Abstract
The risk of dementia and Alzheimer's disease (AD) probably results from an interaction between genetic and environmental factors. The aim of this study was to investigate the effects and putative interactions between the apoE ε4 allele and lifestyle related risk factors for dementia and AD. Participants of the Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) study were derived from random, population-based samples previously studied in 1972, 1977, 1982 or 1987. After an average follow-up of 21 years, 1449 individuals (72.5%) aged 65–79 years were re-examined in 1998. The apoE ε4 allele was an independent risk factor for dementia/AD even after adjustments for sociodemographic, lifestyle and vascular factors (odds ratio [OR]= 2.83, 95% confidence interval [CI]ε1.61–4.97). Physical inactivity, alcohol drinking and smoking increased the risk of dementia/AD particularly among the apoE ε4 carriers. Furthermore, low–moderate intake of polyunsaturated, and moderate–high intake of saturated fats were associated with an increased risk of dementia/AD more pronouncedly among apoE ε4 carriers. Composite effect of the lifestyle factors was particularly seen among the ε4 carriers (OR = 11.42, 95% CI = 1.94–67.07 in the 4th quartile). Physical inactivity, dietary fat intake, alcohol drinking and smoking at midlife are associated with the risk of dementia and AD, especially among the apoE ε4 carriers. The apoE ε4 carriers may be more vulnerable to environmental factors, and thus, lifestyle interventions may greatly modify dementia risk particularly among the genetically susceptible individuals.
Keywords: dementia, Alzheimer's disease, ApoE, physical activity, dietary fat intake, alcohol drinking, smoking
Introduction
Until recently, advanced age, family history and apolipoprotein E (apoE) ε4 allele have been the only established risk factors for Alzheimer's disease (AD). The apoE ε4 allele is the most important currently known genetic risk factor for AD, and it may represent roughly half of the genetic risk for AD [1, 2]. Dominance of the genetic hypotheses for AD has lead to somewhat fatalistic attitude towards the possibilities to prevent or postpone the onset of it. However, this picture has recently started to change due to increasing evidence that modifiable risk factors for dementia and AD also exist [3–7]. Consequently, AD, the major form of dementia is currently considered to be a disease of multi-factorial origin, resulting from an interaction between the genetic susceptibility and environmental risk factors. Recent studies have linked dementia and AD with several lifestyle-related factors including physical activity [4, 8–12], dietary fat and fish oil intake [6, 13–18], alcohol drinking [5, 19–22] and smoking [23–26]. Further, some studies have also suggested that the apoE ε4 carrier status could be a possible effect modifier for the associations between lifestyle/vascular risk factors and dementia [4–6, 11, 14, 16–18, 20, 22, 27–30], but results have varied between the studies. The aim of our study was to investigate further the effects and putative interactions between the apoE ε4 allele and lifestyle-related risk factors at midlife including physical inactivity, dietary fat intake, alcohol drinking and smoking for the development of dementia and AD later in life.
Materials and methods
Participants
The participants of the Cardiovascular risk factors, Aging and Dementia (CAIDE) study were randomly selected from the survivors of four separate, independent, population-based random samples examined within the North Karelia Project and the FINMONICA study. These surveys were carried out to assess the levels of cardiovascular risk factors in two eastern provinces of Finland; North Karelia and Kuopio. The study design has been described in detail elsewhere [31].
After being examined once at midlife (either in 1972, 1977, 1982 or 1987), 2000 randomly selected individuals, aged 65–79 years by the end of 1997, were invited for a re-examination during 1998 (Fig. 1). Altogether 1449 people (72.5%) participated in the re-examination; 900 (62.1%) were women and 549 (37.9%) were men. The mean age (SD) at midlife examination was 50.6 (6.0) years (range 39–64), and 71.6 (4.1) years (range 65–79) at the re-examination. The mean duration of follow-up was 21 years (SD 4.9). The study was approved by the local ethics committee, and written informed consent was obtained from all participants.
Fig. 1.
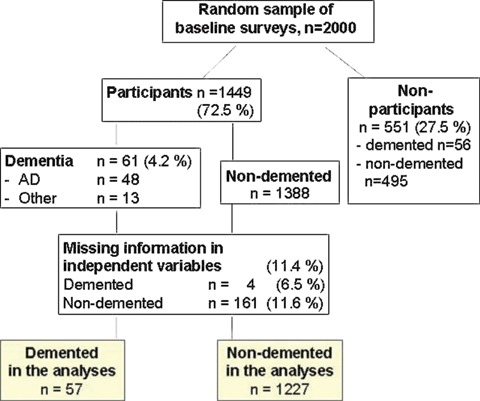
Formation of the study population.
Midlife examination
The survey methods used during the baseline (midlife) visit were carefully standardized and comply with international recommendations. They followed the WHO MONICA protocol of 1982 and 1987; the methods used in 1972 and 1977 were comparable. In brief, the baseline survey procedures included a self-administered questionnaire on health behaviour, health status and medical history. Participants’ blood pressure (BP), height and weight were measured, and body mass index (BMI) was calculated. A venous blood sample was taken to determine serum cholesterol concentrations. In addition, the participants were queried about the presence of various locomotor disorders, which was defined as the existence of at least one of the following: rheumatoid arthritis, arthritis, arthropathy or arthralgia of the joints of the extremities or the back.
