Abstract
Homing and regenerative potential of endogenous bone marrow cells (BMC) in myocardial infarction (MI) is a controversial issue. Using human placental alkaline phosphatase (hPLAP) as genetic marker for cell tracking, we examined the influx of bone marrow-derived cells during tissue repair after the induction of MI over a study period of 17 weeks in wild-type inbred Fischer 344 rats, lethally irradiated and reconstituted with bone marrow (BM) from transgenic F344 rats expressing hPLAP under the control of the ubiquitous R26 promoter. During the early phases of tissue repair, hPLAP-positive macrophages, endothelial cells, fibroblasts and also myofibroblast-like cells were recruited from BM. However, only some hPLAP-positive endothelial cells, fibroblasts and myofibroblast-like cells persisted until 17 weeks after MI. With the exception of a single cell, there was no evidence of BM-derived cardiomyocytes throughout the study. Rather, some local cardiac progenitor cells appeared to differentiate into cardiomyocytes in the peri-infarct regions. In conclusion, our data show that the inflammation-induced influx of BM-derived cells into the infarction area is restricted to leukocytes, endothelial cells, fibroblasts and myofibroblast-like cells. Our long-term analysis casts doubt on the hypothesis that circulating BM-derived mesenchymal precursor cells participate in cardiomyogenesis after MI.
Keywords: regeneration, tissue repair, stem cells, myocardial infarction, human placental alkaline phosphatase, bone marrow cells
The adult mammalian heart was previously considered to be a terminally differentiated, postmitotic organ. However, some years ago, data from sex-mismatched heart transplantation studies challenged this notion. In female transplants, a considerable frequency of Y chromosome-positive cardiomyocytes of recipient origin was found [1]. In contrast, other similarly designed studies found no [2] or very little [3, 4] evidence of recipient-derived cardiomyocytes in sex-mismatched heart transplantations. Data from sex-mismatched heterotopic heart transplantations in rats [5] confirmed the very low regeneration rates seen in the majority of transplantation studies [3, 4]. Much of this controversy may be due to methodological problems with the histological detection of the Y chromosome in tissue sections. Therefore, it is still controversial whether bone marrow-derived cells are able to regenerate myocardial tissue after myocardial injury by differentiating into functional cardiomyocytes.
There is good evidence that bone marrow cells (BMC) can differentiate into cardiomyocytes in vitro[6]. In addition, the existence of cells residing in bone marrow (BM) and expressing early cardiac markers has been demonstrated [7]. However, in vivo experimental studies aimed at elucidating the regeneration potential of BMC after myocardial infarction (MI) showed conflicting results [8–12]. Tracking of BM-derived cells in these studies was performed by reconstitution of lethally irradiated animals with BMC of transgenic (tg) donors expressing green fluorescent protein (GFP) [9–12] or bacterial β-galactosidase (LacZ)[8] as genetic markers. However, lacZ and GFP have several important disadvantages for histological cell tracking. Although lacZ can easily be detected histochemically, it is heat labile, and the enzyme activity is destroyed during paraffin embedding. Therefore, pre-embedding staining methods or immunohistochemistry are necessary to visualize the marker. GFP can easily be visualized in living cells because of its fluorescent properties. However, the fluorescent properties of GFP are greatly diminished during normal paraffin embedding, and sensitive detection of GFP in paraffin sections requires either sophisticated equipment or immunohistochemical detection methods [13]. Thus, the usefulness of GFP for histological studies is limited.
The current study employs human placental alkaline phosphatase (hPLAP) as genetic marker. hPLAP retains its enzymatic activity after paraffin as well as plastic embedding, and allows sensitive tracing of labelled cells in the total absence of background staining [14, 15]. Because hPLAP is a heat-stable enzyme, endogenous alkaline phosphatases can be blocked by heat inactivation. The robust detection methods for this marker enzyme overcome the problems with fluorescent marker proteins such as GFP and non-specific tissue autofluorescence. Here, we used hPLAP as genetic marker to examine the role of endogenous BMC in long-term cardiac repair after myocardial anterior wall infarction. We lethally irradiated inbred wild-type Fischer 344 (F344) rats, and reconstituted them with BM from hPLAP transgenic (hPLAP-tg) F344 donors overexpressing hPLAP under the R26 promoter [14]. hPLAP-tg rats show ubiquitous, uniform and stable expression of this genetic marker [14]. Subsequently, we examined the homing of endogenous hPLAP-labelled BMC after experimentally induced MI in these BM-transplanted (hPLAP-BMT) rats. It has been shown that irradiation and co-isogeneic BMT prior to induction of MI have no effect on the inflammatory processes during infarct repair [11]. Therefore, it is thought that co-isogeneic BMT does not influence the remodelling processes after MI.
