Abstract
Androgen receptor (AR) is expressed in both stromal and epithelial cells of the prostate. The majority of studies on AR expression and function in prostate cancer is focused on malignant epithelial cells rather than stromal cells. In this study, we examined the levels of stromal AR in androgen-dependent and -independent prostate cancer and the function of stromal AR in prostate cancer growth and invasion. We showed that stromal AR levels were decreased in the areas surrounding cancerous tissue, especially in androgen-independent cancer. Using two telomerase-immortalized human stromal cell lines, one AR-positive and the other AR-negative, we demonstrated that stromal cells lacking AR stimulated cell proliferation of co-cultured prostate cancer cells in vitro and enhanced tumour growth in vivo when co-injected with PC3 epithelial cells in nude mice. In contrast, stromal cells expressing AR suppressed prostate cancer growth in vitro and in vivo. In parallel with cancer growth, in vitro invasion assays revealed that stromal cells lacking AR increased the invasion ability of PC3 cell by one order of magnitude, while stromal cells expressing AR reduced this effect. These results indicate a negative regulation of prostate cancer growth and invasion by stromal AR. This provides potentially new mechanistic insights into the failure of androgen ablation therapy, and the reactivation of stromal AR could be a novel therapeutic approach for treating hormone refractory prostate cancer.
Keywords: androgen receptor, prostate cancer, stroma, androgen ablation
Introduction
The tumour stromal environment has a critically active role in prostate cancer initiation and progression [1–4]. In the prostate, the stroma is composed of a heterogeneous population of cells, which includes smooth muscle cells, fibroblasts and myofibroblasts.
In a three-dimensional co-cultured system in vitro, stromal cells from benign prostate tissue can maintain the glandular structure of benign prostatic epithelium, indicating that stromal cells can dictate the behaviour of prostate epithelial cells [5]. In cancer areas, the stromal cells are largely remodelled from fibroblasts to myofibroblasts to form ‘reactive stroma’[6–8]. While cancer stromal cells can influence many aspects of cancer growth, motility, invasion and metastasis, the results of several studies examining the role of stromal cells in prostate cancer growth are controversial and have indicated both growth-inhibitory [9–11] and growth-promoting [12–14] effects.
Androgen and AR play an important role in prostate cancer oncogenesis and progression [15, 16]. The majority of studies on AR in prostate cancer have focused on malignant epithelial cells rather than stromal cells. Prostatic stromal cells are heterogeneous in terms of AR expression. It was reported that stromal AR expression decreases in prostate cancer [17, 18]. To better understand the functions of stromal AR in human prostate tumourigenesis and cancer progression, we established two telomerase-immortalized prostate stromal cell lines, one AR-positive and another AR-negative. Using these cell lines, we examined the function of stromal AR in prostate cancer growth and invasion in in vitro indirect stromal-epithelial co-cultured system. In addition, we examined the effect on prostate tumour growth by co-injecting these cell lines with PC3 epithelial cells into an in vivo nude mouse xenograft model. We showed that stromal AR inhibited prostate cancer growth and invasion. These findings are of great significance as they not only provide insight into the failure of androgen ablation treatment for prostate cancer but also offer the rationale supporting a novel modality in the treatment of prostate cancer.
Materials and methods
Establishing two immortalized prostate stromal cell lines: PshTertAR (AR-positive) and PshTert (AK-negative)
Primary stromal cells were transfected with a retroviral vector containing a full-length hTert cDNA and selected in hygromycin (100 μg/ml). Individual hygromycin-resistant colonies were expanded into cell lines and telomerase activity was measured using the TRAPeze telomerase detection kit (Promega, Madison, WI, USA). The cell lines with high telomerase activities were further expanded and analysed. To establish stromal cell lines stably expressing AR, AR was introduced into PShTert cells using retroviral vector pBabeAR. Three individual clones expressing AR were used in the experiments. Cells transfected with empty pBabe vector were used as control.
Electron microscopy and cytogenetics
Ultra thin sections were stained with uranyl acetate and lead citrate and analysed using a Zeiss EM-10 electron microscope with samples prepared as described [19]. Cytogenetic GTG-banded metaphase analysis was performed according to a standard protocol [20].