In this study, physical activity was considered as leisure time physical activity lasting at least 20–30 min. each time and causing sweating and breathlessness. The responses to the question concerning physical activity were dichotomized similarly to our previous study [4] into categories: (i) active (= persons who participated leisure time physical activity at least two times a week, n= 529) and (ii) sedentary (= persons who participated leisure time physical activity less than two times a week, n= 755). The active group was used as the reference category in the current analyses. Dietary fat intake was determined as daily fat intake from spreads, and the amount of polyunsaturated (PUFA) and saturated (SFA) fatty acids were calculated from these data. Dietary fat intake was classified into quartiles [6] (PUFA: 1st quartile n= 304, 2nd quartile n= 342, 3rd quartile n= 314, 4th quartile n= 324; SFA: 1st quartile n= 338, 2nd quartile n= 323, 3rd quartile n= 355, 4th quartile n= 268). The highest quartile served as the reference category in the analyses concerning PUFA intake and the lowest in the analyses for SFA. A yearly alcohol drinking habit was determined in the 1972 and 1977 questionnaires only, and in this study the analyses including alcohol data were based on the subsample of the persons who participated in 1972 or 1977 (70% of them participated in the re-examination in 1998). Alcohol drinking was considered as the times person drank alcohol per year. For the current study alcohol drinking was classified into three categories as in our previous study [5]: (i) never drinkers (= persons who never drank alcohol, n= 276), (ii) infrequent drinkers (= persons who drank alcohol less than once a month, n= 391) and (iii) frequent drinkers (= persons who drank alcohol once a month or more often, n= 278). The never drinkers served as the reference category in the analyses concerning alcohol drinking. Smoking was dichotomized into: (i) never smokers (n= 722) and (ii) ever smokers (n= 562) according to smoking status at midlife, and the never smokers were used as the reference category in the current study.
Re-examination
During the re-examination in 1998, the survey methods used were identical to those applied in the previous surveys. Furthermore, the participants were studied for their apoE genotypes by use of PCR and Hhal digestion [32]. For the current study, the apoE genotypes were dichotomized into: (i) apoE ε4 carriers (= persons with at least one ε4 allele in the genotype, n= 452) and (ii) apoE ε4 non-carriers (no ε4 alleles in the genotype, n= 832). Cognitive status was determined, and participants who scored 24 or less on the Mini-Mental State Examination [33] at the screening phase (n= 294) were referred for further examinations, including thorough neurological, cardiovascular and neuropsychological examinations. Altogether, 61 participants were diagnosed as having dementia according to the Diagnostic and Statistical Manual of Mental Disorders, (4th edition) criteria [34], and 48 of them fulfilled the diagnostic criteria of AD according to National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association criteria [35] (Fig. 1).
The analyses were restricted to the participants having no missing data on outcome, any of the lifestyle-related variables or the covariates. For dementia, the number of participants was 1284 (57 cases) in the analyses for physical inactivity, dietary fat intake and smoking, and 945 (43 cases) in the analyses for alcohol drinking. For AD, the number of participants was 1272 (45 cases) in the analyses for physical inactivity, dietary fat intake and smoking, and 935 (33 cases) in the analyses for alcohol drinking.
Statistical analyses
Differences among the persons according to their apoE ε4 carrier status were analysed with chi-square test and Student's t-test as appropriate. The associations between midlife physical inactivity, dietary fat intake, alcohol drinking and smoking, and the subsequent development of dementia and AD were investigated with multiple logistic regression analyses. We carried out analyses stratified by apoE ε4 carrier status adjusted for potential confounders in the investigated associations including age, sex, education, follow-up time, BMI, total serum cholesterol, systolic blood pressure (SBP) and history of myocardial infarction, stroke and diabetes mellitus. The models for physical activity were additionally adjusted for locomotor disorders (may limit participation in physical activity), while analysis of subtypes of dietary fat intake were additionally adjusted for other subtypes of fats (PUFA and SFA). In addition to the stratified analyses we conducted analyses with variables combining the apoE ε4 carrier status with each of the lifestyle-related factor separately. The combined variables included all possible combinations between each lifestyle-related factor and the apoE ε4 carrier status.
The putative multiplicative interactions between the apoE ε4 carrier status and various lifestyle-related factors were investigated by adding an interaction term for each lifestyle-related factor separately into the model. In chronic disease epidemiology, a corroborated view is that risk factors that act independently have an additive rather than synergistic effect [36]. This means that when no causal interaction exists, the total effect of risk factors is equal to the sum of the effects of the separate risk factors. This implies that there is an interaction as a departure from additivity (often called additive interaction) [36] when the total effect of risk factors is smaller or larger than the sum of the separate effects. Therefore, in addition to multiplicative interaction, we also calculated additive interactions using relative excess risk from interaction (RERI) as a measure of overall risk [36]. We chose RERI because it shows the size of the interaction in a way that is comparable to an interaction term, which allows us to compare the results from multiplicative and additive interactions. When the RERI was calculated, the distributional differences between risk and odds were taken into account [37]. Standard errors for the additive interaction were calculated with the delta method [38]. When there is no additive interaction, RERI is equal to zero. In this study, RERI > 0 shows that there is a larger difference among the apoE ε4 carriers.
