Abstract
Aged rats recover poorly after unilateral stroke, whereas young rats recover readily possibly with the help from the contralateral, healthy hemisphere. In this study we asked whether anomalous, age-related changes in the transcriptional activity in the brains of aged rats could be one underlying factor contributing to reduced functional recovery. We analysed gene expression in the periinfarct and contralateral areas of 3-month- and 18-month-old Sprague Dawley rats. Our experimental end-points were cDNA arrays containing genes related to hypoxia signalling, DNA damage and apoptosis, cellular response to injury, axonal damage and re-growth, cell lineage differentiation, dendritogenesis and neurogenesis. The major transcriptional events observed were: (i) Early up-regulation of DNA damage and down-regulation of anti-apoptosis-related genes in the periinfarct region of aged rats after stroke; (ii) Impaired neurogenesis in the periinfarct area, especially in aged rats; (iii) Impaired neurogenesis in the contralateral (unlesioned) hemisphere of both young and aged rats at all times after stroke and (iv) Marked up-regulation, in aged rats, of genes associated with inflammation and scar formation. These results were confirmed with quantitative real-time PCR. We conclude that reduced transcriptional activity in the healthy, contralateral hemisphere of aged rats in conjunction with an early up-regulation of DNA damage-related genes and pro-apoptotic genes and down-regulation of axono- and neurogenesis in the periinfarct area are likely to account for poor neurorehabilitation after stroke in old rats.
Keywords: rat, aging, stroke, gene expression
Introduction
Studies of stroke in experimental animals have identified a variety of interventions with marked neuroprotective effects, but most of these approaches have failed to show benefit in aged human stroke victims. One possible explanation for this discrepancy between laboratory and clinical investigations is the role that age plays in the recovery of the brain from insult.
Although it is well known that aging is a major risk factor for stroke [1] the majority of experimental studies of stroke have been performed on young animals, and therefore may not fully replicate the effects of ischaemia on aged neural tissue. Therefore, studies of experimental stroke in the aged animal are likely to have more clinical relevance, both for understanding cellular responses to stroke and for identification of beneficial interventions [2–5].
Young rats recover much better than aged rats within 2 weeks from stroke [4] and it has been proposed that an increased tissue repair capacity of young rats may underlie the rapid recovery from ischaemic damage. However, axonal growth does not occur until the second week after stroke [5, 6], calling into question the role of brain plasticity mechanisms in supporting tissue repair and functional recovery in the first few days after stroke. An alternative explanation is that more robust activation of the contralateral hemisphere explains the age-related difference in recovery [7–10].
DNA array technology may provide insight into the mechanisms underlying differences between old and young animals in rate and extent of brain repair and regeneration after stroke. Studies of focal cerebral ischaemia have identified a series of key molecular events and a number of changes in gene regulation following infarct [11–16]. These studies revealed changes in transcriptional activity of a variety of genes related to stress response, inflammation, acute- and delayed cell death. However, these studies have not examined changes in gene expression both in the ipsilateral and contralateral hemisphere to the infarct, and thus do not address the possibility that age related differences in recovery are related to contralateral genetic mechanisms.
In the present study we addressed this question by using defined cDNA arrays. These arrays included genes related to cell injury and survival, cell growth, the hypoxia signalling pathway (including DNA damage), inflammatory-related processes and apoptosis. By comparing gene expression changes in young versus aged rats, our goal was to identify post-infarct changes in gene expression following middle cerebral artery occlusion (MCAO) in aged rats that may give us clues as to transcriptional events underlying reduced functional recovery after stroke in aged rats.
Materials and methods
Animals
Eighteen hours prior to surgery, male 3-month-old (young) and 18-month-old (aged) Sprague-Dawley rats were fasted but allowed free access to water to minimize variability in ischaemic damage that can result from varying plasma glucose levels. MCAO was perfomed as described below. Survival times for the study were: 3 days (n= 8 young; n= 7 aged rats) and 14 days (n= 10 for young; n= 10 for the aged rats).
All experiments were approved by the University Animal Experimentation Ethics Board as meeting the ethical requirements of the German National Act on the Use of Experimental Animals.
Reversible occlusion of the middle cerebral artery
Blood flow through the middle cerebral artery was transiently interrupted in deeply anaesthetized rats as previously described [2, 4]. The right middle cerebral artery was slowly lifted with a tungsten hook attached to a micromanipulator until blood flow through the vessel was completely stopped. Both common carotid arteries were then occluded by tightening pre-positioned thread loops. The sharply decreased blood flow was monitored with a Laser Doppler (Periflux 5000, Perimed, Sweden) by positioning the optic tube on the temporal bone of rat skull. After 90 min., the middle cerebral artery and the common carotid arteries were re-opened, allowing full reperfusion of the brain. It should be noted that occlusion of the carotid arteries alone for 90 min. has no noticeable hypoxic effect on the brain. With this model, the infarct is located mostly in the sensorimotor cortex and only rarely in the striatum. If striatal infarction did occur, these animals were excluded from this study.
Following survival times of 3 and 14 days, the rats were deeply anaesthetized and perfused with buffered saline followed by buffered, 4% freshly depolymerized paraformaldehyde. The brain was removed, post-fixed in 4% buffered paraformaldehyde for 24 hrs, cryoprotected in 20% sucrose prepared in 10 mmol/l phosphate buffered saline, flash-frozen in isopentane and stored at (70°C until sectioning.
For real time PCR, seven additional brains per age group and time-point were perfused with buffered saline, cut into 2 mm slices that were dipped in triphenyltetrazolium chloride (TTC) to allow visualization of the infarct core. This procedure allowed us to microdissect the periinfarcted area of cortex and the corresponding cortex area of contralateral healthy hemisphere that were then stored at –70°C until use.
RNA isolation
Total RNA was isolated from the microdissected tissue using TRIzol reagent (Invitrogen life technologies, Germany) as described by the manufacturer, followed by DNase 1 (Ambion, Kaufungen, Germany) digestion and further purified using RNeasy Mini extraction kit (Qiagen, Hilden, Germany). To avoid RNA degradation because of secondary hypoxia due to the microdissection procedure, we flash-froze the tissue immediately after microdissection. Purified total RNA was used for cDNA array assay and real time PCR quantification.
cDNA array assay
To analyse gene regulation, we employed commercially available defined oligo microarrays containing known rat genes arranged in three categories: stem cells, hypoxia signalling pathway and apoptosis cDNA microarrays. These arrays contain 258, 96 and 96 known genes respectively (SuperArray, Bethesda, MD) and were processed according to the manufacturer's instructions. In addition, each individual array contains four housekeeping genes and one negative control. Housekeeping genes were included to confirm the integrity of RNA and correct loading of different samples. The list of genes can be found at http://www.superarray.com/ArrayList.php
Briefly, 3 μg of total RNA was annealed with a random primer at 70°C for 3 min., reverse transcribed at 37°C for 25 min. and amplified by linear polymerase reaction (LPR kit, SuperArray, Frederick, MD, USA) with gene-specific primers in the presence of Biotin-UTP to produce labelled cRNA. The PCR program was 85°C for 5 min.; 30 cycles (85°C, 1 min.; 50°C, 1 min.; 72°C, 1 min.); 72°C for 5 min. After 1 hr pre-hybridization, membranes were hybridized with denatured biotin-labelled cRNA overnight, washed, incubated with streptavidin-alkaline phosphatase conjugate and exposed to X-ray film. The scanned images were processed using web-based GEArray Expression Analysis Suite software (Super Array), data were extracted and gene expression profiles were analysed.
After normalization to housekeeping genes, two sets of data for each age group, given as fold change were generated: that is we compared ipsilateral (periinfarct, pi) versus ipsilateral sham controls (ctrl) and contralat-eral (cl) versus contralateral sham controls. Finally, the results are given as the mean of two experiments. Only those genes whose expression is equal to or more than 1.5-fold change were considered as differentially regulated. For down-regulated genes, the threshold was set to 0.5.
Real time quantitative PCR
For real-time PCR, 2 μg of total RNA was reverse-transcribed using random hexamers and the reverse transcription reagents (Superarray, Bethesda, MD). PCR reaction was set up by mixing 10 ng of cDNA, rat primers (Superarray), Master mix (Superarray) and SYBR Green I (Molecular Probes). Real-time PCR amplification was performed as follows: one cycle of 15 min. at 95°C and 45 cycles in three steps each (95°C for 30 sec., 55°C for 30 sec. and 72°C for 30 sec.) using a realtime PCR cycler (SDS 7700, Applied Biosystems, Darmstadt, Germany). At the end of amplification cycles, primer specificity was checked by appearance of single bands of PCR products in a 1% agarose gel. A standard curve was generated by plotting the log10 [target dilution] of template on the X-axis against the Ct value from serial dilutions of target DNA on the Y-axis.
The relative expression level of genes of interest and housekeeping gene was determined based on the standard curve equation generated for each individual gene. To normalize, the expression level of gene of interest was divided by the expression level of the housekeeping gene from the same sample. Finally, the fold change of an individual gene was calculated by dividing the normalized gene expression level of this gene from experimental sample by the normalized gene expression level of this gene from sham control both for the ipsilateral and the contralateral sides. That is, fold change = ([gene of interest/housekeeping gene]experiment]/[gene of interest/housekeeping gene]sham). In addition, we compared baseline gene expression by calculating the ratio of contralateral sham young versus contralateral old rats. The sequences of primers used for RT-PCR are given in Table 1s (supplemental data).
Table 1.
Ratio (± SD) of baseline gene expression levels in old versus young sham control rats
| Gene | Category | Ratio (old versus young) |
|---|---|---|
| Casp 7 | Terminal phase of apoptosis | 2.08 ± 0.13 |
| Cat | Antioxidant, ROS scavenging | 0.42 ± 0.01 |
| Sod2 | Antioxidant, ROS scavenging | 0.51 ± 0.02 |
| Fabp7 | Fatty acid-binding protein 7; lipid metabolism. | 2.09 ± 0.28 |
| Inhibin-β | Growth factor, also known as Activin β | 0.43 ± 0.05 |
| Igf1r | Insulin-like growth factor receptor | 0.27 ± 0.01 |
| Cdh5 | Vascular endothelial cadherin | 1.93 ± 0.08 |
| Icam5 | Intercellular adhesion molecule 5 | 1.82 ± 0.07 |
| Gjb1 | Gap junction membrane channel protein β 1 | 0.36 ± 0.04 |
| Nkx2.2 | Transcription factor; oligodendrocytes | 0.39 ± 0.04 |
The calculated ratio is based on RT-PCR results and is given as mean ± SD (n= 7).
Statistical significance level was set at P < 0.05.
Results
Basal transcriptional activities in the cortex of sham-operated young and aged animals
Since baseline differences between gene expression in young and old control rats might affect levels found after infarction, we first summarize the principal findings in control animals.
We studied a total of 442 genes representing growth factors, growth and differentiation-related transcription factors (258 genes), hypoxia signalling pathway (96 genes) and apoptosis arrays (96 genes).
In control animals, the levels of the apoptotic gene Casp7 are increased in the sensorimotor cortex of aged rats (Table 1). Since Casp7 is implicated in the terminal stages of apoptosis, this result suggests that apoptosis is increased in the brains of aged rats. A similar observation has been made in the cortices of aged Fischer 344 rats [17, 18].
Changes in the mRNAs levels for two major enzymes responsible for reactive oxygen species (ROS) scavenging, catalase (CAT) and superoxide dismutase (SOD) were significantly decreased in the brains of aged rats (Table 1), which is indicative of a reduced capacity to remove radicals from the aging brain.
Fatty acid-binding protein 7 mRNA, which is up-regulated following injury to the axons, [19] was also increased in the aged rat brain (Table 1) suggesting damaged myelin sheets in aged rats [20].
Differentially regulated genes in the post-ischaemic rat brain
We found sixty-one genes (13.8%) that were differentially regulated in the post-ischaemic rat brain (Fig. 1). Of these, 28 genes (6.1% of the total number of genes) were up-regulated. Within the up-regulated genes, 11 genes were increased in both age groups, while nine were up-regulated only in the young rats and eight only in the old. Twenty genes representing 4.5% of the regulated genes were down-regulated. Thirteen genes showed both up- and down-regulation in the two age groups (Fig. 1).
Fig. 1.
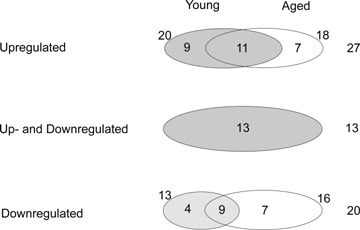
Diagram shows the number of up- and down-regulated genes in young and aged rat brain. The genes showing both up- and down-regulation during their time course are included in a separate circle.