Cell culture, dual luciferase reporter assay and cell proliferation assays using cell counting in Transwell co-cultured system
The PC3 prostate cancer epithelial cell line and stromal cell lines were maintained in RPMI1640 with 10% FBS supplemented with 1% penicillin and streptomycin. Dual luciferase reporter assays were performed with the luciferase reporter with 4 × ARE and normalized with pRL internal control, as described previously [21]. Cell proliferation assays were performed by counting the number of cells every other day for 8 days. For co-culture experiments, epithelial cells were grown in Transwell inserts above stromal cells in 6-well plates while the stromal cells were seeded on the lower plate. All experiments were performed in triplicate.
Matrigel invasion assays in indirect co-culture system
Stromal cells were added to the lower chamber [22] of BD Biocoat Matrigel Invasion Chamber (BD Bioscience, Bedford, MA, USA). PC3 cell suspensions (5 × 104) in 0.5 ml DMEM with 0.1% BSA were placed on the insert. After 24 hrs of incubation, the number of invasive PC3 cells on the lower surface of the filter membrane was determined by Diff Quik staining and counted under a high power (400×). The average of the number of cells from three representative high-power fields was recorded.
Nude mice xenograft with subcutaneous epithelial and stromal cell co-injection
Male nude mice (4- to 5-weeks old) were purchased from NCI and maintained in accordance with the IACUC approved protocol. For subcutaneous injection into the flank region of male nude mice, 1 × 106 stromal cells and 1 × 106 epithelial cells were used. A total of 10 mice were used for each experimental group. The tumours were measured with calliper every 3 days. The tumours were removed from the mice and weighed at the endpoint after the mice were killed.
Western blot analysis and immunohistochemistry (IHC)
Whole cell extracts, performed with cells lysis buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 25 mM NaF and 10 μM ZnCl2), were subjected to SDS-PAGE and transferred to a nitrocellulose membrane for Western blot analysis using AR antibody, as described previously [23]. IHC was performed on 44 cases of androgen-dependent and 22 cases of androgen-independent prostate cancer, as described earlier [21]. Hormone-resistant (AI) samples were derived from patients who underwent transurethral resection of prostate (TURP) at least 6 months after surgical orchiectomy. Hormone naïve (AD) specimens were derived from patients who were diagnosed with prostate cancer by TURP, having high grade (Gleason 8 or higher) and volume of disease. For scoring, only spindle stromal cells in between and surrounding the glands (either benign or cancer) were considered.
Statistical analysis
Statistical analyses for IHC, cell proliferation and invasion assays were performed with pairwise t tests. P-values less than 0.05 were considered statistically significant.
Results
Stromal AR levels are decreased in prostate cancer, especially androgen-independent cancer
We determined the levels of AR in prostate stroma in 44 cases of androgen-dependent and 22 cases androgen-independent prostate cancer by IHC with affinity purified polyclonal AR antibodies. The 44 patients that underwent radical prostatectomy for prostate cancer ranged from 49 to 78 years in age, with Gleason scores (cancer differentiation) from 5 to 9 and tumour stages ranging from pT2a to pT3b. We scored the three, 100-cell areas of benign and cancerous prostate to determine the relative percentages of stromal cells that were AR-positive and AR-negative, respectively. The levels of stromal AR expression were expressed as an average percentage of AR-positive stromal cells. Similar to previous reports, there was a statistically significant decrease of stromal AR expression (P < 0.001) in the areas of prostate cancer compared with benign prostate (Fig. 1A and B) [17, 18]. In androgen-dependent cancer, there was up to a 6% decrease in stromal AR expression (Fig. 1C). When stratified with Gleason score, we observed a trend of greater decrease of AR-positive stromal cells in cancerous areas compared to benign areas with increased tumour grade (Fig. 1C). When comparing androgen-dependent and -independent tumours we observed a statistically significant, 3-fold decrease from 4% to 12%, of AR-positive stromal cells that were associated with androgen-independent prostate cancer (Fig. 1D). These results indicate that lower levels of AR in prostate stroma are associated with poorer differentiation and androgen independency.
Fig. 1.