Furthermore, a composite lifestyle variable was created from all lifestyle-related risk factors to investigate how clustering of lifestyle-related risk factors at midlife affects the risk of dementia and AD later in life. First, all studied lifestyle-related factors were entered simultaneously in a single fully adjusted logistic regression model. Secondly, the value for each factor in the composite variable was assigned according to the β-coefficients resulting from the logistic regression. Moreover, the β-coefficients were summed to form a variable representing the composite effect of the studied lifestyle-related factors. After that, the composite variable was categorized into quartiles (1st quartile was used as the reference category), and the association for the risk of dementia and AD was investigated among all participants, stratified by the apoE ε4 carrier status and combining the composite lifestyle variable with apoE ε4 carrier status as done independently for all lifestyle factors. The level of significance was P < 0.05 in all analyses. The analyses were conducted using SPSS for Windows, release 15.0 and STATA, release 9.1.
Results
Socio-demographic and clinical characteristics
There were 452 (35.2%) apoE ε4 carriers (one or two 4 alleles) and 832 (64.8%) non-carriers (no ε4 alleles) in the current study population. The apoE ε4 carriers were somewhat younger both at midlife and at the re-examination, and they also had higher total cholesterol level than the non-carriers (Table 1). Other socio-demographic and clinical characteristics did not differ significantly between the groups. The apoE ε4 carriers had higher odds to develop dementia (crude model: odds ratio [OR]= 2.28, 95% confidence interval [95% CI]= 1.34–3.90), and adjustment for demographic and vascular factors did not change this association (OR = 2.83, 95% CI = 1.61–4.97).
Table 1.
Association of ApoE ε4 carrier status with demographic and clinical characteristics characteristic
| ApoE ε4 carriers (n= 452) | ApoE ε4 non-carriers (n= 832) | P-value | |
|---|---|---|---|
| Age, year | |||
| • Midlife, years | 49.6 (5.7) | 50.3 (6.0) | 0.03 |
| • Re-examination, years | 70.8 (3.7) | 71.3 (4.1) | 0.02 |
| Follow-up time, years | 21.0 (4.9) | 21.2 (4.7) | 0.46 |
| Education, years | 8.8 (3.5) | 8.7 (3.5) | 0.55 |
| Sex, men (%) | 41.8 | 37.1 | 0.10 |
| Midlife characteristics | |||
| SBP, mmHg | 144.2 (18.9) | 143.9 (20.0) | 0.78 |
| DBP, mmHg | 89.3 (10.5) | 89.1 (11.0) | 0.77 |
| BMI, kg/m2 | 26.6 (3.5) | 26.4 (3.7) | 0.31 |
| Cholesterol, mmol/l | 6.82 (1.2) | 6.66 (1.2) | 0.02 |
| Physically active,% | 39.4 | 42.2 | 0.33 |
| PUFA, g/day | 2.5 (4.0) | 2.8 (4.6) | 0.33 |
| SFA, g/day | 11.9 (15.5) | 12.7 (13.2) | 0.36 |
| Alcohol | |||
| Infrequent drinkers,% | 41.1 | 41.5 | 0.73 |
| Frequent drinkers,% | 28.0 | 30.2 | 0.59 |
| Smokers,% | 45.8 | 42.7 | 0.28 |
| Late-life characteristics | |||
| Dementia,% | 6.9 | 3.1 | 0.002 |
| AD,% | 5.8 | 2.3 | 0.001 |
| Diabetes,% | 5.8 | 6.4 | 0.66 |
| Stroke,% | 7.5 | 7.5 | 0.96 |
| Ml,% | 14.4 | 13.9 | 0.83 |
Chi-square and t-tests were used. Abbreviations: AD, Alzheimer's disease; BMI, body mass index; DBP, diastolic blood pressure; Ml, myocardial infarction;
SBP, systolic blood pressure; SFA, saturated fatty acids from spreads; PUFA, polyunsaturated fatty acids from spreads. Significant differences are bolded.
Lifestyle-related factors and dementia according to the apoE ε4 carrier status
Physical inactivity and moderate intake of dietary SFA were each found to approximately double the odds of dementia (Table 2). The associations between other lifestyle-related factors and dementia did not achieve a statistical significance in the analyses for all participants.
Table 2.