The cumulative number of genes that were up- or down-regulated 1.5-fold for each time-point is shown for the ipsilateral (periinfarct, pi) sensorimotor cortex in Fig. 2A and the contralat-eral (cl) sensorimotor cortex in Fig. 2B. From these data, it can be inferred that at day 3 after stroke the major age-specific transcriptional effects in the periinfarcted area were (i) differential regulation (both up- and down-regulation) of apoptotic genes and (ii) a 50% decrease in the number of regulated stem cell-related genes (Fig. 2A). At day 3 after ischaemia, the contralateral, healthy hemisphere of young rats is much more active, at transcriptional level, than that of the aged rats, especially at the level of stem cell-coding genes. At this time-point, age-specific transcriptional events were (i) absence of regulation of hypoxia signalling-related genes and (ii) down-regulation of apoptosis-related genes (Fig. 2B). At day 14 after stroke we noted a persistent down-regulation of stem cell-related genes in the contralateral (healthy) sensorimotor cortex of aged rats (Fig. 2B).
Fig. 2.
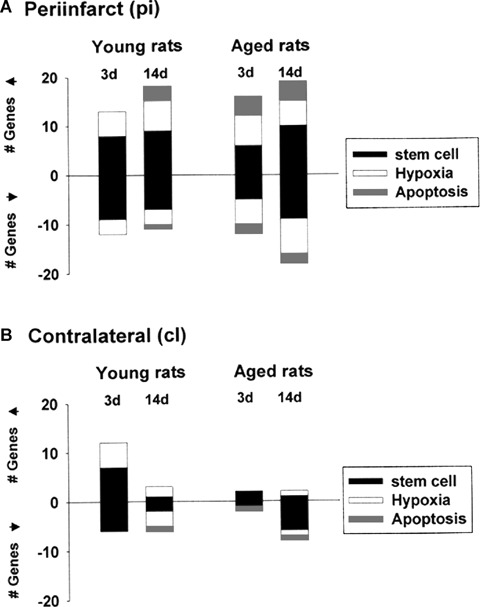
Time course of changes in the numbers of up- and down-regulated genes in post ischaemic brain. The numbers of genes up- and down-regulated more than 1.5-fold in the periinfarct (A) or contralateral area (B) at two time-points of young or aged rats were presented in the form of bar graphs. Stem cell, hypoxia signalling pathway and apoptosis-related genes are presented as black, empty and grey boxes, respectively.
Up-regulated genes in the ipsilateral sensorimotor cortex of young rats
Temporary occlusion of the middle cerebral artery caused in the first week after stroke the up-regulation of several hypoxia-related genes in the perilesional cortex of young rats (Table 2). Among them we noted vigorous increases in glutathione peroxi-dase (Gpx1), a gene necessary for antioxidative defence [21]. Genes that were moderately up-regulated in response to energy deprivation included the uncoupling protein 2 (Ucp2), a gene with a key role in antioxidative defence by regulating mitochondrial gluthatione availability [22]. The extent of neuroprotection was also moderately helped by the up-regulation of cystatin B (cstb), a non-caspase cysteine protease inhibitor shown to protect neurons against apoptotic death [23]. In the second week after stroke, the expression of Gpx1 and Ucp2 genes remained at high levels. In addition, the antioxidative capacity was further increased by up-regulation of gluthathione peroxidase 1, CAT and Sssca1 (a gene coding for Sjogren's syndrome/scleroderma autoantigen) [24].
Table 2.
Hypoxia signalling pathway-related gene expression profiles
| Gene name | Genbank Accession no. | Description | Fold change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3-months-old rat | 18-months-old rat | |||||||||
| Day 3 | Day 14 | Day 3 | Day 14 | |||||||
| pi/ctrl | cl/ctrl | pi/ctrl | cl/ctrl | pi/ctrl | cl/ctrl | pi/ctrl | cl/ctrl | |||
| Hypoxia related gene | ||||||||||
| Col1a1 | NM_007742 | Pro-collagen, type I, α 1 | 3.69 | 7.02 | 4.44 | 17.18 | ||||
| cstb | NM_007793 | Cystatin .B | 1.52 | 1.52 | 1.56 | |||||
| Gpx1* | NM_008160 | Glutathione peroxidase 1 | 5.38 | 5.18 | 3.07 | 3.01 | ||||
| Mmp14 | NM_008608 | Matrix metallopeptidase 14 (membrane-inserted) | 1.55 | |||||||
| Ucp2* | NM_011671 | Uncoupling protein 2 (mitochondrial, proton carrier) | 2.20 | 2.20 | 3.86 | 3.86 | 4.39 | |||
| Rps2 | NM_008503 | Ribosomal protein S2 | 2.43 | 0.65 | 2.47 | 1.93 | ||||
| Sod2* | NM_013671 | Superoxide dismutase 2, mitochondrial | 1.63 | 0.66 | 0.38 | 0.63 | ||||
| Cat* | NM_009804 | Catalase | 2.39 | |||||||
| Sssca1 | NM_020491 | Sjogren's syndrome/scleroderma Autoantigen 1 homologue (human) | 2.12 | |||||||
| Tgfb1 | NM_011577 | Transforming growth factor, β 1 | 2.33 | 2.33 | ||||||
| Pea15 | NM_011063 | Phosphoprotein enriched in astrocytes 15 | 1.57 | |||||||
| IL6 | NM_031168 | Interleukin 6 | 2.13 | |||||||
| Prpf40a | NM_018785 | PRP40 pre-mRNA processing factor 40 homologue A (yeast) | 2.25 | 2.65 | ||||||
| Chga* | NM_007693 | Chromogranin A | 0.44 | 0.39 | 0.33 | |||||
| Gap43* | NM_008083 | Growth associated protein 43 | 0.65 | 0.61 | ||||||
| Vegfa* | NM_009505 | Vascular endothelial growth factor A | 0.64 | 1.50 | 0.56 | |||||
| Bhlhb2 | NM_011498 | Basic helix-loop-helix domain containing, class B2 | 0.23 | |||||||
| Gpi1 | NM_008155 | Glucose phosphate isomerase 1 | 0.57 | 0.61 | 0.54 | 0.42 | ||||
| Npy | NM_023456 | Neuropeptide α | 0,30 | 0.50 | 0.39 | |||||
| Camk2g | NM_178597 | Calcium/calmodulin-dependent protein kinase II gamma | 0.41 | |||||||
| Plod3 | NM_011962 | Pro-collagen-lysine, 2-oxoglutarate 5-dioxygenase 3 | 0.41 | |||||||
| Tuba1* | NM_011653 | Tubulin, α 1 | 0.59 | |||||||
Fold change is calculated as the ratio of experiment to sham control after they were normalized to housekeeping genes obtained from two independent experiments. Genes whose average fold induction was equal to or higher than 1.5 and equal or lower than 0.66 were selected. Genes were listed according to up or down-regulation of genes and alphabetic order.
Represents genes were not previously reported in the after stroke rat brain. Genes which have been validated by RT-PCR were marked by colour. pi, periinfarct, lesional hemisphere; cl, contralateral, healthy hemisphere; ctrl, sham control.
Another gene change that may serve to promote tissue recovery is the up-regulation of transforming growth factor β1 (TGF-β1) in the perilesional area of young rats [25]. Tgfb1 is a pleiotropic cytokine with potent neurotrophic and immunosuppressive properties that is up-regulated after injury [26].
Genes related to apoptosis were not up-regulated at day 3 (Table 3). By day 14, however, the number of genes involved in apoptosis like ataxia telangiectasia-mutated homologue, Atm [27] and growth arrest and DNA damage-inducible 45α, (Gadd45a) [28] had increased in young rats.
Table 3.
Apoptosis-related gene expression profiles
| Gene name | Genbank Accession no. | Description | Fold change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3-months-old rat | 18-months-old rat | |||||||||
| Day 3 | Day 14 | Day 3 | Day 14 | |||||||
| pi/ctrl | cl/ctrl | pi/ctrl | cl/ctrl | pi/ctrl | cl/ctrl | pi/ctrl | cl/ctrl | |||
| Apoptosis-related gene | ||||||||||
| Atm | NM_007499 | Ataxia telangiectasia mutated homologue (human) | 2.64 | 1.97 | ||||||
| Gadd45* | NM_007836 | growth arrest and DNA-damage-inducible 45 α | 1.81 | 3.56 | 3.84 | |||||
| Hus1 | NM_008316 | Hus1 homologue (S. pombe) | 1.52 | 2.78 | 1.64 | |||||
| Mdm2 | NM_010786 | transformed mouse 3T3 cell double minute 2 | 2.67 | |||||||
| Tnfrsf7 | NM_001033126 | Tumour necrosis factor receptor superfamily, member 7 | 4.60 | |||||||
| Casp7* | NM_007611 | Caspase 7 | 0.49 | 0,22 | 3.34 | |||||
| Traf1 | NM_009421 | TNF receptor-associated factor 1 | 0.29 | 0,39 | 0.49 | 0.43 | ||||
| Traf4 | NM_009423 | TNF receptor-associated factor 4 | 0.50 | |||||||
| Trp53 | NM_011640 | Transformation-related protein 53 | 0.62 | |||||||
Fold change is calculated as the ratio of experiment to sham control after they were normalized to housekeeping genes obtained from two independent experiments. Genes whose average fold induction was equal to or higher than 1.5 and equal or lower than 0.66 were selected. Genes were listed according to up- or down-regulation of genes and alphabetic order.
Represents genes were not previously reported in the after stroke rat brain. Genes that have been validated by RT-PCR were marked by colour. pi, periinfarct, lesional hemisphere; cl, contralateral, healthy hemisphere; ctrl, sham control.
In the first week after stroke, major transcriptional events also included up-regulation of genes related to neuronal and oligodendrocyte survival, neuronal injury, axonal myelination and neurogenesis. Those changes related to survival include up-regulation of fibroblast growth factor 22 (Fgf22) [29], nerve growth factor β (Ngfb) [30] and insulin-like growth factor receptor (Igfr1) [31]. Related to neuronal injury is also fatty acid-binding protein 7 (Fabp7) [19]. Changes related to myelination include oligodendrocyte transcription factor 1 (Oligo1)[31], NK2 transcription factor related locus 2 Drosophila (Nkx2–2_predicted) [32] and gap junction membrane channel protein β 1(Gjb1). Two up-regulated factors related to neurogenesis are Frizzled homologue 8 (Fzd8) [33] and Gata-binding protein (Gata2) [34] (Table 4).
Table 4.
Stem cell-related gene expression profiles
| Gene name | Genbank Accession no. | Description | Fold change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3-months-old rat | 18-months-old rat | |||||||||
| Day 3 | Day 14 | Day 3 | Day 14 | |||||||
| pi/ctrl | cl/ctrl | pi/ctrl | cl/ctrl | pi/ctrl | cl/ctrl | pi/ctrl | cl/ctrl | |||
| Stem cell related genes | ||||||||||
| Fabp7* | NM_021272 | fatty acid-binding protein 7, brain | 3.40 | 4.17 | 7.48 | 8.07 | ||||
| Fgf22 | NM_023304 | Fibroblast growth factor 22 | 2.28 | 2.15 | ||||||
| Fzd8 | NM_008058 | frizzled homologue 8 (Drosophila) | 4,61 | 2.37 | 0.61 | |||||
| Gata2 | NM_008090 | Gata-binding protein 2 | 2.17 | 0.36 | 0.44 | 0.40 | ||||
| Igf1r* | NM_010513 | Insulin-like growth factor 1 receptor | 2.70 | 0.56 | 0.62 | |||||
| Ngfb | NM_013609 | Nerve growth factor, β | 2.09 | 2.03 | 2.00 | |||||
| Nkx2–2* | NM_010919 | NK2 transcription factor related, locus 2 (Drosophila) | 1.59 | 2.98 | 2.82 | |||||
| Oligo1 | NM_016968 | oligodendrocyte transcription factor 1 | 2.38 | 1.65 | 1.81 | 1.86 | 1.51 | |||
| Gjb1* | NM_008124 | Gap junction membrane channel protein β 1 | 2.35 | 3.12 | 0.38 | |||||
| Ptch1 | NM_008957 | Patched homologue 1 | 1.81 | 0.36 | ||||||
| Cst3 | NM_009976 | cystatin C | 3.11 | 5.27 | 1.67 | |||||
| Gcm2 | NM_008104 | Glial cells missing homologue 2 (Drosophila) | 2.04 | 2.21 | ||||||
| Igf2 | NM_010514 | insulin-like growth factor 2 | 7.38 | 14.61 | ||||||
| Cdh5* | NM_009868 | Cadherin 5 | 1.59 | 2.30 | ||||||
| Ptprc | NM_011210 | protein tyrosine phosphatase, receptor type, C | 3.67 | 5.73 | ||||||
| Ptges3 | NM_019766 | prostaglandin E synthase 3 (cytosolic) | 2.60 | 1.56 | 2.09 | 0.66 | ||||
| Tgfbr1 | NM_009370 | transforming growth factor, β receptor I | 7.41 | 6.78 | ||||||
| Cdkn1b | NM_009875 | Cyclin-dependent kinase inhibitor 1B | 3.97 | |||||||
| Bmpr2* | NM_007561 | Bone morphogenetic protein receptor, type 2 | 0.51 | 0.60 | ||||||
| Ctnna2 | NM_009819 | Catenin, α 2 | 0.41 | 0.1 | 0.17 | 0.35 | ||||
| Ctnnd2 | NM_008729 | Catenin, delta 2 | 0.50 | 0.33 | 0.27 | 0.26 | 0.57 | 0.17 | ||
| Ctnnd2 | NM_008729 | Catenin, delta 2 | 0.50 | 0.33 | 0.27 | 0.26 | 0.57 | 0.17 | ||
| Fgfr1* | NM_010206 | Fibroblast growth factor receptor 1 | 0.34 | 0.57 | 1.77 | |||||
| Icam5* | NM_008319 | Intercellular adhesion molecular 5, telecephalin | 0.43 | 0.66 | 1.55 | |||||
| Inhbb* | NM_008381 | Inhibin β-B | 0.58 | 2.30 | 0.65 | 0.60 | 0.53 | |||
| Itgb5* | NM_010580 | Integrin β 5 | 0.40 | |||||||
| Myh6 | NM_010856 | Myosin, heavy polypeptide 6, cardiac muscle, α | 0.66 | 0.29 | 0.30 | |||||
| Nefl | NM_010910 | neurofilament, light polypeptide | 0.30 | 0.54 | 0.66 | 0.41 | ||||
| Shh | NM_009170 | Sonic hedgehog | 0.22 | 0.22 | 0.22 | 0.22 | 0.51 | |||
| Foxg1 | NM_008241 | Forkhead box G1 | 0.49 | 0.62 | ||||||
| Mtap2 | XM_894407 | Microtubule-associated protein 2 | 0.64 | |||||||
Fold change is calculated as the ratio of experiment to sham control after they were normalized to housekeeping genes obtained from two independent experiments. Genes whose average fold induction was equal to or higher than 1.5 and equal or lower than 0.66 were selected. Genes were listed according to up- or down-regulation of genes and alphabetic order.