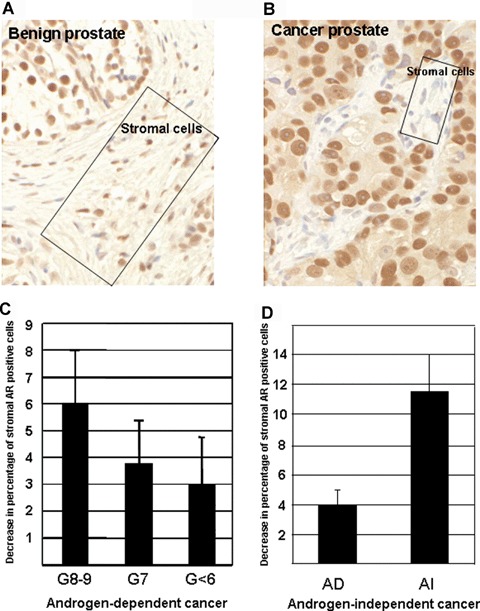
Decreased AR levels in pro-static stroma are associated with androgen- independent prostate cancer by IHC. Hundred stromal cells were scored for AR expression in three areas for each case in both cancerous and benign regions. Rectangles in A and B highlight representative regions of stroma in each tissue section. (A). AR- positive stromal cells. (B). Decreased AR positive stromal cells in cancer. (C), Decreased AR-positive stromal cells correlate with tumour differentiation in androgen-dependent cancer. G < 6 as well differentiated (n= 14), G7 as moderately differentiated (n= 22), and G8–9 (n= 8) as poorly differentiated cancer. (D). Dramatic decrease of stromal AR in androgen-independent cancer.
Construction of immortalized, AR-positive and AR-negative prostate stromal cell lines
The reports in the existing literature on the effect of prostatic stromal cells on cancer cell growth are inconclusive. The seemingly conflicting data could be the result of the intrinsic heterogeneity of prostate stromal cells, which can be comprised of smooth muscle, fibroblasts and myofibroblasts, as well as AR-positive and AR-negative stromal cells. Thus, it is important to establish a well-characterized stromal cell line for use in our study on stroma–epithelium interaction. We first established an immortalized stromal cell line from prostate with benign prostatic hyperplasia, termed PShTert, stably expressing the human telomerase catalytic subunit hTert. ELISA assay confirmed that there was a 2.5-fold increase in telomerase activity compared with parental primary cells.
Morphologically, the PShTert cells are markedly elongated and lie singly without any attachment to neighbouring cells. Under electron microscopy, the cytoplasm is characterized by presence of microfilaments located subplasmalemmally, with elongated monofilament densities scattered among them, and occasionally along the plasma membrane (Fig. 2A), often intimately associated with the rough endoplasmic reticulum (rER) (Fig. 2A). These observations suggest that these cells are myofibroblasts. IHC showed diffuse, strongly positive stain for Vimentin (Fig. 2A, inset), strong SMA staining in 25% of cells, and negative staining for Desmin, and together these data support the myofibroblastic nature of the PShTert stromal cells. Cytogenetically, these cells have a normal karyotype 46XY. Western blot analysis showed that AR was not expressed in PShTert cells (Fig. 2B, lane 1). The PShTert cells are non-tumourigenic using anchorage-independent assays and tumour growth in nude mice xenografts (either via subcutaneous or sub renal capsular route).
Fig. 2.
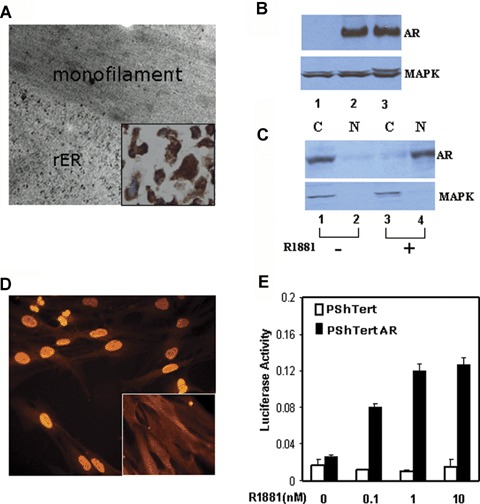
Characterization of hTert-immortalized AR-negative (PShTert) and AR-positive (PShTertAR) stromal cell lines. (A), Electron micrograph of the hTert-immortalized prostate stromal cell line, showing microfilaments and rough endoplasmic reticulum (rER). Inset: PShTert cells are Vimentin positive. (B). Expression of AR in PShTertAR (lane 2) but not in PShTert (lane 1) stromal cells by Western blot analysis. Whole cell extracts were subjected to SDS-PAGE and AR was detected by Western blotting with anti-AR antibody. PC3AR, a PC3 cell line stably expressing AR, served as positive control (lane 3). MAPK was used as a loading control. (C), Cytoplasmic expression (lane 1, upper panel), in the absence of androgen, and nuclear expression (lane 4, upper panel), in the presence of androgen, of AR was measured by Western blot analysis using nuclear or cytoplasmic fractions of PShTertAR cells. MAPK kinase served as cytoplasmic protein control (lanes 1 and lane 3, lower panel). N = nuclear fraction, C = cytoplasmic fraction. (D). AR expression in PShTertAR stromal cells by immunofluorescent microscopy showing weak cytoplasmic expression of AR in the absence of androgen (nuclear exclusion, inset) and strong nuclear staining in the presence of androgen. (E), AR in PShTertAR cells is responsive to androgen stimulation in a dual luciferase assay with an AR promoter-driven reporter. PShTert (white columns) and PShTertAR (black columns) transfected with the reporter constructs were subjected to increasing concentrations of the synthetic androgen analogue R1881.