Lifestyle-related factors and the risk of dementia
| All (n= 1284) | ApoE ε4 carrier (n= 452) | ApoE ε4 non-carrier (n= 832) | |
|---|---|---|---|
| Physical activity | |||
| • Active | 1 | 1 | 1 |
| • Sedentary | 2.07 (1.12–3.86) | 2.43 (0.99–5.96) | 1,66 (0.68–4.05) |
| Dietary PUFA intake | |||
| • 1st quartile | 2.13 (0.73–6.20) | 1.95 (0.37–10.40) | 1.93 (0.45–8.38) |
| • 2nd quartile | 0.84 (0.27–2.63) | 0.71 (0.12–4.28) | 0.77 (0.16–3.69) |
| • 3ri quartile | 1.45 (0.50–4.17) | 1.76 (0.33–9.31) | 0.95 (0.23–3.86) |
| • 4th quartile | 1 | 1 | 1 |
| Dietary SFA intake | |||
| • 1st quartile | 1 | 1 | 1 |
| • 2nd quartile | 2.54 (1.13–5.68) | 4.34 (1.28–14.68) | 1.65 (0.51–5.26) |
| • 3rd quartile | 1.42 (0.54–3.75) | 2.08 (0.39–10.97) | 1.11 (0.30–4.12) |
| • 4th quartile | 2.64 (0.64–10.83) | 11.29 (0.82–155.94) | 1.13 (0.18–6.93) |
| Alcohol drinking* | |||
| • Never | 1 | 1 | 1 |
| • Infrequent | 1.29 (0.55–3.01) | 3.78 (0.94–15.24) | 0.50 (0.14–1.75) |
| • Frequent | 1.81 (0.70–4.65) | 7.42 (1.51–36.38) | 0,62 (0.17–2.33) |
| Smoking | |||
| • Non-smokers | 1 | 1 | 1 |
| • Smokers | 1.11 (0.57–2.20) | 1.97 (0.74–5.28) | 0.63 (0.23–1.75) |
Values are odds ratios (96% confidence intervals). The analyses are adjusted for age, sex, follow-up time, education, body mass index, serum cholesterol, systolic blood pressure, myocardial infarction, stroke and diabetes mellitus (the analyses for dietary fats are additionally adjusted for other subtypes of dietary fats and the analyses for physical activity additionally for locomotor disorders). Analyses for all are aditionally adjusted for ApoE4 carriers status.
Analyses for alcohol drinking: n= 945 (in the analyses for all), n= 343 (in the analyses for ApoE ε4 carriers) and n= 602 (in the analyses for ApoE ε4 non-carriers)
In the stratified analyses, the associations between dementia and moderate SFA intake, frequent alcohol drinking and physical inactivity were more pronounced among the apoE ε4 carriers than non-carriers. Furthermore, there was also a tendency towards increased odds of dementia for high-dietary SFA intake, infrequent alcohol drinking and smoking among the apoE ε4 carriers. However, the confidence intervals became somewhat wider in these analyses reflecting restricted sample size. For the apoE ε4 non-carriers, none of the lifestyle factor-dementia associations were significant (Table 2).
Combined effect of lifestyle factors and the apoE carrier status
Figure 2 shows the combined effect of the apoE ε4 carrier status and various lifestyle-related factors for dementia.
Fig. 2.
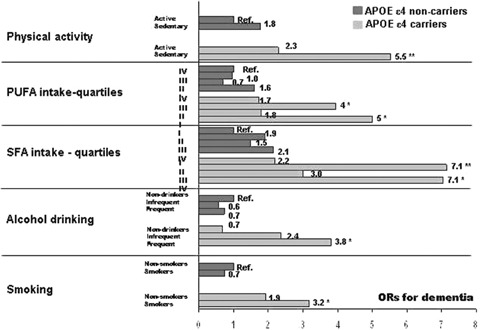
Combined effects of various lifestyle-related factors and ApoE ε4 carrier status for dementia. The values are odds ratios from logistic regression analysis adjusted with age, sex, follow-up time, education, body mass index, serum cholesterol, systolic blood pressure, and the history of myocardial infarction, stroke, and diabetes mellitus (the analyses for dietary fats are additionally adjusted for other subtypes of dietary fats and the analyses for physical activity additionally for locomotor disorders). Significant results are marked with * (P < 0.05) and ** (P < 0.001).
Physical inactivity: Compared with the apoE ε4 non-carriers who were physically active at midlife, the ‘sedentary ε4 non-carriers’ had an OR 1.77 (95% CI = 0.74–4.26) for dementia, the ‘active ε4 carriers’ 2.30 (95% CI = 0.81–6.49), and the ‘sedentary ε4 carriers’ 5.53 (95% CI = 2.31–13.23) after all the adjustments. However, there were neither significant multiplicative nor additive interactions between physical inactivity and the apoE ε4 carrier status for the odds of dementia.
Dietary PUFA and SFA intake: Compared with the apoE non-carriers who were in the high PUFA intake group, the persons in the ‘apoE ε4 carrier and low PUFA intake’ group had an OR 5.00 (95% CI = 1.45–17.42) for dementia. Similarly, compared to the apoE ε4, non-carriers who were in the low SFA intake group, the persons in the ‘apoE ε4 carrier and high SFA intake’ group had an OR 7.05 (95% CI = 1.34–37.06). There was no evidence for either a significant multiplicative or additive interaction between each subtype of dietary fats and the apoE ε4 carrier status for dementia (results not shown).
Alcohol drinking: Compared with the ‘apoE ε4 non-carrier and never drinker’ group the ‘apoE ε4 carrier and never drinker’ group had an OR 0.67 (95% CI = 0.17–2.73), ‘apoE ε4 carrier and infrequent drinker’ group 2.36 (95% CI = 0.83–6.73) and ‘apoE ε4 carrier and frequent drinker’ group 3.82 (95% CI = 1.14–12.75) for dementia. There was a statistically significant multiplicative (P-values: infrequent drinking = 0.04, frequent drinking = 0.03) and additive interaction (infrequent drinking: RERI = 1.97, P < 0.001, frequent drinking: RERI = 2.95, P < 0.001) between the apoE ε4 carrier status and alcohol drinking for dementia risk.