Represents genes were not previously reported in the after stroke rat brain. Genes that have been validated by RT-PCR were marked by colour. pi, periinfarct, lesional hemisphere; cl, contralateral, healthy hemisphere; ctrl, sham control.
We also noted high levels of mRNA coding for pro-collagen type I, α (Col1a1), a gene associated with vascular remodelling [35] by providing a substrate for neurite outgrowth, respectively [36]. Likewise, the matrix metalloproteinase 14 gene (Mmp14), which is critical to creating a permissive growth environment for neurites [36], was moderately increased in the infarcted area (Table 2).
In the second week following stroke, several genes returned to control values (Fgf22, Fzd8, Gata2 and Igfr1) while several other new genes were up-regulated. These include cystatin C (Cst3), a gene that has been linked to glial development [37] and glial cells missing homologue (Gcm2), a transcription factor that is involved in specification and differentiation of certain neuronal and glial lineages [38, 39]. Also up-regulated in the second week were the neuronal survival factors like insulin-like growth factor 2 (Igf2) [40] and inhibin β B (Inhbb) [41] (Table 4).
Finally, the gene coding for cadherin 5 (Cdh5), was increased between day 3 and day 14 after stroke. Since Cdh5 has cell-adhesion activity and is specifically expressed in vascular endothelial cells, this finding suggests that the blood-brain barrier of aged rats is still compromised (Table 4).
Dow-regulated genes in the ipsilateral sensorimotor cortex of young rats
In the first week after stroke, major transcriptional events included down-regulation of genes involved in neurogenesis, axonal growth and maturation, dendritic injury, axonal migration and growth, and in cell adhesion. For example, neural cell precursor proliferation sonic hedgehog homologue (Shh) [42–44] was down-regulated. Genes involved in axonal growth and maturation that were also down-regulated include neurofilament light chain (Nefl) [45, 46], chromogranin A [47] and growth-associated protein GAP-43 [48]. Bone morphogenetic protein receptor type 2 (Bmpr2) [49], which is involved in dendritic injury and dendritogenesis also showed decreased activity. Also down-regulated were α and δ catenins (Ctnna2 and Ctnnd2)[50], necessary for axonal migration, intercellular adhesion molecule 5 (telencephalin) [51] and integrin β 5, a vitronectin receptor [52] (Table 4).
In the second week after stroke, down-regulated genes included fibroblasts growth factor receptor 1 (Fgfr1), a gene implicated in axonal growth [53], and basic helix-loop-helix domain containing class B2 (Bhlhb2), a gene that may play a role in regulating neuronal differentiation during development and adaptive neuronal plasticity and neurite outgrowth in the adult [54] (Table 4). Glucose phosphate isomerase (Gpi) was also decreased. Gpi is a housekeeping gene encoding for phosphoglucose isomerase that has been found to be identical to neuroleukin, which has neurotrophic and lymphokine properties [55]. Neuropeptide Y (Npy) was also decreased at this time-point. Npy is a gene involved in regulation of energy balance (Table 2), and which forms part of the ‘lipostat’ system along with leptin and corticotropin-releasing hormone.
In addition, genes implicated in axonal growth/migration like Shh [43] and catenin α 2 and catenin δ 2 remained at low levels at all time-points studied. Finally, genes important for synaptic transmission such as myosin heavy polypeptide 6 (Myh6) [56] was down-regulated at all time-points studied (Table 4).
Young rats, contralateral sensorimotor (undamaged) cortex
In young rats 3 days after stroke, 12 genes (2.7% of the total) were up-regulated and six (1.3%) were down-regulated within the contralateral sensorimotor cortex. By day 14, the number of up-regulated genes decreased from 12 to three (0.7%), while the number of down-regulated genes increased (Fig. 2B).
Among the genes up-regulated at 3 days were several related to oligodendrocytes regeneration, re-myelination and communication, including Oligo1, NK2 transcription factor related locus 2 Drosophila (Nkx2–2_predicted) and gap junction membrane channel protein β 1(Gjb1; see Table 4). In the second week, a gene coding for a phosphoprotein enriched in astrocytes (PEA-15) was up-regulated. PEA-15 is a 15 kD acidic serine-phosphorylated protein which is expressed in different cell types, especially in the central nervous system (Table 2). Recent data indicates that PEA-15 has an anti-apoptotic role, presumably within astrocytes [57].
Genes down-regulated in the first week in the contralateral hemisphere of the young rats included intercellular adhesion molecule5 (telencephalin) (Icam5) and Nefl. The first of these is involved in neuritic outgrowth [58] while the second is required for axonal maturation [59]. Myh6 [56], a gene important for synaptic transmission, was also decreased in this region in the first week (Table 4).
In the second week after stroke, calcium-/calmodulin-dependent protein kinase II (Camk2g) and Gpi were both down-regulated. Camk2g is important for dendritogenesis [60], and the Gpi-coding product has neurotrophic properties [55] (Table 2).
Up-regulated genes in the ipsilateral sensorimotor cortex of aged rats
Temporary occlusion of the middle cerebral artery caused the up-regulation of several hypoxia-related genes in the perilesional cortex of aged rats (Table 2). Unique to aged rats was the up-regulation of ribosomal protein 2 (Rps2), which under normal cellular conditions is a substrate for PRMT3 (protein arginine methyltransferase 3) that catalyse the formation of asymmetric dimethylarginine [61]. A likely mechanism for this is that Rps2 is synthesized by reactive astrocytes that are present in greater number in the perilesional area of aged rats [4].
Gpx1, a gene necessary for antioxidative defence, was up-regulated in old rats, but at a lower level than observed in young rats. Further, tissue protection was achieved by a strong up-regulation of the Ucp2, a gene with a key role in energy availability and antioxidative defence by regulating mitochondrial gluthatione availability. Cystatin B (cstb) was also up-regulated, although less strongly than Ucp2. Cstb is a non-caspase cysteine protease inhibitor shown to protect neurons against apoptotic death.
In the second week after stroke, the expression of Gpx1 and Ucp2 genes remained at high levels in the contralateral hemisphere of old rats. However, in contrast to the young rats, the antioxidative capacity was not supplemented by increases in CAT and SOD gene expression. Furthermore, tissue recovery was not supported by increases in Tgfb1, a cytokine whose expression was associated with increased neuroprotection by suppressing inflammation, promotes neurogenesis and prevents apoptosis (Table 2).
In contrast to the young rats at day 3, aged rats exhibited strong up-regulation of genes involved in DNA damage, cell cycle arrest and apoptosis (Table 3). In particular, aged rats rapidly up-regulated genes such as growth arrest and DNA-damaged inducible 45 α (Gadd45α), telangiectasis mutated homologue (human) (Atm_mapped), Hus1 homologue (S. pombe) (Hus1_predicted), transformed mouse 3T3 cell double minute 2 (Mdm2), caspase 7 and tumour necrosis factor receptor superfamily member 7 (Tnfrsf7, also called CD27). These genes are required for DNA damage-induced apoptosis. These changes are probably related to the simultaneous decrease of anti-apoptotic genes expression such as Traf1, Traf 4 and Trp53 [62, 63] in the same region. Caspase 7, an additional pro-apoptotic gene, was also strongly activated in the second week after stroke.
In addition, inflammatory- and stroke-related genes, prostaglandin E synthase 3 (Ptges3) [64], protein tyrosine phosphatase, receptor type C (Ptprc) also known as leukocyte-common antigen (LCA) or CD45 [65] (Table 4) and interleukin 6 (Il6) [66] were increased in the aged, but not the young, perilesional cortex (Tables 2 and 4). In particular, increased expression of Ptprc and Ptges3 genes persisted in the second week after stroke (Table 4). Closely related to increased inflammation, there is a robust overexpression of the gene coding for transforming growth factor receptor type I (TGFR1) thought to be implicated in fibrosis and therefore, scar formation [67] (Table 4).
Aged rats still have the capability to mount a robust cytoproliferative response to cerebral ischaemia [68]. Similar to the young rats, the aged rats displayed vigorous increases in pro-collagen type I, α I (Col1a1), a gene associated with hypoxia-induced vascular remodelling and neurite outgrowth. Interestingly, the levels of Col1a1 mRNA vastly exceeded those of young rats in the second week after stroke (Table 2).
Down-regulated genes in the ipsilateral sensorimotor cortex of aged rats
In the first week after stroke, several genes with a role in axonal outgrowth and neurogenesis were down-regulated in the damaged hemisphere of old rats. These included Shh, an axonal chemoattractant [42–44] and Fgfr1, an axonal growth factor [53]. In contrast, Fgfr1 was not down-regulated at 3 days after stroke (Table 4). Also, down-regulated was the gene encoding chromogranin A (Chga) which is required for neurogenesis [47] (Table 2). RNA-splicing factor Prp40, a negative regulator of Notch signalling, was up-regulated in old, but not young rats [69]. Because the Notch pathway is a versatile regulator of axonal growth [70], this finding suggests that axonal and dendritic growth in aged rats is severely restricted. Other genes that were down-regulated include growth-associated protein 43, tubulin α1 (Tub1a), Bmpr2 and microtubule-associated protein 2 (Mtap2). GAP43 is required for neurogenesis, axonal growth and maturation [71]. Bmpr2 is involved in dendritic injury and dendritogenesis [49]. Mtap2 and Nefl genes are required for axonal maturation (Table 4). Genes coding for neuronal survival factors, such as Inhbb, were also down-regulated in the second week after stroke, a decisive time-point for successful regeneration [71] (Table 4). Along this line, pro-collagen-lysine, 2-oxoglutarate 5-dioxygenase 3 (Plod3; synonym, LH3) that is critical for growth cone migration [72], and Gpi were down-regulated at all time-points studied [41]. Neurogenesis was further impaired by down-regulation of Tuba1 that in necessary for proper neuronal migration [73] and down-regulation of genes necessary for axonal migration, such as α and δ catenins [50] and cell adhesion like intercellular adhesion molecule 5 (Icam5, also called telencephalin) [58] (Table 4).
In the second week after stroke, down-regulated genes included Fgfr1 that was implicated in axonal growth [53]. In addition, genes implicated in neurogensis and axonal growth/migration like Shh [43] and catenin α 2 and catenin β 2 (Table 4) and Npy(Table 2), a gene involved in regulation of energy balance, were kept at low levels at all time-points studied. Surprisingly, in contrast to the young rats, the gene encoding Myh6, and which is important for synaptic transmission [56], was not down-regulated after stroke in the brains of aged rats.
Regulated genes in the contralateral (undamaged) sensorimotor cortex of aged rats
Generally, the healthy contralateral sensorimotor cortex of the aged rats was transcriptionally less active than that of the young rats, especially in the first week after stroke. Aged rats had six-fold fewer up-regulated genes than young rats at all time-points studied. However, the number of down-regulated genes in aged rats in the second week after stroke was 6-fold greater than the number down-regulated in first week (Fig. 2B). The most prominent change in aged rats was a robust increase in the expression of the anti-proliferation gene, cyclin-dependent kinase inhibitor 1B (Cdkn1b; synonyms: p27, p27Kip1) (Table 4), which inhibits the activity of cyclin-CDK complexes and plays a central role in neuronal migration, that is Cdkn1b up-regulation would impair neurogenesis [74]. RNA-splicing factor Prp40, a negative regulator of notch signalling [69], was also up-regulated. The Notch pathway is a versatile regulator of cell fate specification, growth, differentiation and patterning processes in metazoan organisms. In particular, in the developing nervous system the Notch signalling pathway specify axonal growth cones [70]. Several changes in gene regulation were observed that could enhance the Notch pathway, including down-regulation of genes required for neurogenesis and axonal growth/migration (Ctnnd2, Foxg1, Ptges3, Gata2, Fzd8, Sod2, Traf1, Shh) and of one gene required for neuronal survival factors (Inhbb).