Next, we transduced the AR-negative PShTert with pBabeAR retroviral vector and selected stable clonal cell lines expressing AR, termed PShTertAR. Western blot analysis revealed AR expression (Fig. 2B, lane 2). Western blot analysis performed with nuclear and cytoplasmic extracts further showed AR expression in cytoplasmic fraction (Fig. 2C, lane 1) in the absence of androgen and in nuclear fraction (Fig. 2C, lane 4) in the presence of androgen. Immunofluorescent analysis showed weak cytoplasmic expression of AR (Fig. 2D, inset) in the absence of androgen and strong nuclear expression of AR (Fig. 2D) in the presence of androgen. To determine if the ectopic AR is functional, we performed in vivo dual luciferase assays [21], which showed that there was a ligand-dependent transcriptional activation in the presence of androgen (Fig. 2E). These experiments confirm the presence of functional AR in PShTertAR cells.
PShTertAR, compared to PShTert, inhibits prostate cancer cell proliferation both in vitro and in vivo
To determine the effects of AR-positive and AR-negative stromal cells on prostate cancer cell growth in vitro, we performed proliferation assays using transwell indirect co-culture assays with PC3 and PShTert or PC3 and PShTertAR cells by cell counting. The results showed that, in the presence of androgen, co-culture with PshTertAR resulted in an inhibition of PC3 cell proliferation compared to PC3 cell growth when cultured alone (P < 0.045, Fig. 3A). In contrast, co-culture with the AR-negative PShTert cells resulted in an enhancement of the growth rate of PC3 cells, compared to PC3 grown alone (P < 0.03, Fig. 3A). Flow cytometric analysis revealed that PC3 cells co-cultured with PShTertAR showed 20% S-phase cells, decreased from the 27% S-phase cells measured in PC3 cells co-cultured with PShTert cells. We examined the expression of cell cycle genes, including cyclin A, cyclin B, p21 and p27, and the expression of Skp2 was decreased in PC3 cells co-cultured with PShTertAR compared with PC3 cells co-cultured with PShTert cells. Together, these results reveal distinct growth-regulatory effects of AR-positive and AR-negative stromal cells, and they indicate the presence of paracrine factors that are differentially regulated by stromal AR. Interestingly, when co-cultured in androgen-free media, both PShTert and PshTertAR stimulated the growth of PC3 cells (Fig. 3B). This is an important observation because these results indicate that under androgen ablation therapy, both AR-positive and AR-negative stromal cells would exert growth stimulatory effects. These stromal effects will counteract the apoptotic effects of androgen ablation and may lead to androgen independence.
Fig. 3.