Smoking: Compared with the ‘apoE ε4 non-carriers and never smokers’, the ‘apoE ε4 non-carriers and ever smokers’ had an OR 0.73 (95% CI = 0.29–1.81), the ‘apoE ε4 carriers and never smokers’ 1.92 (95% CI = 0.88–4.19) and the ‘apoE ε4 carriers and ever smokers’ 3.17 (95% CI 1.37–7.35) for dementia. The multiplicative and additive interactions between smoking and the apoE ε4 carrier status were non-significant (results not shown).
Effect of sex: There was no statistically significant multiplicative interaction between sex and lifestyle factors or between sex and apoE ε4 carrier status (P-values for interaction terms between lifestyle factors and sex varied between P= 0.24 and P= 0.99, and for interaction term between apoE and sex P= 0.74) for dementia risk.
Composite effect of lifestyle on dementia risk
The 4th quartile of the composite lifestyle variable was associated with an increased risk of dementia among all participants (OR = 6.22, 95% CI = 2.13–18.13), and the risk in the 3rd quartile also approached statistical significance (OR = 2.71, 95% CI = 0.90–8.16). As expected, in the fully adjusted analyses combining the composite lifestyle variable with apoE ε4 carrier status the associations were more pronounced among apoE ε4 carriers: the OR was 4.80 (95% CI = 0.83–27.75) for the 2nd quartile, 6.40 (95% CI = 1.04–39.54) for the 3rd quartile, and 11.42 (95% CI = 1.94–67.07) for the 4th quartile. In contrary, the results for the composite variable were not significant among the apoE ε4 non-carriers (results not shown). Furthermore, in the stratified analyses the mean of the composite variable differed significantly between demented and non-demented among the apoE ε4 carriers (2.29 ± 0.76 versus 1.80 ± 0.91, P= 0.007), while such a difference was not found among the apoE ε4 non-carriers (0.35 ± 0.46 versus 0.22 ± 0.45, P= 0.20).
Lifestyle-related factors and AD
Analyses concerning lifestyle risks (individual lifestyle factors and their composite effects) for AD yielded virtually similar results than the analyses for all dementias. Also here, the associations were stronger among the apoE ε4 carriers (data not shown).
Discussion
This study showed that physical inactivity, high dietary fat intake, alcohol drinking and smoking are associated with the risk of dementia and AD especially among the apoE ε4 carriers. Each individual lifestyle-related risk factor was associated with an increased risk of dementia and AD; the increase varied from three times increased risk among the apoE ε4 carriers who smoked to sevenfold increase among the apoE ε4 carriers with high-dietary SFA intake. Further, also clustering of the lifestyle-related risk factors strongly increased the risk of dementia especially among the apoE ε4 carriers.
The possible effect modification of the apoE carrier status for various environmental factors-dementia associations has been suggested in previous epidemiological studies [4–6, 11, 14, 16–18, 20, 22, 25–30]. Results from our group, as well as some others, have supported the hypothesis that the apoE ε4 carriers are more vulnerable to various hazardous lifestyle related and vascular risk factors [4–6, 14, 16, 20, 29, 30, 39]. However, some studies have shown opposite results [11, 17, 18, 22, 25, 26] while others found no effect modification [9, 13, 15, 24] between the apoE ε4 carrier status and lifestyle-related factors. It is important to notice that almost all previous studies have been conducted on elderly cohorts (baseline age 55 years or more) and/or had relatively short follow-up times (ranging from 2 to 6 years). Effect of the apoE ε4 allele on dementia is suggested to attenuate with increasing age [2], which might explain the negative results concerning effect modification in some studies. Furthermore, subclinical dementia and other factors may have affected the individuals’ lifestyle at baseline in studies with shorter follow-up times. This kind of bias (i.e. reverse causality) is less likely to have occurred in our study where the assessment of the lifestyle-related factors was performed at midlife, on an average 21 years before the diagnosis of dementia. Additionally, midlife risk factors can be considered to be of special interest as the neurobiological changes of AD may start to develop decades before dementia can be clinically diagnosed [40]. Apart from our previous studies [4–6], only few epidemiological studies exist with follow-up times up to 20 years investigating the effects of lifestyle-related factors and taking also into account the possible effect modification by the apoE ε4 allele. One study focusing on smoking and with a follow-up time comparable to that of our study did not find any difference in the effect of smoking on the risk of dementia regarding to the apoE ε4 carrier status [24]. Interestingly, apoE ε4 may also modify effects of some drug treatments; a greater benefit has been suggested for the apoE ε4 carriers with acetylcholinesterase inhibitor donepezil in mild cognitive impairment [41], and atorvastatin in AD patients [42].
