation. These studies demonstrate that operation in intact platelets of the cyclooxygenase pathway, through use of endogenous or exogenous substrate, leads to a very rapid, irreversible inactivation of this enzyme. The lipoxygenase pathway is also progressively impaired, but much less rapidly than the cyclooxygenase enzyme and much less markedly on use of exogenous compared to endogenous substrate. The possible consequences of these physiological processes of spontaneous inactivation are considered.
Keywords: lipoxygenase, arachidonate, phospholipases, aggregation, self-regulation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bills T. K., Smith J. B., Silver M. J. Metabolism of [14C]arachidonic acid by human platelets. Biochim Biophys Acta. 1976 Feb 23;424(2):303–314. doi: 10.1016/0005-2760(76)90198-3. [DOI] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Selective release of archidonic acid from the phospholipids of human platelets in response to thrombin. J Clin Invest. 1977 Jul;60(1):1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Duncombe W. G., Flower R. J., Parsons M. F., Vane J. R. The distribution and metabolism of arachidonic acid in rabbit platelets during aggregation and its modification by drugs. Br J Pharmacol. 1977 Feb;59(2):353–366. doi: 10.1111/j.1476-5381.1977.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrabose K. A., Lapetina E. G., Schmitges C. J., Siegel M. I., Cuatrecasas P. Action of corticosteroids in regulation of prostaglandin biosynthesis in cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Jan;75(1):214–217. doi: 10.1073/pnas.75.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan R. W., Paxton J., Kuehl F. A., Jr Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976 Dec 10;251(23):7329–7335. [PubMed] [Google Scholar]
- Feinstein M. B., Becker E. L., Fraser C. Thrombin, collagen and A23187 stimulated endogenous platelet arachidonate metabolism: differential inhibition by PGE1, local anesthetics and a serine-protease inhibitor. Prostaglandins. 1977;14(6):1075–1093. doi: 10.1016/0090-6980(77)90286-6. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Peller J. D., Krick T. P., White J. G. Cyclic AMP and platelet prostaglandin synthesis. Prostaglandins. 1977 Jul;14(1):39–50. doi: 10.1016/0090-6980(77)90155-1. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Reimers H. J., Mustard J. F., Packham M. A. Sodium arachidonate can induce platelet shape change and aggregation which are independent of the release reaction. Science. 1976 Jun 4;192(4243):1011–1012. doi: 10.1126/science.1273582. [DOI] [PubMed] [Google Scholar]
- Knapp H. R., Oelz O., Roberts L. J., Sweetman B. J., Oates J. A., Reed P. W. Ionophores stimulate prostaglandin and thromboxane biosynthesis. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4251–4255. doi: 10.1073/pnas.74.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Chandrabose K. A., Cuatrecasas P. Ionophore A-23187- and thrombin-induced platelet aggregation: independence from cycloxygenase products. Proc Natl Acad Sci U S A. 1978 Feb;75(2):818–822. doi: 10.1073/pnas.75.2.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Schmitges C. J., Chandrabose K., Cuatrecasas P. Regulation of phospholipase activity in platelets. Adv Prostaglandin Thromboxane Res. 1978;3:127–135. [PubMed] [Google Scholar]
- Lapetina E. G., Schmitges C. J., Chandrabose K., Cuatrecases P. Cyclic adenosine 3',5'-monophosphate and prostacyclin inhibit membrane phospholipase activity in platelets. Biochem Biophys Res Commun. 1977 Jun 6;76(3):828–835. doi: 10.1016/0006-291x(77)91575-3. [DOI] [PubMed] [Google Scholar]
- Malmsten C., Granström E., Samuelsson B. Cyclic AMP inhibits synthesis of prostaglandin endoperoxide (PGG2) in human platelets. Biochem Biophys Res Commun. 1976 Jan 26;68(2):569–576. doi: 10.1016/0006-291x(76)91183-9. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Ullman H. L., Safier L. B. Lipid composition of subcellular particles of human blood platelets. J Lipid Res. 1969 Jan;10(1):108–114. [PubMed] [Google Scholar]
- Minkes M., Stanford N., Chi M. M., Roth G. J., Raz A., Needleman P., Majerus P. W. Cyclic adenosine 3',5'-monophosphate inhibits the availability of arachidonate to prostaglandin synthetase in human platelet suspensions. J Clin Invest. 1977 Mar;59(3):449–454. doi: 10.1172/JCI108659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P., Bronson S. D., Wyche A., Sivakoff M., Nicolaou K. C. Cardiac and renal prostaglandin I2. Biosynthesis and biological effects in isolated perfused rabbit tissues. J Clin Invest. 1978 Mar;61(3):839–849. doi: 10.1172/JCI108998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett W. C., Jesse R. L., Cohen P. Initiation of phospholipase A2 activity in human platelets by the calcium ion ionophore A23187. Biochim Biophys Acta. 1976 Jan 18;486(1):209–213. doi: 10.1016/0005-2760(77)90086-8. [DOI] [PubMed] [Google Scholar]
- Pickett W. C., Jesse R. L., Cohen P. Trypsin-induced phospholipase activity in human platelets. Biochem J. 1976 Nov 15;160(2):405–408. doi: 10.1042/bj1600405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Deykin D. The mobilization of arachidonic acid in platelets exposed to thrombin or ionophore A23187. Effects of adenosine triphosphate deprivation. J Clin Invest. 1977 Aug;60(2):495–498. doi: 10.1172/JCI108801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. J., Smith J. B., Ingerman C., Kocsis J. J. Arachidonic acid-induced human platelet aggregation and prostaglandin formation. Prostaglandins. 1973 Dec;4(6):863–875. doi: 10.1016/0090-6980(73)90121-4. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Lands W. E. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry. 1972 Aug 15;11(17):3276–3285. doi: 10.1021/bi00767a024. [DOI] [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R. Prostaglandin E2 biosynthesis by rabbit renomedullary interstitial cells in tissue culture. Mechanism of stimulation by angiotensin II, bradykinin, and arginine vasopressin. J Biol Chem. 1977 Mar 25;252(6):2069–2071. [PubMed] [Google Scholar]