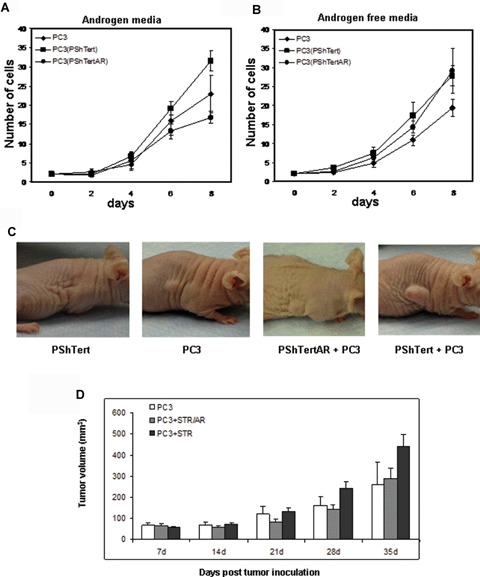
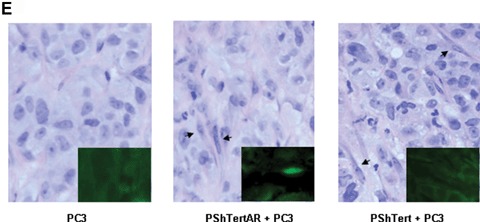
Stromal AR inhibits prostate cancer growth in vitro and in vivo. PC3 cells were co-cultured with PShTert (squares) or PShTertAR (circles), or grown alone (diamonds), for 8 days, in medium containing 10-nM synthetic androgen R1881 (3A) or in androgen-free medium (3B). Assays were performed in triplicate. (A) PShTertAR inhibited PC3 cell growth in the presence of androgen. (B) Both PShTert and PShTertAR stimulated PC3 cell growth in androgen-free media. (C) Effects of stromal cells on subcutaneous tumour growth in nude mice xenografts, 32 days after injection. No tumour growth with stromal cells (PShTert) only; middle, smaller tumour growth with PC3 cells only and PC3 plus PShTertAR; and enhanced tumour growth after co-injection of PShTert and PC3 cells in xenograft experiments. (D) Tumour volume after co-injection of PC3 together with PShTert (black columns) and PC3 together with PShTertAR (grey columns), compared with PC3 alone (white columns). Error bar represents SEM value. The P-value, calculated from the t-statistics on a particular day described by the same statistical distribution. Each column represents measurements of 10 tumours. (E) Histology and immunofluorescent stains of representative tumour sections. Haematoxylin and eosin (H&E) stain showing sections of tumour xenografts from PC3, PC3 with PShTertAR and PC3 with PShTert, mimicking poorly differentiated human prostate cancer. AR immunofluorescent staining of tumour xenografts of PC3 (left inset), PC3 with PShTertAR cells (middle inset), and PC3 with PShTert cells (right inset), 400× magnification.
Next, we investigated if AR-positive and AR-negative stromal cells affect tumour cell growth in vivo in nude mice xenografts. The mixture of PC3 (5 × 106) and PShTert or PShTertAR (5 × 106) cells was injected subcutaneously in the flank region of nude male mice. The controls were mice injected with PC3 (5 × 106) or PShTert (5 × 106) alone. In agreement with the results of the in vitro co-culture experiments, co-injection of PC3 and PShTert resulted in the development of tumours in the mice xenografts that were up to twice as large as when PC3 was injected alone (Fig. 3C). Co-injection of PC3 and PShTertAR resulted in statistically significant reductions (starting day 21 after injection) of tumour growth compared to PC3 co-injected with PShTert (Fig. 3D). Histology of the tumour showed stromal cells supporting the growth of cancer cells in a way that mimicked poorly differentiated human prostate cancer. Immunofluorescent analysis of representative PC3/PShTertAR tumours confirmed the presence of AR-expressing stromal cell (Fig. 3E, mid-inset). PC3 cell and PShTert cells are negative for AR staining indicating the specificity. These results demonstrate that stromal cells expressing AR suppress prostate cancer growth in vivo compared to stromal cells lacking AR stimulate prostate cancer tumour growth.
AR-positive stromal cells inhibit prostate cancer cell invasion in vitro compared to AR-negative stromal cells
In addition to uncontrolled growth, invasion is another characteristic of cancer cells. To determine the role of stromal AR in prostate cancer invasion, we performed Matrigel invasion assay while indirectly co-culturing PC3 cells with PShTert or PShTertAR cells [22], in the presence or absence of androgen analogue R1881. We counted the number of PC3 cells invaded through the Matrigel membrane (Fig. 4A). When PC3 cells were assayed alone in the absence of stromal cells, addition of synthetic androgen ligand R1881 decreased their invasive ability. This is consistent with reports in literature [24]. While levels of AR in the PC3 cell line are undetectable by Western blot, a small sub-population of the PC3 cell line is AR-positive. When co-cultured with PShTert, the invasiveness of PC3 cells increased roughly one order of magnitude (Fig. 4B, lane 6 versus lane 2) (P= 0.0002), while the relative effect of ligand addition was preserved (Fig. 4B, lanes 5–6 versus lanes 1–2). The invasiveness of PC3 cells was approximately 50% lower when co-cultured with PShTertAR (P= 0.003); still, addition of ligand decreased the invasiveness. The number of invasive cells was normalized to total number of stromal cells grown at 24 hrs in the co-cultured invasion assays. These data are analogous to the results of our indirect co-culture proliferation assay and consistent with the scenario that either AR negatively regulates one invasion-enhancing paracrine factor or AR differentially regulates one invasion-enhancing factor and one invasion-inhibiting factor.