Accumulating evidence has implicated that the apoE ε4 allele influences not only AD, but also atherosclerosis and several other neurological disorders. A common molecular mechanism by which the apoE ε4 allele contributes to neurodegeneration has been suggested. Interestingly, apoE has been linked to all the major features in AD pathogenesis including β-amyloid generation and clearance, neurofibrillary tangle formation, oxidative stress, apoptosis, dysfunction in lipid transport and homeostasis, modulation of intracellular signalling and synaptic plasticity [43]. In all cases, the presence of the apoE ε4 allele has been shown to exacerbate these disturbances, in contrast to the protection seen with other apoE isoforms [43]. Further, apoE plays a key role in the maintenance and repair of neurons, and the apoE ε4 allele carriers show a poor compensation of neuronal loss in different brain regions [44]. Also treatment of neuronal cultures with reconstituted apoE3 enhances neurite outgrowth, whereas apoE4 treatment decreases synaptic plasticity [45]. Some studies have suggested that apoE4 transgenic mice might be more susceptible to such environmental factors that can be considered detrimental or stressing [46, 47]. Further, several reports have shown different deficit in apoE ε4 knock-in mice compared with those carrying the ε3, including altered astroglial organization [48], long-term potentiation [49], amyloid precursor protein (APP) processing [50] and cholinergic deficits [51]. Consequently, the lack of function observed in the apoE4, compared with other apoE isoforms, could act in concert with environmental deleterious factors and lead to neuropathological processes. The current study together with our recent experimental demonstration showing that combination of apoE deficiency and long-term high cholesterol diet induces neurodegenerative changes in mice [39] support this hypothesis.
Despite the relatively large cohort, the sample size in our study may still be considered to be limited especially concerning stratified analyses and interactions. However, this should not be a problem for significant associations found, but the lack of associations or interactions should be interpreted more cautiously, since they can result from lack of power if effect sizes are not very high. For stratified and multi-variable analyses, it would be desirable to have as large sample sizes as possible. Nevertheless, the number of the demented persons was almost equal among the apoE ε4 carriers and non-carriers, and it is the number of end-points that is important for statistical power. Thus, it is unlikely that lack of power would be the only explanation for the significant associations found for the apoE ε4 carriers alone. Moreover, similar patterns for all lifestyle risk factors studied further support the true effect modification by the apoE ε4 carrier status, which is also biologically plausible. The proportion of apoE ε4 carries is known to be somewhat higher in Finland (35% in our study) and Scandinavia compared to some other European countries (ranging from 10% up to 40%). The higher proportion of ε4 carriers in the population increases the statistical power and may make it easier to detect associations compared to more diverse populations. Thus, the relative homogeneity of our sample offers an advantage for the current analyses. Moreover, the similarity between the previous results obtained using Finnish cohorts versus other European or American populations concerning ApoE and environmental risk factors for dementia and AD [4–6, 14, 16, 20, 29, 30] supports the generalizability of the results from this study. The extent to which possible differences in risk factors profiles among different ethnic groups determine their absolute risks of dementia and AD need to be determined in further studies.
The design of our study as a large and representative population-based study including both men and women increases the credibility of our findings. However, our results may be somewhat compromised by survival bias. It has been shown previously that the apoE ε4 carrier status [52], physical inactivity [53], unhealthy dietary pattern [53], smoking [53] and heavy acute load of alcohol [54] are associated with increased premature mortality. Thus, if we assume that among the deceased there were more persons with the apoE ε4 allele and unhealthy lifestyle, and that they were more likely to be demented as well, then our results would not overestimate but rather underestimate the true dementia risk associated with these lifestyle-related factors and the apoE ε4 carrier status. Furthermore, the limitation in most large studies concerning lifestyle is the reliability of the data. Even though the level of each lifestyle-related factor cannot be determined exactly (e.g. absolute amount and quality of alcohol), ranking of individuals into different categories of lifestyle risk exposure, as done in our study, is possible. Concerning alcohol drinking, it might also be that it is the drinking pattern which together with apoE ε4 carrier status forms a hazardous combination. At the time of the midlife assessment of the current study, it was a common habit in Finland to drink reasonable seldom (most drinkers drank only once of twice per month in the current data), but still, a large quantity of alcohol per each drinking session (i.e. binge drinking). This binge drinking habit might specifically interact with apoE ε4 and therefore, the increase in the dementia risk might be more pronouncedly seen among those persons carrying the apoE ε4 allele and classified as frequent drinkers in our study. These issues need to be further clarified in large cohort studies with more detailed information about alcohol consumption.
Our findings suggest that physical activity, sufficient intake of PUFA and avoiding excess SFA intake, alcohol drinking and smoking may decrease the risk or postpone the onset of dementia especially among the apoE ε4 carriers. These findings provide an optimistic outlook for genetically susceptible individuals; adopting healthy lifestyle options may even reduce the risk in an apoE ε4 allele carrier close or equal to that in an apoE ε4 non-carrier. Therefore, while healthy lifestyle can be recommended for all, it should be the major preventive strategy especially for people who have the genetic susceptibility for dementia and AD. Even if genetic screening for the apoE ε4 allele might seem to be justified for high-risk individuals [55] or persons who are willing to know their carrier status (e.g. persons with family history of AD) as the recent results indicate [56], it can still be argued that such a genetic testing may not be appropriate at the population level at the moment, as the healthy lifestyle intervention will be beneficial also for the apoE ε4 non-carriers to reduce at least the risk of other major chronic diseases such as cardiovascular diseases, type 2 diabetes and certain cancers. However, it should also be noticed that if apoE genotyping is performed, it should never be done in isolation from a proper intervention plan to enhance healthy lifestyle and without information about the meaning of the test result [57]. Nevertheless, these results add rationale for possibilities to create tailor-made interventions and clinical trials for cognitive disorders according to appropriate carrier status.