All experimental procedures were conducted in compliance with prevailing animal welfare regulations, and were approved by the Ethical Committee of the University of Veterinary Medicine Vienna. Twenty-six wild-type female F344 rats received a lethal dose of whole body irradiation at the age of 18 weeks in a single dose of 8 Gray using a linear accelerator (6MV, Primus, Siemens, Vienna, Austria). Six to 8 hrs after irradiation, animals were reconstituted by intravenous administration of 4 × 106 BMC isolated from co-isogeneic hPLAP-tg littermates as described previously [15]. Three weeks later, rats underwent sham surgery (n= 3) or induction of MI (n= 4–6 per time point) under medetomidine/midazolam/fentanyl anaesthesia and controlled ventilation with 100% oxygen. Following left lateral thoracotomy, the left coronary artery (LCA) was ligated for 30 min. to induce myocardial ischaemia, and then reperfused. Sham-operated (SHAM) animals underwent the identical procedure except ligation of the artery. Rats were killed 2, 5 and 17 weeks after MI by exsanguination from the abdominal aorta under ketamine/xylazine anaesthesia. Because hPLAP enzyme activity is sensitive to formaldehyde fixation [15], hearts were fixed in 40% ethanol, and embedded in paraffin.
Our previous data suggested that hPLAP is ubiquitously expressed in hPLAP-tg rats [14, 15]. An obvious prerequisite for the current study was that hPLAP-tg cardiomyocytes express the transgene. Figure 1A shows intensely stained myocardium from a hPLAP-tg rat, demonstrating that the marker enzyme is strongly expressed in cardiomyocytes of hPLAP-tg rats. In accordance with our earlier results in hPLAP-BMT rats [15], we found only hPLAP-positive BM-derived capillary endothelial cells in the heart muscle of SHAM animals, but no hPLAP-positive cardiomyocytes as judged by cell morphology (Fig. 1B). For double staining of hPLAP-positive cardiomyocytes and endothelial cells, we combined histochemical hPLAP staining after heat inactivation and immunohistochemical staining with tomato lectin (Fig. 1C) for endothelial cells, and against alpha-sarcomeric actin (αSA, Fig. 1D) for cardiomyocytes. Double staining revealed hPLAP-positive endothelial cells but no hPLAP-positive cardiomyocytes, showing that bone marrow is a source of capillary endothelial cells but not of cardiomyocytes in the healthy heart (Fig. 1A–C).
Fig. 1.
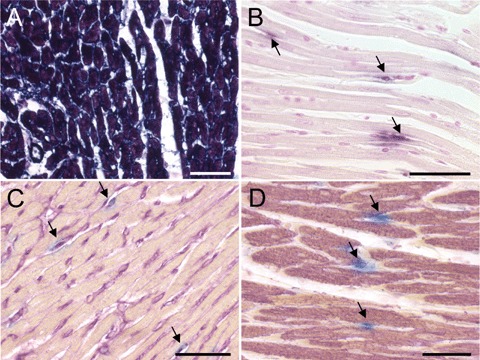
Paraffin sections of hearts from hPLAP-tg and sham-operated wild-type F344 rats reconstituted with bone marrow (BM) from hPLAP-tg F344 donors. (A and B) Sections stained for hPLAP activity after blocking of endogenous alkaline phosphatases by heat pretreatment (30 min. at 65°C), and incubation with BCIP/NBT (Sigma, Deisenhofen, Germany) over night at room temperature, as described previously [15]. (A) Myocardium of a hPLAP-tg rat shows intense hPLAP-staining in cardiomyocytes, demonstrating strong expression of the marker enzyme in transgenic cardiomyocytes. (B) Myocardium of a SHAM hPLAP-BMT rat stained for hPLAP activity, 2 weeks after surgery. The hPLAP-positive endothelial cell-like cells are marked by arrows. (C and D) Double staining of hPLAP enzyme activity (blue) and tomato lectin (purple, C) or alpha-sarcomeric actin (αSA, purple, D), showing BM-derived hPLAP-positive capillary endothelial cells (arrows) but no double positive cardiomyocytes in SHAM rats, 2 weeks after surgery. Double staining of myocardial tissue was performed as follows: In a first step, histochemical detection of hPLAP-positive cells was performed after heat inactivation of endogenous alkaline phosphatases (AP) by incubating the slides for 5 hrs with the AP substrate Vector Blue (Vector, Burlingame, CA, USA) at room temperature in the dark. After quenching of endogenous peroxidase activity by using 3% H2O2 in phosphate-buffered saline (PBS) for 15 min., slides were incubated with 20% horse or rabbit serum (Vector) for 20 min. Thereafter, slides were incubated with mouse anti-αSA (Sigma) diluted 1:500 in PBS containing 5% horse serum at 4°C over night. Bound antibody was detected by incubation with biotinylated rat adsorbed horse antimouse antibody (Vector) 1:50 for 1 hr at room temperature. Staining was accomplished by applying ABC-peroxidase complex (Vector) for 30 min., followed by incubation with the peroxidase substrate VIP (Vector) for 0.5–min., resulting in purple staining of positive cells. For detection of endothelial cells after histochemical hPLAP staining and peroxidase blocking, slides were incubated with the biotinylated tomato lectin Lycopersicon esculentum (Vector) 1:200 for 2 hrs at room temperature. Visualization was performed by ABC kit as described above. No counterstaining was carried out and slides were mounted with gelatin (Merck, Darmstadt, Germany). Five-μm-thick paraffin sections of 40% ethanol-fixed hearts. Bar = 50 μm.