Fig. 4.
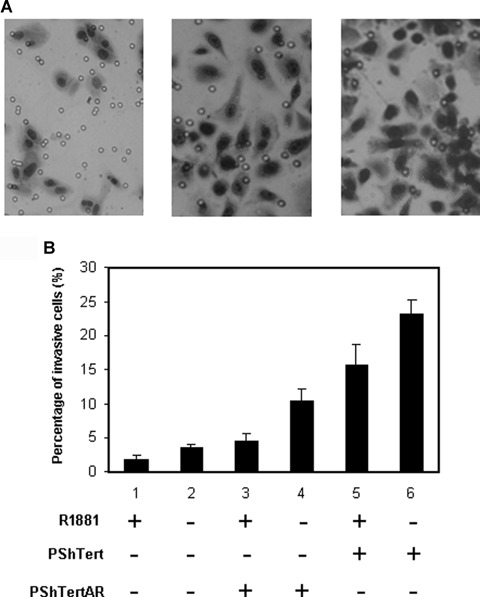
Effects of stromal AR on prostate cancer cell invasion. (A) Matrigel invasion assay while indirectly co-culturing PC3 cells with PShTert or PShTertAR cells[22], in the presence or absence of androgen analogue R1881. The number of PC3 cells invaded through the Matrigel membrane was counted. (B) Stromal AR reduced invasion ability of prostate cancer PC3 cells inMatrigel invasion assays co-cultured with stromal cells. Percentage of invasive cells graphed, with each column representing three independent counts. While stromal cells increased PC3 cell invasion ability (lanes 3–6 versus 1–2), introduction of AR in stromal cells reduced invasion ability (lane 5–6 versus 3–4).
Discussion
In this work, we demonstrated that stromal AR decreases the growth and invasive ability of prostate cancer cells. Stromal cells positive for AR expression decreased the growth of PC3 cells compared to stromal cells negative for AR expression when assayed in an indirect co-cultured system. We also employed LNCaP cells to determine the growth regulatory effects of stromal AR in the indirect co-cultured system. The results are similar to our findings with PC3 cells (Supplement Fig. 1). We showed stromal cells, either positive or negative for AR, promoted LNCaP cell growth at a comparable level to PC3 cells in androgen-free media. In the presence of androgen, while AR-negative stromal cells promoted LNCaP cell growth at comparable level to PC3 cells, AR-positive stromal cells repressed the growth of LNCaP cells, though to somewhat of a lesser degree than the level of PC3 cells. One of the explanations for this observation is that since androgen and AR enhance LNCaP cell growth, the end result is due to the inhibition of epithelial cells by stromal AR and additional stimulation of epithelial cell by androgen and AR.
Since the in vitro cell proliferation and invasion assays were performed under an indirect, co-cultured system (i.e. the stromal and epithelial cells were not in direct contact), the distinct effects of AR-positive stromal cells and AR-negative stromal cells on cell growth and invasion strongly suggest the involvement of paracrine pathways. Of note, we also observed a decreased growth of prostate stromal cells with the introduction of AR (Li and Lee, unpublished results); however, this will not change our interpretation that AR inhibits PC3 cell growth in vitro since the control is PC3 without stromal cells. In addition, the difference in tumour size between PC3 only and PC3 plus AR-positive stromal cells is at reduced level in xenograft experiments. Potentially, the observed differences between in vitroversusin vivo data are due to variation in the direct contact between tumour cells and stromal cells and the involvement of angiogenesis in the xenografts, which may result in differences in the mechanism by which stromal cells affect PC3 cells. Nevertheless, our in vitro and in vivo data support the hypothesis that compared with stromal cells negative for AR, AR-positive stromal cells inhibit epithelial cell growth. In Matrigel invasion assays in the presence of R1881, PC3 cells showed reduced invasion capacity when co-cultured with AR-positive compared to AR-negative stromal cells. A difference in PC3 cell invasion between the presence of PSTert and PSTertAR stromal cells in the absence of R1881 is observed, possibly suggesting an androgen-independent function of AR.