Verification of microarray data by real-time quantitative PCR
To confirm the microarray data, real-time quantitative PCR was performed. We randomly selected Bmpr2, brain fatty acid-binding protein, Cdh5, fibroblast growth factor 1 receptor, inhibin β, insulin-like growth factor 1 receptor, integrin β 5 and intercellular adhesion molecular 5 from the stem cell-related cDNA array (Fig. 3). We also selected Chga, Ucp2, Gpx1, SOD2, CAT, growth-associated protein 43, tubulin (1, vascular endothelial growth factor A from hypoxia signalling pathway (Fig. 4). In addition, we chose caspase 7 and Gadd45a from the apoptosis array (Fig. 5). The expression pattern of these genes from real-time PCR matched well with the expression profile obtained by microarray analysis.
Fig. 3.
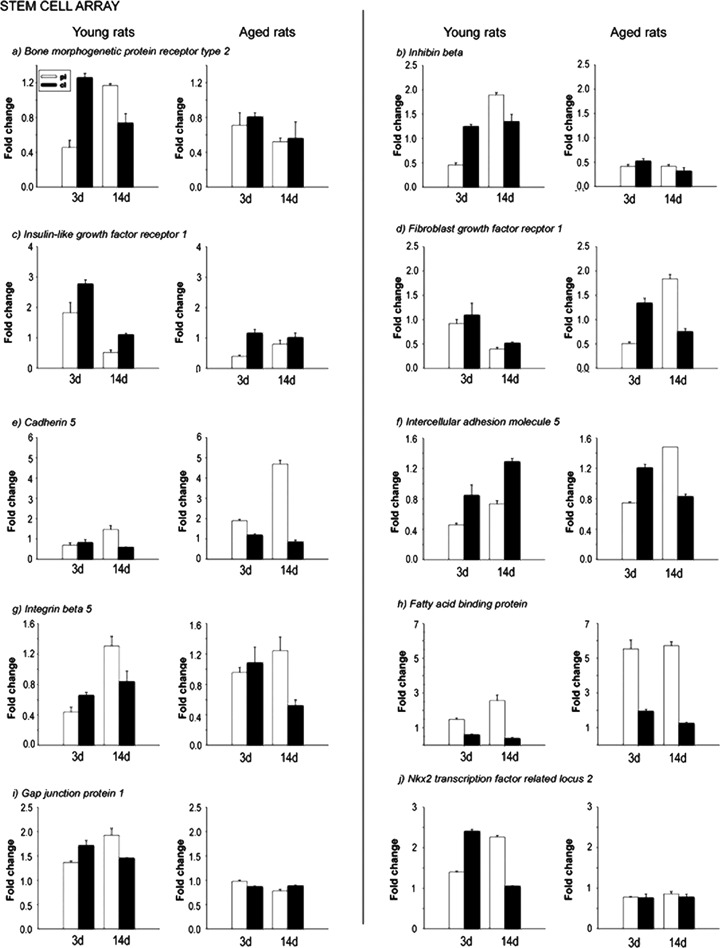
Validation of altered stem cell-related gene expression by realtime quantitative PCR. Selected genes identified from the macroarray analysis were further confirmed by real-time PCR. The expression profiles are presented in the form of column graphs. The numbers on the vertical axis represent the average fold change. The horizontal axis denotes the post stroke time-points. pi, periinfart; cl, contralateral; 3d, after stroke day 3; 14d, after stroke day 14.
Fig. 4.
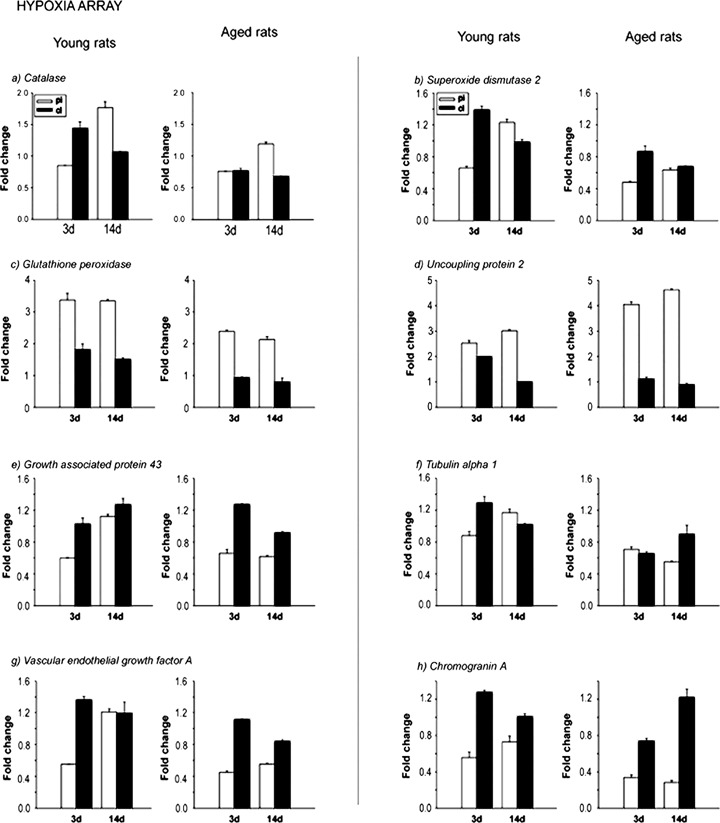
Validation of altered hypoxia signalling pathway-related gene expression by real-time quantitative PCR. Selected genes identified from the macroarray analysis were further confirmed by real-time PCR. The expression profiles are presented in the form of column graphs. The numbers on the vertical axis represent the average fold change. The horizontal axis denotes the post stroke time-points. pi, periinfart; cl, contralateral; 3d, after stroke day 3; 14d, after stroke day 14.
Fig. 5.
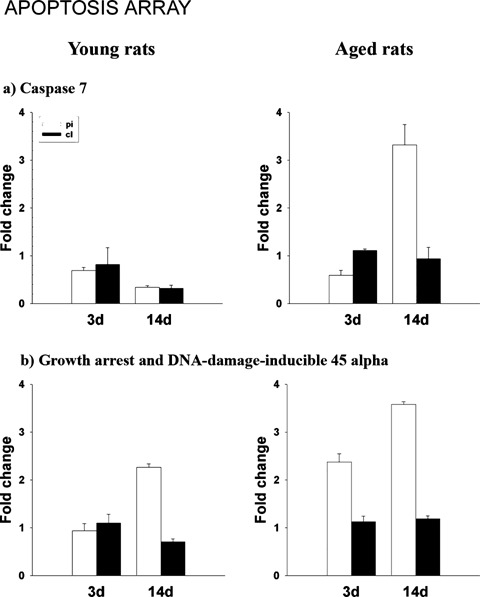
Validation of altered apoptosis-related gene expression by realtime quantitative PCR. The selected genes identified from the microarray analysis were further confirmed by real-time PCR. The expression profiles are presented in the form of column graphs. The numbers on the vertical axis represent the average fold change. The horizontal axis denotes the after stroke time-points. pi, periinfart; cl, contralateral; 3d, after stroke day 3; 14d, after stroke day 14.
Discussion
It has been hypothesized that the ipsilateral and the contralateral hemispheres work in synergy to promote functional recovery after stroke in young rats. Using categorized DNA arrays we found inappropriate gene regulation in response to stroke both in the ipsilateral and the contralateral hemisphere of aged rats. The gene expression profile in the brains of post-stroke aged rats is indicative of increased cell death due to DNA damage and apoptosis, especially in the first week after stroke. Similarly, we report persistent down-regulation of genes that are required for neurogenesis after stroke in aged rats.
Up-regulation for DNA damage and down-regulation of anti-apoptosis-related genes in the periinfarcted region of aged rats after stroke
We [4], and others [75–77] found that aged rats were more severely impaired by stroke and showed diminished functional recovery as compared with young rats. Therefore, in the following discussion we will assume that the time course of gene changes occurring in the brains of young rats represents the optimal response to stroke.
Early up-regulation of genes related to DNA damage, cell cycle arrest and apoptosis after cerebral ischaemia in aged rats
The first week after stroke is crucial for successful recovery in human stroke victims and in animal models of stroke. This period is associated with both immediate and late neuronal death that will determine the extent of overall cell loss in the hypoperfusion area (formerly designated the ‘penumbra’).
Our results indicate that one major genetic event that may lead to increased cellular death in the hypoperfusion area in aged rats is the rapid up-regulation of a group of genes related to DNA damage, cell cycle arrest and apoptosis. In the first 3 days after stroke there was a massive up-regulation of DNA damage-related genes like Gadd45a, Hus1, Mdm2 and Tnfrsf7 in the perilesional cortex of aged rats as compared to young at the same time-point. Cell death in aged rat brains was accelerated due to down-regulation of anti-apopotic genes, such as Traf1, Traf4 and Trp53. In contrast, young rats did not express DNA damage in the first 3 days after stroke. By day 14, the number of DNA damage-related genes in the perilesional cortex of young rats too increased to equal those of aged rats that were kept at high levels at all time-points (summarized in supplemental Fig. 1A).
It has been proposed that Mdm2 is an indicator of DNA damage in the brain early after an ischaemic insult in a similar way to Gadd45_ [78]. The role of Hus1 and Atm in the post stroke rat brain are not known. The DNA damage-induced chromatin binding has been shown to depend on the activation of the checkpoint kinase Atm, and is thought to be an early checkpoint signalling event [79].
Another dramatic change we observed was a 4-fold increase in the down-regulation of anti-apoptotic genes Traf 1, Traf4 and Trp53 in the lesioned area of post-ischaemic aged rats brains. In the contralateral hemisphere we noted the early increase in the expression of the anti-apoptotic gene Cstb in the brains of young as compared to aged rats.
Trp53 belongs to the tumour suppressor p53 superfamily. Mutations in this locus affect cell-cycle regulation and apoptosis. After brain trauma, Traf1 and Traf4 were polyubiquitinated in lipid rafts. Subsequently, the signalling complex contained activated caspase-8, thus initiating apoptosis [80].
Tnfrsf7 and caspase 7 (Casp 7) play an important role in mediating CD27-binding protein induced apoptosis [81]. At 14 days after stroke, both of these were strongly up-regulated in the perilesional area of aged but not young rats. Since Casp7 is already increased in control aged rat brains, we infer that ischaemia will exacerbate a death mechanism that is already operational in aged brains.
There was no change in the contralateral hemisphere of either age group in regulation of DNA damage or apoptosis-related genes. There was only one pro-apoptotic gene, Casp7, that was down-regulated in young rats and one down-regulated gene in the aged rats, Traf1 (summarized in supplemental Fig. 1B).
Genes associated with inflammation and scar formation are strongly up-regulated in aged rats
Ischaemia/reperfusion injury is associated with the proliferation and hypertrophy of astrocytes and with inflammatory responses. In aged rats there were in total five up-regulated inflammation-related genes in the peiinfarcted region (Ptprc, Ptges3, Tgfbr1, IL6, Rps2), while in the young rats there was only one up-regulated gene, Tgfb1.
Ptges3 may contribute to production of prostaglandin E2 from arachidonic acid. It was also found to be significantly increased in the rat brain after challenge with lipopolysaccharide (LPS) [64]. In the present study, increased levels of this inflammatory gene Ptges3 persisted into the 2nd week post-stroke. The inflammatory cytokine IL-6 is clearly implicated in the response to central nervous system (CNS) injury, but whether this response is neuroprotective or pathological is uncertain [82, 83].
Aged, but not young, rats exhibited up-regulation of Rps2, which under normal cellular conditions is a substrate for PRMT3 . A likely explanation of the up-regulation we observed is that Rsp2 is synthesized by reactive astrocytes [84] which are present in great number in the perilesional area of aged rats [4].
TGF-β1 was up-regulated only in young rats. Tgfb1 is a pleiotropic cytokine with potent neurotrophic and immunosuppressive properties that is up-regulated after injury. In vitro, Tgfb1 protects neurons against excitotoxicity by inhibiting t-PA-potentiated NMDA-induced neuronal death by up-regulation of type-1 plasminogen activator inhibitor (PAI-1) in astrocytes 4. In addition, Tgfb1 has been recently characterized as an antiapoptotic factor in a model of staurosporine-induced neuronal death [25]. It has recently been demonstrated that Tgfb1 suppresses inflammation and promotes survival in adult CNS after axonal injury [26] (as summarized in supplemental Fig. 2).
In general, genes influencing inflammatory processes were not significantly modified in the contralateral hemisphere of either young or aged rats.