Acknowledgments
The study was supported by EVO 5772720 from Kuopio University Hospital, grant IIRG-04-1345 from Alzheimer Association, Academy of Finland grants 103334, 206951 and 120676, the Gamla Tjänarinnor Foundation, the SADF (Insamligsstiftelsen för Alzheimer- och Demensforskning), Juho Vainio Foundation, the Finnish Cultural Foundation and Yrjö Jahnsson Foundation.
References
- 1.Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25:641–50. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 2.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer's disease: A meta-analysis. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- 3.Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer's disease in later life. Longitudinal, population based study. BMJ. 2001;322:1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rovio S, Kåreholt I, Helkala E-L, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–10. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 5.Anttila T, Helkala E-L, Viitanen M, Kåreholt I, Fratiglioni L, Winblad B, Soininen H, Tuomilehto J, Nissinen A, Kivipelto M. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329:539–42. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laitinen M, Ngandu T, Rovio S, Helkala E-L, Uusitalo U, Viitanen M, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M. Fat intake at midlife and risk of dementia and Alzheimer's disease: a population-based study. Dement Ger Cogn Disord. 2006;22:99–107. doi: 10.1159/000093478. [DOI] [PubMed] [Google Scholar]
- 7.Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- 8.Laurin D, Verreault R, Lindsay J, Mac Pherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–53. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 10.Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Chen CH, Petot GJ, Debanne SM. Patients with Alzheimer's disease have reduced activities in midlife compared with healthy control-group members. PNAS. 2001;98:3440–5. doi: 10.1073/pnas.061002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podewills LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–51. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 12.Larson EB, Wang L, Bowen JD, Mc Cormick WC, Teri L, Crane P, Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Int Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 13.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Schneider J, Wilson RS. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–6. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 15.Engelhart MJ, Geerlings MI, Ruitenberg AM, van Swieten JC, Hofman A, Witteman JCM, Breteler MMB. Diet and risk of dementia: does fat matter? The Rotterdam Study. Neurology. 2002;59:1915–21. doi: 10.1212/01.wnl.0000038345.77753.46. [DOI] [PubMed] [Google Scholar]
- 16.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–63. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 17.Huang TI, Zandi PP, Tucker KL, Fitzpatric AL, Kuller LH, Fried LP, Burke GL, Carlson MC. Benefits of fatty fish on dementia risk are stronger for those without APOE ε4. Neurology. 2005;65:1409–14. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 18.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alpérovitch A. Dietary patterns and risk of dementia. The Three-City cohort study. Neurology. 2007;69:1921–30. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 19.Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, Renaud S, Breteler MB. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol. 1997;153:185–92. [PubMed] [Google Scholar]
- 20.Ruitenberg A, van Swieten JC, Wittemann JCM, Mehta KM, van Duijn CM, Horman A, Breteler MMB. Alcohol consumption and risk of dementia: the Rotterdam study. Lancet. 2002;359:281–6. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 21.Huang W, Qiu C, Winblad B, Fratiglioni L. Alcohol consumption and incidence of dementia in a community sample aged 75 years and older. J Clin Epidemiol. 2002;55:959–64. doi: 10.1016/s0895-4356(02)00462-6. [DOI] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. JAGS. 2004;52:540–6. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 23.Almeida OP, Hulse GK, Lawrence D, Flicker L. Smoking as a risk factor for Alzheimer's disease: contrasting evidence from a systematic review of case-control and cohort studies. Addiction. 2002;97:15–28. doi: 10.1046/j.1360-0443.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 24.Tyas SL, White LR, Petrovitch H, Ross GW, Foley DJ, Heimovitz HK, Launer LJ. Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol Aging. 2003;24:589–96. doi: 10.1016/s0197-4580(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 25.Merchant C, Tang MX, Albert S, Manly J, Stern Y, Mayeux R. The influence of smoking on the risk of Alzheimer's disease. Neurology. 1999;52:1408–12. doi: 10.1212/wnl.52.7.1408. [DOI] [PubMed] [Google Scholar]
- 26.Ott A, Slooter AJC, Hofman A, van Harskamp F, Witteman JCM, van Broeckhoven C, van Duijn CM, Breteler MMB. Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351:1840–3. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- 27.Peila R, White LF, Petrovich H, Masaki K, Ross GW, Havlik RJ, Launer LJ. Joint effect of the APOE gene and midlife systolic blood pressure on late-life cognitive impairment. The Honolulu-Asia Aging Study. Stroke. 2001;32:2882–9. doi: 10.1161/hs1201.100392. [DOI] [PubMed] [Google Scholar]
- 28.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies. The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–62. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 29.Bunce D, Kivipelto M, Wahlin Å. Apolipoprotein E, B vitamins, and cognitive function in older adults. J Gerontol. 2005;60B:41–8. doi: 10.1093/geronb/60.1.p41. [DOI] [PubMed] [Google Scholar]
- 30.Martins IJ, Hone E, Foster JK, Sujram-Lea SI, Gnejc A, Fuller SJ, Nolan D, Gandy SE, Martins RN. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol Psych. 2006;11:721–36. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 31.Vartiainen E, Puska P, Jousilahti Pr, Korhonen HJ, Tuomilehto J, Nissinen A. Twenty-years trends in coronary risk factors in North-Karelia and in other areas of Finland. Int J Epidemiol. 1994;23:495–504. doi: 10.1093/ije/23.3.495. [DOI] [PubMed] [Google Scholar]
- 32.Tsukamoto K, Watanabe T, Matsushima T, Kinoshita M, Kato H, Hashimoto Y, Kurokawa K, Teramoto T. Determination by PCR-RFLP of ApoE genotype in a Japanese population. J Lab Clin Med. 1993;121:598–602. [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 36.Rothman KJ. Modern Epidemiology. Boston: Little, Brown; 1986. [Google Scholar]
- 37. http://www.sweold.se/working-papers/kareholt2003.pdf.