Temporary occlusion of the LCA resulted in large areas of ischaemic myocardium, as shown by Masson trichrome (MT) staining (Fig. 2A–C). As expected, serial sections stained by histochemistry (HC) for hPLAP showed co-localization of the infarcted heart muscle and the hPLAP-positive BM-derived cell infiltrate (Fig. 2D–F). Infiltration of the damaged myocardium by BM-derived cells decreased over time, and only few of the cells recruited from BM were found in the scar at the end of the study (Fig. 2D–I).
Fig. 2.
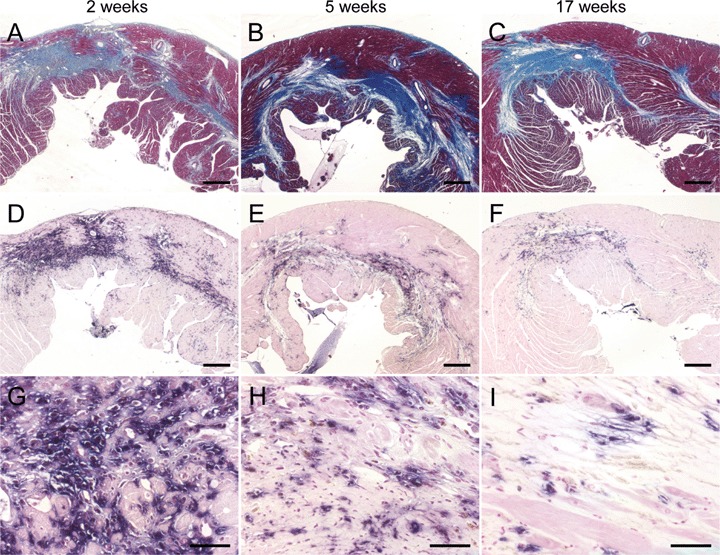
Size of myocardial lesions and bone marrow (BM)-derived cellular infiltrate as a function of time after myocardial infarction (MI) in wild-type F344 rats reconstituted with BM from hPLAP-tg F344 rats. (A–C) Representative Masson trichrome (MT)-stained heart sections at 2, 5 and 17 weeks after MI. MT staining (Sigma) was performed using routine protocols. (D–I) Histochemical staining of hPLAP enzyme activity using NBT/BCIP as substrate after heat pretreatment as described in Fig. 1. (A–F) Co-localization of myocardial lesions (A–C) and the hPLAP-positive, BM-derived cellular infiltrate (D–F) in serial sections. It is evident that infiltration of hPLAP-positive cells decreased with time, whereas the size of the scar remained largely unchanged. Five-μm-thick paraffin sections of 40% ethanol-fixed hearts. (A–F) Bar = 500 μm, (G–I) bar = 50 μm
To examine further the nature of the cells recruited from BM, we performed double stainings (Figs 3–5), and quantified single and double positive cells in the infarct and peri-infarct regions (Fig. 6). Heart sections were stained for hPLAP by immunohistochemistry (IHC) or HC, and, subsequently, for CD68, vimentin, smooth muscle actin (SMA), connexin 43, αSA by IHC or by tomato lectin. CD68 was used as a marker for macrophages, vimentin as marker for early differentiated cardiomyocytes [16], endothelial cells and fibroblasts, SMA as marker for myofibroblasts, smooth muscle cells and precursors of cardiomyocytes [17], connexin 43 and αSA as markers for mature cardiomyocytes, and tomato lectin as marker for endothelial cells.