Neuroprotection, including antioxidative defence and neuronal survival factors is diminished in the perilesional cortex of aged rats
Neuroprotection conferred by antioxidative defence
In the young rats the number of genes conferring neuroprotection in the damaged hemisphere exceeded by a factor of 2 those expressed in the aged rats at all time-points. The degree of neuroprotection in aged rats was further decreased by a 2-fold increase in the number of down-regulated genes coding for antioxidative defence and neuronal survival factors so that, in total, aged rats had a 4-fold decrease in the number of genes conferring neuroprotection (summarized in supplemental Fig. 3A).
In the contralateral hemisphere the effect on neuroprotective genes expression was even more damaging in aged rats, which did not express any protective gene, while young rats up-regulated seven such genes. In the second week after stroke there was no difference in the number of expressed gene numbers in the two age groups (summarized in supplemental Fig. 3B).
Oxidative damage may be caused by ROS, including superoxide [85, 86]. Counteracting oxidative stress through up-regulation of mitochondrial antioxidants is one of the cell survival mechanisms operating shortly after cerebral ischaemia. Failure to increase the expression of antioxidant systems may increase the sensitivity to oxidative stress [14, 87] and contribute to poor recovery after cerebral ischaemia.
In the first week after stroke, the antioxidative capacity in young rats was supported by up-regulation of Ucp2 (mitochondrial, proton carrier)(Ucp2) and Gpx1. For example, mice lacking Gpx1 (Gpx1−/− mice) which is indicative of reduced protection, have increased infarct volumes after stroke [88]. In the second week after stroke, the antioxidative capacity of young rats was further improved by up-regulation of CAT, which has been intensively studied as an antioxidant in Sjogren's syndrome/scleroderma pathology. The expression of Sssca1 gene may reflect the generation of faulty proteins by oxidation [24].
The antioxidative capacity of aged rats was reduced as compared to young rats. SOD2, mitochondrial (Sod2), another component of the antioxidant system, was down-regulated in the perinfarcted area of aged rats while Cat and Sssca1 were not up-regulated at all in aged animals.
Our finding of decreased levels of both CAT and SOD2 implies both a decline in the antioxidative capacity and synaptic plasticity with increasing age [89]. Taken together, these data suggest that the antioxidative system is not fully operational in aged rats (supplemental Fig. 3A and B, grey bars).
Neuroprotection due to increased expression of neuronal survival factors
Another age difference we detected was the more robust activation in young rats of genes coding for neuronal survival factors. Thus, in the first week after stroke, genes such as Fgf22, Ngfb, Igf1r1, Olig1 and Gjb1 were up-regulated in young, but not aged rats (as summarized in supplemental Fig. 3A, black bars).
Injury-related regulated genes have been implicated in numerous cellular processes, including proliferation, migration, differentiation and survival [90]. In particular, Fgf22 plays a significant role as a neuronal and oligodendrocyte survival factor [29]. Moreover, using clustering of synaptic vesicles in cultured neurons from mouse brain as an assay, FGF22 was identified as a major active organizing species [91]. In line with our results, Fgf22 was up-regulated in the skin of young but not aged mice in a model of skin injury [29].
Insulin-like growth factor-I (IGF-I) has been shown to be a potent agent in promoting the growth and differentiation of oligo-dendrocyte precursors, and in stimulating myelination during development and following injury [31]. Recent evidence shows that endogenous IGF-1 also plays a significant role in recovery from insults such as hypoxia-ischaemia and that giving additional exogenous IGF-1 can ameliorate damage.
During the second week, when recovery at tissue level is evident, young rats exhibited up-regulation of several neuronal survival factors including Ngfb, Igf2, inhibin β (Inhbb) (also called activin), Oligo1, Cyt3, Pea-15 and TGF-β.
There is evidence that each of these factors is related to neuronal survival or growth. For example, Ngfb is a neurotrophic factor that is critically involved in the development and maintenance of both the peripheral and central nervous system [92] and it is up-regulated in response to cerebral ischaemia [93].
Activin is a neuronal survival factor that is rapidly increased after transient cerebral ischaemia and hypoxia in mice [94]. Activin is a member of the TGFB family that is involved in cell differentiation, hormone secretion and regulation of neuron survival [95]. These findings suggest that endogenously activated autocrine loops of activin-Nodal signalling promote ES cell self-renewal [41]. Igf2, also called somatomedin A, is a neuronal survival factor that acts neuroprotectively if administered intracerebroventricularly after cerebral ischaemia [96].
CysC is an endogenous cysteine proteases inhibitor produced by mature astrocytes in the adult brain and is vigourously up-regulated in the ipsilateral hemisphere of both and young rats after stroke. Cystatin-B is expressed by neural stem cells and by differentiated neurons and astrocytes. Mice with a gene deletion of CSTB exhibit increased apoptosis of specific neurons [37, 97].
PEA-15 is a small protein (15 kD) that was first identified as an abundant phosphoprotein in brain astrocytes and subsequently shown to be widely expressed in different tissues and highly conserved among mammals [98].
Neuroprotection is completely absent in the contralateral hemisphere of aged rats after stroke
A major difference between young and aged rats was seen in the number of neuroprotective genes that were up-regulated in the contralateral hemisphere of young versus aged rats. There were in total seven up-regulated such genes in the contralateral hemisphere of young rats, two coding for antioxidative defence (Ucp2 and Sod2) and five coding for growth and survival factors (Fgf22, Ngfb, Oligo1, Gjb1 and Vegfa) (summarized in supplemental Fig. 3B).
Neurogenesis is not fully supported in the periinfarcted area, especially in aged
We found that neurogenesis is not fully supported in the periinfarcted area of both age groups and especially in the aged rats (supplemental Fig. 4A and B).
Both age groups expressed high levels of Fabp7 mRNA in the perilesional area during the 2-week study period. We found that the extent of axonal damage, as indicated by higher levels of expression for Fabp7 mRNA in the perilesional area, is greater in aged rats than in young rats. Since the basal levels of Fabp7 mRNA were also increased in the control aging brain, we hypothesize that the increased levels in Fabp7 transcript in the aged rats brain after injury are in part due to age-associated increases in Fabp7 mRNA levels. In the normal aging brain, myelin sheaths show signs of breakdown [99] and the turnover rates of cerebroside and GM1 increased in senescence. The latter phenomenon may indicate an enhanced myelin turnover in senescence [100]. Previous work also indicated age-related increases in Fabps [101] and implicated Fabp7 as a potential marker of axonal injury after cerebral infarction and, generally, of brain damage [101, 102, 19].
Specific to the aged rats was the up-regulation of myelin-injury specific gene, NK2 transcription factor related, locus 2 (Drosophila)(Nkx2–2). Up-regulation of this gene has been demonstrated in NG2-expressing oligodendrocyte precursor cells surrounding the hypoperfusion area after MCAO [32].
Myosin VI (Myo6) is a minus end-directed actin-based motor component found in neurons that express Trk receptors. It has been reported that Myo6 is necessary for brain-derived neurotrophic factor (BDNF)-TrkB-mediated facilitation of long-term potentiation and synaptic plasticity in postnatal day 12–13 hippocampus [56].
Cerebral ischaemia causes cellular death and dissolution of neuronal networks with subsequent activation of genes involved in cellular adhesion. Specific to the aged rats was the up-regulation genes with cell adhesion activity (Cdh5, Icam5) though the significance of down-regulation for tissue recovery is not presently understood.
After the infarct area is stabilized, repair mechanisms involving stem cells may become active, especially in the second week after stroke. In young rats, there were seven up-regulated genes with important roles in neurogenesis (Fzd8, Gata2, Gcm2, Oligo1, Gjb1, Colla1, Mmp14).
Wnt signalling plays critical biological roles during normal embryonic development and homeostasis in adults. In the canonical pathway, binding of Wnt ligands to the Fzd receptor and the low-density lipoprotein-related receptor (LRP) 5 or LRP6 co-receptor initiates downstream signalling events leading to gene activation by β-catenin and the T cell factor (TCF)-lymphoid enhancer factor (LEF) family transcription factor complex [33].
An essential role of Gata transcription factors in sympathetic neuron development has also been recently described [34].
Gcm2 is a transcription factor whose expression is restricted to post-embryonic stages and that is required for specification and differentiation of certain neuronal and glial lineages [38, 39].
We found a number of genes implicated in re-myelinization, such as Nkx2-2 and Olig1 that were up-regulated in the contralateral hemisphere of young rats at 3 days after ischaemia, but not in aged rats. Both Nkx2–2 and Olig1 are transcription factors that play an important role in the differentiation of oligodendrocyte progenitor cell (OPC) into re-myelinating oligodendrocytes, myelinogenesis and in axonal recognition [103, 104]. In this light, the aged rats are at disadvantage in myelin repair because, as we found, Nkx2–2 gene activity was substantially decreased even in intact aged rats as compared to their younger counterparts [106].
Pro-collagen, type I, α 1 (Col1a1) is a gene associated with hypoxia-induced vascular remodelling [35]. Very likely, neovasculogenesis and indirectly pro-collagen type I α are required for neurite outgrowth, respectively [36]. Surprisingly, in aged rats the levels of pro-collagen, type I, α 1 mRNA vastly exceeded those of young rats in the second week after stroke suggesting that neovasculogenesis is not impaired after stroke in aged rats. Since there is a reported down-regulation of several collagen genes (e.g. Col1a1 and Col3a1) in the aging lung from C57BL/6 mice [107], we infer that the middle-aged rat brain is still capable of robust up-regulation of genes expression upon injury.
The Mmp14 gene, which is critical to creating a permissive growth environment for neurites [36], was up-regulated to modest levels in the periinfarcted area of young rats only.
Down-regulation of axonal growth- and dendritogenesis-related gene expression in both age groups
Axonal re-growth after injuries to the CNS is central to successful functionality of the damaged area and is generally known to be impaired in higher vertebrates. Indeed, in the first week after stroke, no genes important for axonal growth were up-regulated in either age group (supplemental Fig. 4A and B). On the other hand, genes with axonal growth activity like Shh, patched homologue 1 (Ptch1), Fgfr1, catenin α 2 (Ctnna2), catenin δ 2 (Ctnnd2), growth-associated protein 43 (Gap43), α-tubulin (Tuba), forkhead box G1 (Foxg1) and Plod3, were down-regulated in the lesioned cortex of both young and aged rats.
The gene coding for hedgehog homologue (Shh) is a signalling pathway component that plays a role in neurite outgrowth and neurogenesis by regulating proliferation of adult neural stem cells [44]. Shh belongs to a family of signalling glycoproteins implicated in embryonic development. Shh displays inductive, proliferative, neurotrophic and neuroprotective activities on various neural cells and signals through a receptor complex associating Patched (Ptch1) and Smoothened (Smo) [94].
Specific to the aged rats was the down-regulation of several axonal growth and neurogenesis-specific genes including Gata2, Gjb1, Foxg1, Plod3, Mtap2 and Tuba1. Gata2 and Gata3 have been demonstrated, in the chick and mouse respectively, to be essential members of the transcription factor network controlling sympathetic neuron development [34].
Gjb1, a component of gap junctions, was strongly up-regulated at 3 days after ischaemia in young, but down-regulated in aged rats, suggesting poor cell–cell communication in the perilesional area in the brains of aged rats. Previous work showed that Gjb1, also known as Cx22, is expressed in oligodendrocytes and facilitated cell–cell communication [107].
Foxg1 is an evolutionarily conserved, winged-helix transcriptional factor that controls telencephalic neurogenesis, suggesting that Foxg1 controls precursor proliferation via regulation of Fgf signalling and differentiation via regulation of Bmp signalling.
Axonal growth cone migration is further impaired by down-regulation of genes that are important to establish appropriate migration cues such as the gene coding for Plod3. Plod3 has a role in matrix remodelling and modifies collagen IV residues to render them growth-permissive [72]. The down-regulation of these genes suggests that failure to grow axons may have many causes, each acting at different levels.
Other genes involved in neuritic outgrowth and differentiation were also down-regulated. These included basic helix-loop-helix domain containing, class B2 (Bhlhb2), Chga, Icam5 (telencephalin), synaptic transmission required gene, myosin and heavy polypeptide 6 (Myh6). Bhlhb2 is up-regulated in the hippocampus after transient forebrain ischaemia and may play a role in regulating neuronal differentiation during development and adaptive neuronal plasticity and neurite outgrowth in the adult [54, 108].
The Chga gene was down-regulated in the first week after stroke in young rats and at all time-points in the aged. Activation of Chga promoter in hippocampal progenitor cells led to neurite outgrowth, indicative of a role for Chga in neuronal differentiation [47]. Icam5 (telencephalin) is a dendrite-expressed membrane glycoprotein of telencephalic neurons in the mammalian brain. Additionally, the Icam5/actinin interaction is involved in neuritic outgrowth [58].
The growth and morphological differentiation of dendrites are critical events in the establishment of proper neuronal connectivity and neural function. The expression of Bmpr2 is required for BMP-dependent induction of the dendritic arbour in cortical neurons (Bmpr2) [49].