- 38.Assman SF, Hosmer DW, Lemeshow S, Mundt KA. Confidence intervals for measures of interaction. Epidemiology. 1996;7:286–90. doi: 10.1097/00001648-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Rahman A, Akterin S, Flores-Morales A, Crisby M, Kivipelto M, Schultzberg M, Cedazo-Minguez A. High cholesterol diet induces tau hyperphosphorylation in apolipoprotein E deficient mice. FEBS Lett. 2005;579:6411–6. doi: 10.1016/j.febslet.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer's disease: what is new since Alzheimer? Eur Arch Psychiatry Clin Neurosci. 1999;249:S14–22. doi: 10.1007/pl00014168. [DOI] [PubMed] [Google Scholar]
- 41.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Alzheimer's Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. NEJM. 2005;352:2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 42.Sparks DL, Connor DJ, Sabbagh MN, Petersen RB, Lopez J, Browne P. Circulating cholesterol levels, apolipopro-tein E genotype and dementia severity influence the benefit of atorvastatin treatment in Alzheimer's disease; results of the Alzheimer's Diseaswe Cholesterol-Lowering Treatment (ADCLT) trial. Acta Neurol Scand. 2006;114:S3–7. doi: 10.1111/j.1600-0404.2006.00690.x. [DOI] [PubMed] [Google Scholar]
- 43.Cedazo-Minguez A, Cowburn RF. Apolipoprotein E: a major piece in the Alzheimer's disease puzzle. J Cell Mol Med. 2001;5:254–66. doi: 10.1111/j.1582-4934.2001.tb00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arendt T. Disturbance of neuronal plasticity is a critical pathogenetic event in Alzheimer's disease. Int J Dev Neurosci. 2001;19:231–45. doi: 10.1016/s0736-5748(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 45.Nathan BP, Bellosta S, Sanan DA. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–2. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 46.Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166:883–91. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 47.Levi O, Michaelson DM. Environmental enrichment stimulates neurogenesis in apolipoprotein E3 and neuronal apoptosis in apolipoprotein E4 transgenic mice. J Neurochem. 2007;100:202–10. doi: 10.1111/j.1471-4159.2006.04189.x. [DOI] [PubMed] [Google Scholar]
- 48.Blain JC, Sullivan PM, Poirier J. A deficit in astroglial organization causes the impaired reactive sprouting in human apolipoprotein E4 targeted replacement mice. Neurolbiol Dis. 2006;21:505–14. doi: 10.1016/j.nbd.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Ye GL, Sotak M, Sullivan PM, Pasternak JF, LaDu MJ. ApoE isoform affects LTP in human targeted replacement mice. Neuroreport. 2004;15:2655–8. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- 50.DeMattos RB. Apolipoprotein E dose-dependent modulation of β-amyloid deposition in a transgenic mouse model of Alzheimer's disease. J Mol Neurosci. 2004;23:255–62. doi: 10.1385/JMN:23:3:255. [DOI] [PubMed] [Google Scholar]
- 51.Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci. 2002;22:10539–48. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ewbank DC. The APOE gene and differences in life expectancy in Europe. J Gerontol a Biol Sci Med Sci. 2004;59:16–20. doi: 10.1093/gerona/59.1.b16. [DOI] [PubMed] [Google Scholar]
- 53.De Groot LCMG, Verheijden MW, de Henauw S, Schroll M, van Staveren WA for the SENECA Investigators. Lifestyle, nurtritional status, health, and mortality in elderly people across Europe: a review on the longitudinal results of the SENECA study. J Gerontol. 2004;59A:1277–84. doi: 10.1093/gerona/59.12.1277. [DOI] [PubMed] [Google Scholar]
- 54.Kauhanen J, Kaplan GA, Goldberg DE, Salonen R, Salonen JT. Pattern of alcohol drinking and progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:3001–6. doi: 10.1161/01.atv.19.12.3001. [DOI] [PubMed] [Google Scholar]
- 55.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–41. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 56.Gooding HC, Linnenbringer EL, Burack J, Roberts JS, Green RC, Biesecker BB. Genetic susceptibility testing for Alzheimer disease: Motivation to obtain information and control as precursors to coping with increased risk. Patient Educ Couns. 2006;64:259–67. doi: 10.1016/j.pec.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Marteau TM, Roberts S, LaRusse S, Green RC. Predictive genetic testing for Alzheimer's disease: Impact upon risk perception. Risk Anal. 2005;25:397–404. doi: 10.1111/j.1539-6924.2005.00598.x. [DOI] [PubMed] [Google Scholar]