Fig. 3.
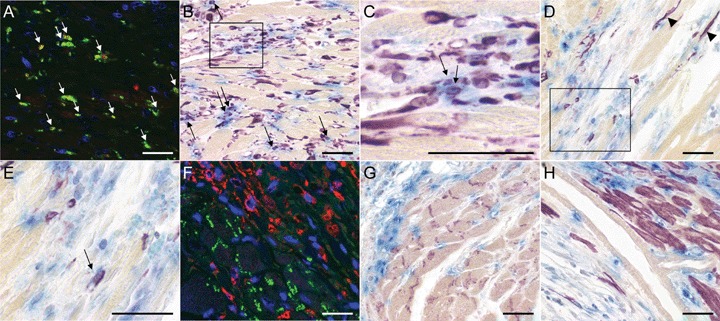
Double staining of the cellular infiltrate in hPLAP-BMT rats, 2 weeks after myocardial infarction (MI). Double positive cells are indicated by arrows. (A) For double fluorescence labelling of hPLAP and CD68 the slides were incubated with mouse anti-hPLAP antibody (Chemicon, Temecula, CA, USA) 1:50 for 2 hrs at 37°C, followed by secondary biotinylated rat adsorbed horse antimouse antibody (Vector) 1:200 for 1 hr at room temperature and Streptavidin Alexa Fluor 568 conjugate (Molecular Probes, Eugene, OR, USA). After blocking with M.O.M. kit (Vector), the sections were incubated with mouse anti-rat CD68 (Serotec, Oxford, UK) 1:50, which was finally detected with Streptavidin Alexa Fluor 488 (Molecular Probes). (F) For double fluorescence labelling of hPLAP and connexin43, slides were incubated with a cocktail of anti-hPLAP antibody 1:100 and rabbit anti-connexin43 (Sigma) 1:2000 for 2 hrs at 37°C. Subsequently, slides were incubated with biotinylated rat adsorbed horse antimouse antibody, and subsequently with a cocktail of goat anti-rabbit Alexa 488 1:100 (Molecular Probes) and Streptavidin Alexa Fluor 568 conjugate 1:100. Thereafter, nuclei were stained with DAPI (Molecular Probes) and mounted with Mowiol. (B–E) and (G–H) For light microscopic double staining of myocardial tissue histochemical hPLAP detection using Vector Blue as substrate was performed as described in Fig. 1, followed by incubation with the primary antibodies mouse anti-vimentin (Dako, Hamburg, Germany) 1:300 (B and C), mouse anti-human smooth muscle actin SMA (Dako) 1:300 (D and E), rabbit anti-connexin 43 (Sigma) 1:2000 (G) and mouse anti-alpha-sarcomeric actin αSA (Sigma) 1:500 (H), diluted in PBS containing 5% horse or rabbit serum. For vimentin staining, sections were microwaved in citrate buffer pH 6 two times 3 min. Bound antibody was detected by incubation with biotinylated rat adsorbed horse antimouse antibody (Vector) 1:50 or biotinylated goat anti-rabbit antibody (Vector) 1:200 for 1 hr at room temperature. Vector VIP (purple) was used as peroxidase substrate for IHC. (A) A large proportion of cells infiltrating the infarcted myocardium 2 weeks after MI stains positive for CD68 (green). The majority of these CD68-positive macrophages also stain for hPLAP (red). (B and C) There is a high number of vimentin-positive cells infiltrating the ischaemic myocardium, and a considerable proportion of these vimentin-positive cells also stains positive for hPLAP. (C) is an enlargement of the boxed area in (B). (D and E) The number of SMA-positive myofibroblast-like cells is much lower than the number of vimentin-positive cells. (E) is an enlargement of the boxed area in (D). (D) Single SMA-positive cardiomyocytes (arrowheads) can be detected in the peri-infarct area, indicating the formation of new cardiomyocytes by local precursor cells. (F–H) Fluorescent and light microscopic double labelling for hPLAP (red fluorescence in F) and connexin 43 (F and G; green fluorescence in F) as well as αSA (H) shows lack of hPLAP-positive cardiomyocytes. Five-μm-thick paraffin sections of 40% ethanol-fixed hearts. Bar = 50 μm.
Fig. 6.