One possible cause of the absence of axonal growth at the lesion site is the opposite expression of genes which otherwise should be co-ordinated. For example, the Fzd8, which is robustly up-regulated in the ipsilateral hemisphere of young rats, does not co-ordinate with the gene expression for α N-catenin, a gene that is down-regulated at the same time-points.
Neurogenesis is impaired in the contralateral (unlesioned) hemisphere of both young and aged rats at all times after stroke
The contralateral hemisphere of young rats seems to support some neurogenesis by up-regulating several genes related to axonal injury (Nkx2-2), glial differentiation (Gcm2), embryonic development (Ptch1) and neurogenesis (Fzd8). Additionally, the synaptic transmission-relevant gene, Myh6 was down-regulated. Aged rats, in contrast, up-regulated genes suppressing cell division (Cdkn1b) and axonal growth (Prp40a), both of which are required for neurogenesis (summarized in supplemental Fig. 4B).
In the second week, a period decisive for axonal growth, the only up-regulated gene in aged rats was PRP40 pre-mRNA processing factor 40 homologue A (Prpf40a).
The neurogenesis-required genes Fzd8 and Foxg1 were down-regulated in old rats only. Aged rats also failed to up-regulate the axonal and myelin injury-related gene (Nkx2-2) and the Gmc2 gene, a gene is important for differentiation of certain glial and neuronal lineages. They also failed to up-regulate Ptch1 that is required for reactivation of developmental mechanisms after injury. Both age groups had many down-regulated neurogenesis-related genes (Gata2, Ctnnd2, Shh).
An overview of major genetic events in the ipsilateral and contralateral hemisphere tentatively associated with functional recovery of the after stroke in young and aged rats is given is Fig. 6 and 7.
Fig. 6.
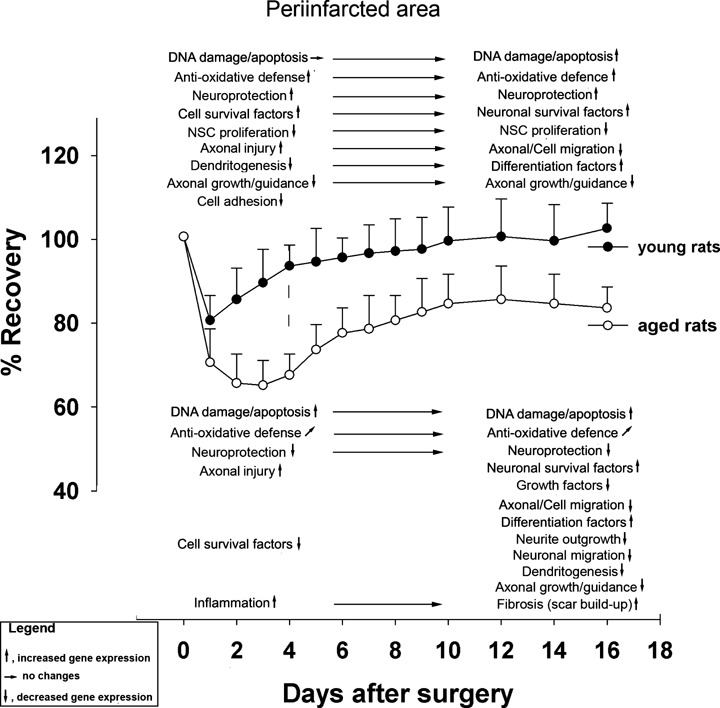
Overview of major genetic events in the ipsilateral hemisphere tentatively associated with functional recovery of the after stroke in young and aged rats.
Fig. 7.
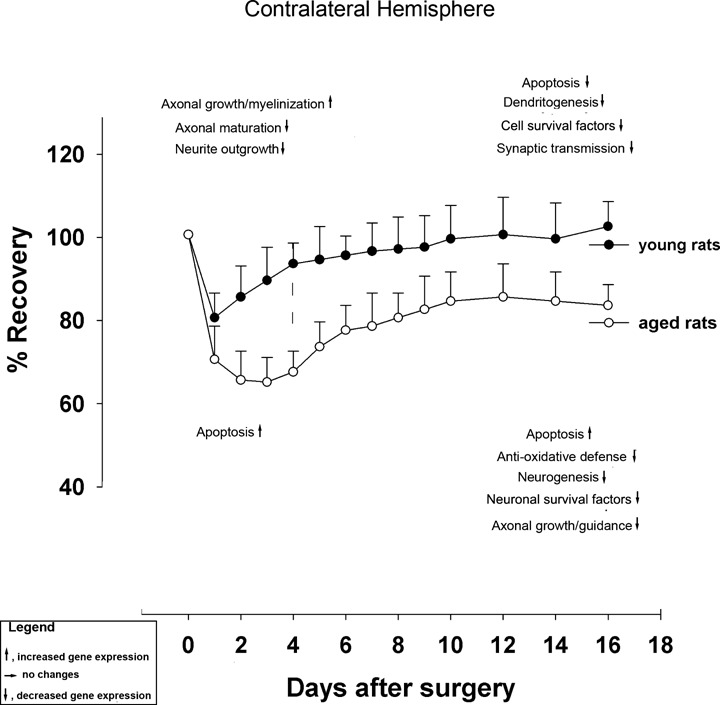
Overview of major genetic events in the contralateral hemisphere tentatively associated with functional recovery of the after stroke in young and aged rats.
The substrates that mediate recovery of motor function after stroke are incompletely understood. Although the effect of age on cerebral ischaemia and recovery after stroke has been the focus of several recent reports [109, 110], the contribution of the contralateral hemisphere to neurorestoration has not been addressed at gene expression level. Our study shows that the contralateral, healthy hemisphere in young rats is much more active at transcriptional level than that of the aged rats at day 3 after ischaemia, especially at the level of stem cell and hypoxia signalling coding genes. A possible explanation for this is the temporary decrease in transcallossal disinhibition allowing misbalanced gene expression in the contralateral hemisphere [111–113]. However, at 3 days after stroke, tissue in the hypoperfusion region remains highly vulnerable to cell death, making it unlikely that the infarcted area could support tissue regeneration and recovery of function. Instead, we hypothesize that the contralateral sensorimotor cortex may contribute to functional recovery by taking over some function of the damaged hemisphere via uncrossed corticospinal tract fibres [7]. Later in the recovery process, regions adjacent to the lesioned area become activated and take over areas previously innervated by the lost neurons [114]. More complete recovery after stroke may ultimately require both the local reorganization of the ipsilateral perilesional cortex and inputs from the contralateral hemisphere [8, 9].
Profiles of gene expression after stroke are likely to be influenced by basal levels, that is by changes gene expression associated with the aging process itself.
Following insult to the brain, old rats are capable of up-regulating gene expression, but the response is often blunted and temporally uncoordinated, in comparison to the response in young rats [115–122].
Many aspects of age-related changes in gene expression in the normal aging brain have been summarized recently [123]. Recently, gene profiling in the hippocampus of young and aged rats revealed that normal aging is associated with down-regulation of axonal growth, cytoskeletal assembly/transport, signalling and lipogenic/uptake pathways, concomitant with up-regulation in immune/inflammatory, lysosomal, lipid/protein degradation, cholesterol transport, TGF and cAMP signalling pathways in the hippocampus [124].
Conclusions
Reduced transcriptional activity in the healthy, contralateral hemisphere of aged rats, in conjunction with an early up-regulation of DNA damage-related genes and pro-apoptotic genes and down-regulation of axono and neurogenesis in the periinfarct area, is likely to account for poor neurorehabilitation after stroke in old rats. Our results suggest several ways to improve functional recovery after stroke in the aged such as transcranial stimulation of the contralateral hemisphere or application of pharmacologic agents aimed at reducing the DNA damage and apoptosis in the periinfarcted area in the first week after stroke.
Acknowledgments
This research was supported by a grant from Bundesministerium fuer Bildung und Forschung to Aurel Popa-Wagner.
References
- 1.Barnett HJ. Stroke prevention in the elderly. Clin Exp Hypertens. 2002;24:563–71. doi: 10.1081/ceh-120015333. [DOI] [PubMed] [Google Scholar]
- 2.Popa-Wagner A, Schroder E, Walker LC, Kessler C. Beta-amyloid precursor protein and ss-amyloid peptide immunoreactivity in the rat brain after middle cerebral artery occlusion: effect of age. Stroke. 1998;29:2196–202. doi: 10.1161/01.str.29.10.2196. [DOI] [PubMed] [Google Scholar]
- 3.Lindner MD, Gribkoff VK, Donlan NA, Jones TA. Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J Neurosci. 2003;23:10913–22. doi: 10.1523/JNEUROSCI.23-34-10913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler Ch, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–4. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- 5.Badan I, Dinca I, Buchhold B, Suofu Y, Walker L, Gratz M, Platt D, Kessler Ch, Popa-Wagner A. Accelerated accumulation of N- and C-terminal betaAPP fragments and delayed recovery of microtubule-associated protein 1B expression following stroke in aged rats. Eur J Neurosci. 2004;19:2270–80. doi: 10.1111/j.0953-816X.2004.03323.x. [DOI] [PubMed] [Google Scholar]
- 6.Markus TM, Tsai SY, Bollnow MR, Farrer RG, O'Brien TE, Kindler-Baumann DR, Rausch M, Rudin M, Wiessner C, Mir AK, Schwab ME, Kartje GL. Recovery and brain reorganization after stroke in adult and aged rats. Ann Neurol. 2005;58:950–3. doi: 10.1002/ana.20676. [DOI] [PubMed] [Google Scholar]
- 7.Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- 8.Serrien DJ, Strens LH, Cassidy MJ, Thompson AJ, Brown P. Functional significance of the ipsilateral hemisphere during movement of the affected hand after stroke. Exp Neurol. 2004;190:425–32. doi: 10.1016/j.expneurol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Foltys H, Krings T, Meister IG, Sparing R, Boroojerdi B, Thron A, Töpper R. Motor representation in patients rapidly recovering after stroke: a functional magnetic resonance imaging and transcranial magnetic stimulation study. Clin Neurophysiol. 2003;114:2404–15. doi: 10.1016/s1388-2457(03)00263-3. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, You SH, Kwon YH, Hallett M, Kim JH, Jang SH. Longitudinal fMRI study for locomotor recovery in patients with stroke. Neurology. 2006;67:330–3. doi: 10.1212/01.wnl.0000225178.85833.0d. [DOI] [PubMed] [Google Scholar]
- 11.Soriano MA, Tessier M, Certa U, Gill R. Parallel gene expression monitoring using oligonucleotide probe arrays of multiple transcripts with an animal model of focal ischemia. J Cereb Blood Flow Metab. 2000;20:1045–55. doi: 10.1097/00004647-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Kastner R, Zhang B, Belayev L, Khoutorova L, Amin R, Busto R, Ginsberg MD. DNA microarray analysis of cortical gene expression during early recirculation after focal brain ischemia in rat. Brain Res Mol Brain Res. 2002;108:81–93. doi: 10.1016/s0169-328x(02)00516-8. [DOI] [PubMed] [Google Scholar]
- 13.Roth A, Gill R, Certa U. Temporal and spatial gene expression patterns after experimental stroke in a rat model and characterization of PC4, a potential regulator of transcription. Mol Cell Neurosci. 2003;22:353–64. doi: 10.1016/s1044-7431(02)00039-8. [DOI] [PubMed] [Google Scholar]
- 14.Kim JB, Piao CS, Lee KW, Han PL, Ahn JI, Lee YS, Lee JK. Delayed genomic responses to transient middle cerebral artery occlusion in the rat. J Neurochem. 2004;89:1271–82. doi: 10.1111/j.1471-4159.2004.02429.x. [DOI] [PubMed] [Google Scholar]
- 15.Kury P, Schroeter M, Jander S. Transcriptional response to circumscribed cortical brain ischemia: spatiotemporal patterns in ischemic vs. remote non-ischemic cortex. Eur J Neurosci. 2004;19:1708–20. doi: 10.1111/j.1460-9568.2004.03226.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim YD, Sohn NW, Kang C, Soh Y. DNA array reveals altered gene expression in response to focal cerebral ischemia. Brain Res Bull. 2002;58:491–8. doi: 10.1016/s0361-9230(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 17.Dorszewska J, Adamczewska-Goncerzewicz Z, Szczech J. Apoptotic proteins in the course of aging of central nervous system in the rat. Respir Physio. Neurobiol. 2004;139:145–5. doi: 10.1016/j.resp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Hiona A, Leeuwenburgh C. Effects of age and caloric restriction on brain neuronal cell death/survival. Ann N Y Acad Sci. 2004;1019:96–105. doi: 10.1196/annals.1297.018. [DOI] [PubMed] [Google Scholar]
- 19.Chang ML, Wu CH, Jiang-Shieh YF, Shieh JY, Wen CY. Reactive changes of retinal astrocytes and Müller glial cells in kainate-induced neuroexcitotoxicity. J Anat. 2007;210:54–65. doi: 10.1111/j.1469-7580.2006.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy EJ, Owada Y, Kitanaka N, Kondo H, Glatz JF. Brain arachidonic acid incorporation is decreased in heart fatty acid binding protein gene-ablated mice. Biochemistry. 2005;44:6350–60. doi: 10.1021/bi047292r. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon RA, Jiang X, Francisco C, Christen S, Vexler ZS, Täuber MG, Ferriero DM. Manipulation of antioxidant pathways in neonatal murine brain. Pediatr Res. 2004;56:656–62. doi: 10.1203/01.PDR.0000139413.27864.50. [DOI] [PubMed] [Google Scholar]
- 22.de Bilbao F, Arsenijevic D, Vallet P, Hjelle OP, Ottersen OP, Bouras C, Raffin Y, Abou K, Langhans W, Collins S, Plamondon J, Alves-Guerra MC, Haguenauer A, Garcia I, Richard D, Ricquier D, Giannakopoulos P. Resistance to cerebral ischemic injury in UCP2 knockout mice: evidence for a role of UCP2 as a regulator of mitochondrial glutathione levels. J Neurochem. 2004;89:1283–92. doi: 10.1111/j.1471-4159.2004.02432.x. [DOI] [PubMed] [Google Scholar]
- 23.Pennacchio LA, Bouley DM, Higgins KM, Scott MP, Noebels JL, Myers RM. Progressive ataxia, myoclonic epilepsy and cerebellar apoptosis in cystatin B-deficient mice. Nat Genet. 1998;20:251–8. doi: 10.1038/3059. [DOI] [PubMed] [Google Scholar]