Our results suggest the involvement of at least two paracrine factors in cancer proliferation (Fig. 3A), one growth promoting (AR-independent), the other growth suppressing (and likely AR-regulated). In addition, our in vitro invasion results are consistent with the presence of at least one paracrine factor (Fig. 3D). Whether the same putative factors are responsible for both the observed growth and invasion regulation cannot be deduced from the current data. Understanding the paracrine regulation of growth, metastasis and androgen independence by stromal cells could lead to their development as a new therapeutic intervention target for prostate cancer, and we are actively investigating this new direction.
While we show stromal AR has inhibitory effects on prostate cancer growth and invasion in androgen media, intriguingly, stromal cells both with and without AR stimulate the growth of cancer cells in androgen-free media (Fig. 3B). Interestingly, we isolated AR-negative, instead of AR-positive stromal cells from primary culture in establishing the immortalized stromal cell lines, while prostate stromal tissue consists of both AR positive and negative stromal cells. This could be explained by slower growing nature of stromal cells positive for AR, possibly serving as a selection power that favours the growth of AR negative cells. These results are consistent with the observation in clinical samples that stro-mal AR is indeed decreased in areas of prostate cancer. Together, these results provide an important new insight into the mechanism in the development of androgen-independent cancer after androgen ablation therapy. Androgen ablation results in the increased death rate of malignant epithelial cells. However, the effect of androgen ablation on stromal cells is likely to prevent the inhibition of growth and invasion exerted by stromal AR. This may, in turn, facilitate the survival, growth, and invasion of a sub-population of the epithelial prostate cancer cells, eventually leading to androgen-independence. We propose that, in addition to androgen ablation, targeting the AR-negative stromal cells would be beneficial to the treatment of prostate cancer patients.
Acknowledgments
We would like to thank Dr. Susan Logan for scientific discussion. We thank Jessie Yu for editorial assistance. This work is supported by DOD and VA Merit Grants to P.L. and NIH grant (DK058024) to M.J.G.
References
- 1.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–85. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 2.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–83. [PubMed] [Google Scholar]
- 3.Edlund M, Sung SY, Chung LW. Modulation of prostate cancer growth in bone microenvironments. J Cell Biochem. 2004;91:686–705. doi: 10.1002/jcb.10702. [DOI] [PubMed] [Google Scholar]
- 4.Condon MS. The role of the stromal microenvironment in prostate cancer. Semin Cancer Biol. 2005;15:132–7. doi: 10.1016/j.semcancer.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Hall JA, Maitland NJ, Stower M, Lang SH. Primary prostate stromal cells modulate the morphology and migration of primary prostate epithelial cells in type 1 collagen gels. Cancer Res. 2002;62:58–62. [PubMed] [Google Scholar]
- 6.Tomas D, Kruslin B. The potential value of (Myo)fibroblastic stromal reaction in the diagnosis of prostatic adenocarcinoma. Prostate. 2004;61:324–31. doi: 10.1002/pros.20109. [DOI] [PubMed] [Google Scholar]
- 7.Singh H, Dang TD, Ayala GE, Rowley DR. Transforming growth factor-beta1 induced myofibroblasts regulate LNCaP cell death. J Urol. 2004;172:2421–25. doi: 10.1097/01.ju.0000138082.68045.48. [DOI] [PubMed] [Google Scholar]
- 8.Untergasser G, Gander R, Lilg C, Lepperdinger G, Plas E, Berger P. Profiling molecular targets of TGF-beta1 in prostate fibroblast-to-myofibroblast trans differentiation. Mech Ageing Dev. 2005;126:59–69. doi: 10.1016/j.mad.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Konig JJ, Romijn JC, Schroder FH. Prostatic epithelium inhibiting factor (PEIF): organ specificity and production by prostatic fibroblasts. Urol Res. 1987;15:145–9. doi: 10.1007/BF00254426. [DOI] [PubMed] [Google Scholar]