- 24.Williams PH, Cobb BL, Namjou B, Scofield RH, Sawalha AH, Harley JB. Horizons in Sjögren's Syndrome Genetics. Clin Rev Allergy Immunol. 2007 doi: 10.1007/s12016-007-8002-9. Oct 26; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buisson A, Lesne S, Docagne F, Ali C, Nicole O, MacKenzie ET, Vivien D. Transforming growth factor-beta and ischemic brain injury. Cell Mol Neurobiol. 2003;23:539–50. doi: 10.1023/A:1025072013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makwana M, Jones LL, Cuthill D, Heuer H, Bohatschek M, Hristova M, Friedrichsen S, Ormsby I, Bueringer D, Koppius A, Bauer K, Doetschman T, Raivich G. Endogenous transforming growth factor beta1 suppresses inflammation and promotes survival in adult CNS. J Neurosci. 2007;27:11201–13. doi: 10.1523/JNEUROSCI.2255-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–50. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Maekawa T, Sano Y, Shinagawa T, Rahman Z, Sakuma T, Nomura S, Licht JD, Ishii S. ATF-2 controls transcription of Maspin and GADD45alpha genes independently from p53 to suppress mammary tumors. 2008;27:1045–54. doi: 10.1038/sj.onc.1210727. [DOI] [PubMed] [Google Scholar]
- 29.Komi-Kuramochi A, Kawano M, Oda Y, Asada M, Suzuki M, Oki J, Imamura T. Expression of fibroblast growth factors and their receptors during full-thickness skin wound healing in young and aged mice. J Endocrinol. 2005;186:273–89. doi: 10.1677/joe.1.06055. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Segura LM, Sanz A, Mendez P. Cross-talk between IGF-I and estradiol in the brain: focus on neuroprotection. Neuroendocrinology. 2006;84:275–9. doi: 10.1159/000097485. [DOI] [PubMed] [Google Scholar]
- 31.Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, Nave KA, Rowitch D, D'Ercole AJ, Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–11. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe M, Hadzic T, Nishiyama A. Transient upregulation of Nkx2.2 expression in oligodendrocyte lineage cells during remyelination. Glia. 2004;46:311–22. doi: 10.1002/glia.20006. [DOI] [PubMed] [Google Scholar]
- 33.Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem. 2006;281:13247–57. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- 34.Tsarovina K, Pattyn A, Stubbusch J, Müller F, van der Wees J, Schneider C, Brunet JF, Rohrer H. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131:4775–86. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- 35.Hänze J, Weissmann N, Grimminger F, Seeger W, Rose F. Cellular and molecular mechanisms of hypoxia-inducible factor driven vascular remodeling. Thromb Haemost. 2007;97:774–87. [PubMed] [Google Scholar]
- 36.Zhang Y, Klassen HJ, Tucker BA, Perez MT, Young MJ. CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J Neurosci. 2007;27:4499–506. doi: 10.1523/JNEUROSCI.0200-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasegawa A, Naruse M, Hitoshi S, Iwasaki Y, Takebayashi H, Ikenaka K. Regulation of glial development by cystatin C. J Neurochem. 2007;100:12–22. doi: 10.1111/j.1471-4159.2006.04169.x. [DOI] [PubMed] [Google Scholar]
- 38.Colonques J, Ceron J, Tejedor FJ. Segregation of postembryonic neuronal and glial lineages inferred from a mosaic analysis of the Drosophila larval brain. Mech Dev. 2007;124:327–40. doi: 10.1016/j.mod.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Soustelle L, Trousse F, Jacques C, Ceron J, Cochard P, Soula C, Giangrande A. Neurogenic role of Gcm transcription factors is conserved in chicken spinal cord. Development. 2007;134:625–34. doi: 10.1242/dev.02750. [DOI] [PubMed] [Google Scholar]
- 40.Dikkes P, Jaffe D, Guo WH, Chao C, Hemond P, Yoon K, Zurakowski D, Lopez MF. IGF2 knockout mice are resistant to kainic acid-induced seizures and neurode-generation. Brain Res. 2007 doi: 10.1016/j.brainres.2007.05.068. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Ogawa K, Saito A, Matsui H, Suzuki H, Ohtsuka S, Shimosato D, Morishita Y, Watabe T, Niwa H, Miyazono K. Activin-Nodal signaling is involved in propagation of mouse embryonic stem cells. J Cell Sci. 2007;120:55–65. doi: 10.1242/jcs.03296. [DOI] [PubMed] [Google Scholar]
- 42.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 43.Mu X, Beremand PD, Zhao S, Pershad R, Sun H, Scarpa A, Liang S, Thomas TL, Klein WH. Discrete gene sets depend on POU domain transcription factor Brn3b/Brn-3.2/POU4f2 for their expression in the mouse embryonic retina. Development. 2004;131:1197–210. doi: 10.1242/dev.01010. [DOI] [PubMed] [Google Scholar]
- 44.Saarimäki-Vire J, Peltopuro P, Lahti L, Naserke T, Blak AA, Vogt Weisenhorn DM, Yu K, Ornitz DM, Wurst W, Partanen J. Fibroblast growth factor receptors cooperate to regulate neural progenitor properties in the developing midbrain and hindbrain. J Neurosci. 2007;27:8581–92. doi: 10.1523/JNEUROSCI.0192-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergeron C, Beric-Maskarel K, Muntasser S, Weyer L, Somerville MJ, Percy ME. Neurofilament light and polyadenylated mRNA levels are decreased in amyotrophic lateral sclerosis motor neurons. J Neuropathol Exp Neurol. 1994;53:221–30. doi: 10.1097/00005072-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez I, Hassinger L, Peter A. Paskevich H, Shine D, Ralph A. Nixon oligodendroglia regulate the regional expansion of axon caliber and local accumulation of neurofilaments during development independently of myelin formation. J Neurosci. 1996;16:5095–105. doi: 10.1523/JNEUROSCI.16-16-05095.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persson P, Manetopoulos C, Lagergren A, Nygren J, Gisler R, Axelson H, Sigvardsson M. Olf/EBF proteins are expressed in neuroblastoma cells: potential regulators of the Chromogranin A and SCG10 promoters. Int J Cancer. 2004;110:22–30. doi: 10.1002/ijc.20092. [DOI] [PubMed] [Google Scholar]
- 48.Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–20. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, Attisano L. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J. 2004;23:4792–801. doi: 10.1038/sj.emboj.7600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uemura M, Takeichi M. Alpha N-catenin deficiency causes defects in axon migration and nuclear organization in restricted regions of the mouse brain. Dev Dyn. 2006;235:2559–66. doi: 10.1002/dvdy.20841. [DOI] [PubMed] [Google Scholar]
- 51.Maruya SI, Myers JN, Weber RS, Rosenthal DI, Lotan R, El-Naggar AK. ICAM-5 (telencephalin) gene expression in head and neck squamous carcinoma tumorigenesis and perineural invasion. Oral Oncol. 2005;41:580–8. doi: 10.1016/j.oraloncology.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Milner R, Crocker SJ, Hung S, Wang X, Frausto RF, del Zoppo GJ. Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins alpha5beta1 and alphavbeta5. J Immunol. 2007;178:8158–67. doi: 10.4049/jimmunol.178.12.8158. [DOI] [PubMed] [Google Scholar]
- 53.Sapieha PS, Peltier M, Rendahl KG, Manning WC, Di Polo A. Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol Cell Neurosci. 2003;24:656–72. doi: 10.1016/s1044-7431(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 54.Rossner MJ, Doerr J, Gass P, Schwab MH, Nave KA. SHARPs: mammalian enhancer-of-split- and hairy-related proteins coupled toneuronal stimulation. Mol Cell Neurosci. 1997;9:460–75. doi: 10.1006/mcne.1997.0640. [DOI] [PubMed] [Google Scholar]
- 55.Kugler W, Breme K, Laspe P, Muirhead H, Davies C, Winkler H, Schröter W, Lakomek M. Molecular basis of neurological dysfunction coupled with haemolytic anaemia in human glucose-6-phosphate isomerase (GPI) deficiency. Hum Genet. 1998;103:450–4. doi: 10.1007/s004390050849. [DOI] [PubMed] [Google Scholar]
- 56.Yano H, Ninan I, Zhang H, Milner TA, Arancio O, Chao MV. BDNF-mediated neu-rotransmission relies upon a myosin VI motor complex. Nat Neurosci. 2006;9:1009–18. doi: 10.1038/nn1730. [DOI] [PubMed] [Google Scholar]
- 57.Mizrak SC, Renault-Mihara F, Párraga M, Bogerd J, van de Kant HJ, López-Casas PP, Paz M, del Mazo J, de Rooij DG. Phosphoprotein enriched in astrocytes-15 is expressed in mouse testis and protects spermatocytes from apoptosis. Reproduction. 2007;133:743–51. doi: 10.1530/REP-06-0281. [DOI] [PubMed] [Google Scholar]
- 58.Nyman-Huttunen H, Tian L, Ning L, Gahmberg CG. alpha-Actinin-dependent cytoskeletal anchorage is important for ICAM-5-mediated neuritic outgrowth. J Cell Sci. 2006;119:3057–66. doi: 10.1242/jcs.03045. [DOI] [PubMed] [Google Scholar]
- 59.Bergeron C, Beric-Maskarel K, Muntasser S, Weyer L, Somerville MJ, Percy ME. Neurofilament light and polyadenylated mRNA levels are decreased in amyotrophic lateral sclerosis motor neurons. J Neuropathol Exp Neurol. 1994;53:221–30. doi: 10.1097/00005072-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Takemoto-Kimura S, Ageta-Ishihara N, Nonaka M, Adachi-Morishima A, Mano T, Okamura M, Fujii H, Fuse T, Hoshino M, Suzuki S, Kojima M, Mishina M, Okuno H, Bito H. Regulation of dendritogenesis via a lipid-raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIgamma. Neuron. 2007;54:755–70. doi: 10.1016/j.neuron.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 61.Swiercz R, Person MD, Bedford MT. Ribosomal protein S2 is a substrate for mammalian PRMT3 (protein arginine methyltransferase 3) Biochem J. 2005;386:85–91. doi: 10.1042/BJ20041466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conti A, Ageunnouz M, La Torre D, Cardali S, Angileri FF, Buemi C, Tomasello C, Iacopino DG, D'Avella D, Vita G, Tomasello F. Expression of the tumor necrosis factor receptor-associated factors 1 and 2 and regulation of the nuclear factor-kappaB antiapoptotic activity in human gliomas. J Neurosurg. 2005;103:873–81. doi: 10.3171/jns.2005.103.5.0873. [DOI] [PubMed] [Google Scholar]
- 63.van de Wetering CI, Knudson CM. Chromosomal instability and supernumerary centrosomes represent precursor defects in a mouse model of T-cell lymphoma. Cancer Res. 2007;67:8081–8. doi: 10.1158/0008-5472.CAN-07-1666. [DOI] [PubMed] [Google Scholar]
- 64.Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxy-genase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem. 2000;275:32775–82. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- 65.Denker SP, Ji S, Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J Neurochem. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, Hashimoto T, Zaroff J, Lawton MT, Marchuk DA, Kwok PY, Young WL. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- 67.Pannu J, Nakerakanti S, Smith E, ten Dijke P, Trojanowska M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282:10405–13. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- 68.Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol. 2007;113:277–93. doi: 10.1007/s00401-006-0164-7. [DOI] [PubMed] [Google Scholar]
- 69.Fedoroff OY, Townson SA, Golovanov AP, Baron M, Avis JM. The structure and dynamics of tandem WW domains in a negative regulator of notch signaling, Suppressor of deltex. J Biol Chem. 2004;279:34991–5000. doi: 10.1074/jbc.M404987200. [DOI] [PubMed] [Google Scholar]
- 70.Endo K, Aoki T, Yoda Y, Kimura K, Hama C. Notch signal organizes the Drosophila olfactory circuitry by diversifying the sensory neuronal lineages. Nat Neurosci. 2007;10:153–60. doi: 10.1038/nn1832. [DOI] [PubMed] [Google Scholar]
- 71.Fenrich KK, Skelton N, MacDermid VE, Meehan CF, Armstrong S, Neuber-Hess MS, Rose PK. Axonal regeneration and development of de novo axons from distal dendrites of adult feline commissural interneurons after a proximal axotomy. J Comp Neurol. 2007;502:1079–97. doi: 10.1002/cne.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Myllylä R, Wang C, Heikkinen J, Juffer A, Lampela O, Risteli M, Ruotsalainen H, Salo A, Sipilä L. Expanding the lysyl hydroxylase toolbox: new insights into the localization and activities of lysyl hydroxylase 3 (LH3) J Cell Physiol. 2007;212:323–9. doi: 10.1002/jcp.21036. [DOI] [PubMed] [Google Scholar]
- 73.Keays DA, Tian G, Poirier K, Huang GJ, Siebold C, Cleak J, Oliver PL, Fray M, Harvey RJ, Molnár Z, Piñon MC, Dear N, Valdar W, Brown SD, Davies KE, Rawlins JN, Cowan NJ, Nolan P, Chelly J, Flint J. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Itoh Y, Masuyama N, Nakayama K, Nakayama KI, Gotoh Y. The cyclin-dependent kinase inhibitors p57 and p27 regulate neuronal migration in the developing mouse neocortex. J Biol Chem. 2007;282:390–6. doi: 10.1074/jbc.M609944200. [DOI] [PubMed] [Google Scholar]
- 75.Kharlamov A, Kharlamov E, Armstrong DM. Age-dependent increase in infarct volume following photochemically induced cerebral infarction: putative role of astroglia. J Gerontol A Biol Sci Med Sci. 2000;55:B135–41. doi: 10.1093/gerona/55.3.b135. [DOI] [PubMed] [Google Scholar]
- 76.Brown AW, Marlowe KJ, Bjelke B. Age effect on motor recovery in a post-acute animal stroke model. Neurobiol Aging. 2003;24:607–14. doi: 10.1016/s0197-4580(02)00129-x. [DOI] [PubMed] [Google Scholar]
- 77.Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral Hemorrhage: effects of Aging on Brain Edema and Neurological Deficits. Stroke. 2004;35:2571–5. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- 78.Tu Y, Hou ST, Huang Z, Robertson GS, MacManus JP. Increased Mdm2 expression in rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1998;18:658–69. doi: 10.1097/00004647-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Roos-Mattjus P, Vroman BT, Burtelow MA, Rauen M, Eapen AK, Karnitz LM. Genotoxin-induced Rad9-Hus1-Rad1 (9-1-1) chromatin association is an early checkpoint signaling event. J Biol Chem. 2002;277:43809–12. doi: 10.1074/jbc.M207272200. [DOI] [PubMed] [Google Scholar]
- 80.Lotocki G, Alonso OF, Dietrich WD, Keane RW. Tumor necrosis factor receptor 1 and its signaling intermediates are recruited to lipid rafts in the traumatized brain. J Neurosci. 2004;24:11010–6. doi: 10.1523/JNEUROSCI.3823-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prasad KV, Ao Z, Yoon Y, WuM X, Rizk M, Jacquot S, Schlossman SF. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sc. USA. 1997;94:6346–51. doi: 10.1073/pnas.94.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rhodes JK, Andrews PJ, Holmes MC, Seckl JR. Expression of interleukin-6 messenger RNA in a rat model of diffuse axonal injury. Neurosci Lett. 2002;335:1–4. doi: 10.1016/s0304-3940(02)00811-x. [DOI] [PubMed] [Google Scholar]
- 83.MacDonald TJ, Pollack IF, Okada H, Bhattacharya S, Lyons-Weiler J. Progression-associated genes in astrocytoma identified by novel microarray gene expression data reanalysis. Methods Mol Biol. 2007;377:203–22. doi: 10.1007/978-1-59745-390-5_13. [DOI] [PubMed] [Google Scholar]
- 84.Penkowa M, Camats J, Hadberg H, Quintana A, Rojas S, Giralt M, Molinero A, Campbell IL, Hidalgo J. Astrocyte-targeted expression of interleukin-6 protects the central nervous system during neu-roglial degeneration induced by 6-aminon-icotinamide. J Neurosci Res. 2003;73:481–96. doi: 10.1002/jnr.10681. [DOI] [PubMed] [Google Scholar]
- 85.Knight JA. Free radicals: their history and current status in aging and disease. Ann Clin Lab Sci. 1998;28:331–46. [PubMed] [Google Scholar]
- 86.Smith MA, Nunomura A, Zhu X, Takeda A, Perry G. Metabolic, metallic, and mitotic sources of oxidative stress in Alzheimer's disease. Antiox Redox Signal. 2000;2:413–20. doi: 10.1089/15230860050192198. [DOI] [PubMed] [Google Scholar]
- 87.Van Remmen H, Qi W, Sabia M, Freeman G, Estlack L, Yang H, Mao Guo Z, Huang TT, Strong R, Lee S, Epstein CJ, Richardson A. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med. 2004;36:1625–34. doi: 10.1016/j.freeradbiomed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 88.Crack PJ, Taylor JM, Ali U, Mansell A, Hertzog PJ. Potential contribution of NF-kappaB in neuronal cell death in the glutathione peroxidase-1 knockout mouse in response to ischemia-reperfusion injury. Stroke. 2006;37:1533–8. doi: 10.1161/01.STR.0000221708.17159.64. [DOI] [PubMed] [Google Scholar]
- 89.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overex- press extracellular superoxide dismutase. J Neurosci. 2006;26:3933–41. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fortin D, Rom E, Sun H, Yayon A, Bansal R. Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligoden-drocyte lineage. J Neurosci. 2005;25:7470–9. doi: 10.1523/JNEUROSCI.2120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–70. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 92.Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–34. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 93.Lindvall O, Ernfors P, Bengzon J, Kokaia Z, Smith ML, Siesjö BK, Persson H. Differential regulation of mRNAs for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proc Natl Acad Sci U S A. 1992;89:648–52. doi: 10.1073/pnas.89.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mukerji N, Damodaran TV, Winn MP. TRPC6 and FSGS: the latest TRP channelopathy. Biochim Biophys Acta. 2007;1772:859–68. doi: 10.1016/j.bbadis.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 95.Kitisin K, Saha T, Blake T, Golestaneh N, Deng M, Kim C, Tang Y, Shetty K, Mishra B, Mishra L. Tgf-Beta signaling in development. Sci STKE. 2007;399:cm1. doi: 10.1126/stke.3992007cm1. [DOI] [PubMed] [Google Scholar]
- 96.Mackay KB, Loddick SA, Naeve GS, Vana AM, Verge GM, Foster AC. Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. J Cereb Blood Flow Metab. 2003;23:1160–7. doi: 10.1097/01.WCB.0000087091.01171.AE. [DOI] [PubMed] [Google Scholar]
- 97.Brännvall K, Hjelm H, Korhonen L, Lahtinen U, Lehesjoki AE, Lindholm D. Cystatin-B is expressed by neural stem cells and by differentiated neurons and astrocytes. Biochem Biophys Res Commun. 2003;308:369–74. doi: 10.1016/s0006-291x(03)01386-x. [DOI] [PubMed] [Google Scholar]
- 98.Sharif A, Canton B, Junier MP, Chneiweiss H. PEA-15 modulates TNFalpha intracellular signaling in astrocytes. Ann N Y Acad Sci. 2003;1010:43–50. doi: 10.1196/annals.1299.006. [DOI] [PubMed] [Google Scholar]
- 99.Peters A. Structural changes that occur during normal aging of primate cerebral hemispheres. Neurosci. Biobehav Rev. 2002;26:733–41. doi: 10.1016/s0149-7634(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 100.Ando S, Tanaka Y, Toyoda Y, Kon K. Turnover of myelin lipids in aging brain. Neurochem Res. 2003;28:5–13. doi: 10.1023/a:1021635826032. [DOI] [PubMed] [Google Scholar]
- 101.De Galarza Bo ER, Atlasovich FM, Ermacora MR, Torea JH, Pasquini JM, Santome JA, Soto EF. Rat brain fatty acid-binding protein during development. Neurochem Int. 1992;21:237–41. doi: 10.1016/0197-0186(92)90153-i. [DOI] [PubMed] [Google Scholar]
- 102.Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9:445–52. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fancy SP, Zhao C, Franklin RJ. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 2004;27:247–54. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 104.Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–65. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li WW, Penderis J, Zhao C, Schumacher M, Franklin RJ. Females remyelinate more efficiently than males following demyelination in the aged but not young adult CNS. Exp Neurol. 2006;202:250–4. doi: 10.1016/j.expneurol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 106.Misra V, Lee H, Singh A, Huang K, Thimmulappa RK, Mitzner W, Biswal S, Tankersley CG. Global expression profiles from C57BL/6J and DBA/2J mouse lungs to determine aging-related genes. Physiol Genomics. 2007;31:429–40. doi: 10.1152/physiolgenomics.00060.2007. [DOI] [PubMed] [Google Scholar]
- 107.Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun Adhes. 2001;8:315–20. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nagata T, Takahashi Y, Sugahara M, Murata A, Nishida Y, Ishikawa K, Asai S. Profiling of genes associated with transcriptional responses in mouse hippocam-pus after transient forebrain ischemia using high-density oligonucleotide DNA array. Brain Res Mol Brain Res. 2004;121:1–11. doi: 10.1016/j.molbrainres.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 109.Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, Simon RP, Greenberg DA. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3:373–7. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 110.He Z, Crook JE, Meschia JF, Brott TG, Dickson DW, McKinney M. Aging blunts ischemic-preconditioning-induced neuroprotection following transient global ischemia in rats. Curr Neurovasc Res. 2005;2:365–74. doi: 10.2174/156720205774962674. [DOI] [PubMed] [Google Scholar]
- 111.Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol. 2005;18:429–34. doi: 10.1097/01.wco.0000168081.76859.c1. [DOI] [PubMed] [Google Scholar]
- 112.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2005;55:400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 113.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–23. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 114.Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–58. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 115.Schauwecker PE, Cheng HW, Serquinia RM, Mori N, McNeill TH. Lesion-induced sprouting of commissural/associational axons and induction of GAP-43 mRNA in hilar and CA3 pyramidal neurons in the hippocampus are diminished in aged rats. J Neurosci. 1995;15:2462–70. doi: 10.1523/JNEUROSCI.15-03-02462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parhad IM, Scott JN, Cellars LA, Bains JS, Krekoski CA, Clark AW. Axonal atrophy in aging is associated with a decline in neurofilament gene expression. J Neurosci Res. 1995;41:355–66. doi: 10.1002/jnr.490410308. [DOI] [PubMed] [Google Scholar]
- 117.Popa-Wagner A, Fischer B, Platt D, Neubig R, Schmoll H, Kessler C. Anomalous expression of microtubule-associated protein 1B in the hippocampus and cortex of aged rats treated with pentylenetetrazole. Neuroscience. 1999b;94:395–403. doi: 10.1016/s0306-4522(99)00204-3. [DOI] [PubMed] [Google Scholar]
- 118.Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci. U S A. 2001a;98:8071–6. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adams MM, Gazzaley AH, Morrison JH. Attenuated lesion-induced N-methyl-D-aspartate receptor (NMDAR) plasticity in the dentate gyrus of aged rats following perforant path lesions. Exp Neurol. 2001b;172:244–9. doi: 10.1006/exnr.2001.7794. [DOI] [PubMed] [Google Scholar]
- 120.Hoff SF, Scheff SW, Cotman CW. Lesion-induced synaptogenesis in the dentate gyrus of aged rats: I. Loss and reacquisition of normal synaptic density. J Comp Neurol. 1982;205:246–52. doi: 10.1002/cne.902050304. [DOI] [PubMed] [Google Scholar]
- 121.Woods AG, Guthrie KM, Kurlawalla MA, Gall CM. Deafferentation-induced increases in hippocampal insulin-like growth factor-1 messenger RNA expression are severely attenuated in middle aged and aged rats. Neuroscience. 1998;83:663–8. doi: 10.1016/s0306-4522(97)00539-3. [DOI] [PubMed] [Google Scholar]
- 122.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Lopez LM, Shick J, Finch CE. Effects of age on gene expression during estrogen-induced synaptic sprouting in the female rat. Exp Neurol. 2000;165:46–57. doi: 10.1006/exnr.2000.7455. [DOI] [PubMed] [Google Scholar]
- 123.Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–20. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 124.Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27:3098–110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