- 10.Kooistra A, Romijn JC, Schroder FH. Stromal inhibition of epithelial cell growth in the prostate: overview of an experimental study. Urol Res. 1997;25:S97–105. doi: 10.1007/BF00941995. [DOI] [PubMed] [Google Scholar]
- 11.Degeorges A, Tatoud R, Fauvel-Lafeve F, Podgorniak MP, Millot G, de Cremoux P, Calvo F. Stromal cells from human benign prostate hyperplasia produce a growth-inhibitory factor for LNCaP prostate cancer cells, identified as interleukin-6. Int J Cancer. 1996;68:207–14. doi: 10.1002/(SICI)1097-0215(19961009)68:2<207::AID-IJC12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Lang SH, Stower M, Maitland NJ. In vitro modelling of epithelial and stromal interactions in non-malignant and malignant prostates. Br J Cancer. 2000;82:990–7. doi: 10.1054/bjoc.1999.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabalin JN, Peehl DM, Stamey TA. Clonal growth of human prostatic epithelial cells is stimulated by fibroblasts. Prostate. 1989;14:251–63. doi: 10.1002/pros.2990140306. [DOI] [PubMed] [Google Scholar]
- 14.Castellon E, Venegas K, Saenz L, Contreras H, Huidobro C. Secretion of prostatic specific antigen, proliferative activity and androgen response in epithelial-stromal co-cultures from human prostate carcinoma. Int J Androl. 2005;28:39–46. doi: 10.1111/j.1365-2605.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 15.Linja MJ, Visakorpi T. Alterations of androgen receptor in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:255–64. doi: 10.1016/j.jsbmb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–90. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 17.Henshall SM, Quinn DI, Lee CS, Head DR, Golovsky D, Brenner PC, Delprado W, Stricker PD, Grygiel JJ, Sutherland RL. Altered expression of androgen receptor in the malignant epithelium and adjacent stroma is associated with early relapse in prostate cancer. Cancer Res. 2001;61:423–7. [PubMed] [Google Scholar]
- 18.Olapade-Olaopa EO, MacKay EH, Taub NA, Sandhu DP, Terry TR, Habib FK. Malignant transformation of human pro-static epithelium is associated with the loss of androgen receptor immunoreactivity in the surrounding stroma. Clin Cancer Res. 1999;5:569–76. [PubMed] [Google Scholar]
- 19.Sidhu GS, Wieczorek R, Cassai ND, Zhu CC. The concept of bronchioloalveolar cell adenocarcinoma: redefinition, a critique of the 1999 WHO classification, and an ultra-structural analysis of 155 cases. Int J Surg Pathol. 2003;11:89–99. doi: 10.1177/106689690301100204. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Perle MA, Scholes JV, Yang GC. Wilms’ tumor in adults: aspiration cytology and cytogenetics. Diagn Cytopathol. 2002;26:99–103. doi: 10.1002/dc.10048. [DOI] [PubMed] [Google Scholar]
- 21.Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, Wang YF, Wang Z. The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol. 2005;19:1792–802. doi: 10.1210/me.2004-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura K, Kitamura M, Miura H, Nonomura N, Takada S, Takahara S, Matsumoto K, Nakamura T, Matsumiya K. Prostate stromal cell-derived hepatocyte growth factor induces invasion of prostate cancer cell line DU145 through tumor-stromal interaction. Prostate. 1999;41:145–53. doi: 10.1002/(sici)1097-0045(19991101)41:3<145::aid-pros1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Gao S, Wang H, Lee P, Melamed J, Li CX, Zhang F, Wu H, Zhou L, Wang Z. Androgen receptor and prostate apoptosis response factor-4 target the c-FLIP gene to determine survival and apoptosis in the prostate gland. J Mol Endocrinol. 2006;36:463–83. doi: 10.1677/jme.1.01991. [DOI] [PubMed] [Google Scholar]
- 24.Bonaccorsi L, Carloni V, Muratori M, Salvadori A, Giannini A, Carini M, Serio M, Forti G, Baldi E. Androgen receptor expression in prostate carcinoma cells suppresses alpha6beta4 integrin-mediated invasive phenotype. Endocrinology. 2000;141:3172–82. doi: 10.1210/endo.141.9.7640. [DOI] [PubMed] [Google Scholar]