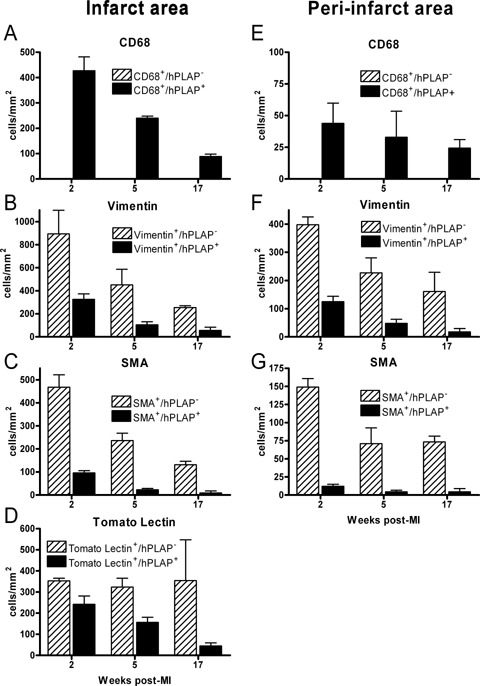
Quantification of the number of CD68 (A and E), vimentin (B and F), SMA (C and G), and tomato lectin (D) single positive cells and of cells double staining for hPLAP and CD68, vimentin, SMA or tomato lectin per mm2 of infarct (left panels) or peri-infarct (right panels) area. Light microscopic double staining of myocardial tissue was performed as described in Figs 1 and 3. Heart sections were stained for hPLAP by histochemistry, and subsequently for CD68, vimentin, and SMA by immunohistochemistry, or by tomato lectin. In each animal, 10 optical fields each in the infarct and peri-infarct region were analysed in 2 slides each with the help of an ocular grid at ×400, using a Zeiss Axioskop II microscope. The peri-infarct region was defined as the region extending 200 μm beyond the border of the infarct region. Each data point is the mean ± S.E.M. of 3–5 animals.
Two weeks after MI, a large proportion of the BM-derived inflammatory cell infiltrate in the infarct area consisted of CD68-positive macrophages (Figs. 3A and 6A). All CD68-positive cells were also hPLAP-positive, i.e. of BM origin. Furthermore, we detected a high number of vimentin-positive cells in the infiltrate at 2 weeks after MI (Figs. 3B and C, 4D and E, 5E and F, 6B). A considerable proportion of these cells was also positive for hPLAP throughout all time points (Figs. 3B and C, 4D and E, 5E and F, 6B), indicating the presence of BM-derived fibroblasts and endothelial cells in the infarcted myocardium. The number of vimentin single positive as well as the number of vimentin-hPLAP double positive cells decreased with time after MI (Figs. 3B, 4D, 5E, 6B). SMA-positive cells with a myofibroblast morphology were not as frequent as vimentin-positive cells throughout the study (Figs. 3D and E, 4F, 6C). In addition, the proportion of SMA-hPLAP double positive cells in relation to SMA single positive cells was less than vimentin-hPLAP double positive cells in relation to vimentin single positive cells, and decreased with time in the infarct area (Figs. 3D and E, 4F, 6C). The SMA- and vimentin-hPLAP double positive cells had a spindle-shaped or endothelial cell phenotype (Figs. 3B—E, 4D–F, 5E and F). A considerable number of hPLAP-positive, BM-derived cells in the infarct region stained positive for the endothelial cell marker tomato lectin, especially at 2 weeks after MI (Fig. 6D). However, the number of endothelial cells of BM origin decreased with time (Figs. 4A, 5A, 6D), and only few of them persisted in the scar until 17 weeks after MI (Figs. 5D, 6D). These findings indicate that BM serves as a major source of endothelial cells, fibroblasts and myofibroblast-like cells during the early phases of cardiac repair. However, only few of the BM-derived myofibroblast-like and endothelial cells persisted in the scar until 17 weeks after MI. A limitation of our data is that SMA is not a specific marker for myofibroblasts. A clear definition of myofibroblasts requires electron microscopy.
Fig. 4.
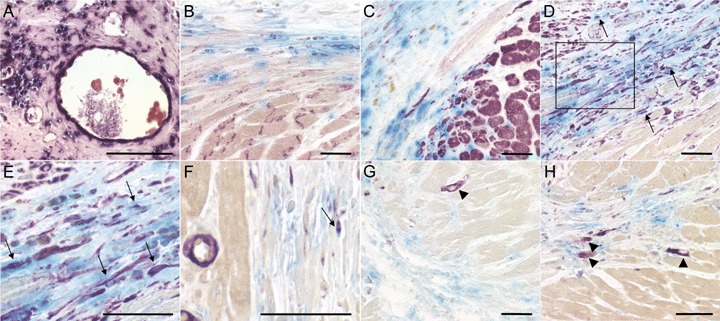
Double staining of the cellular infiltrate in hPLAP-BMT rats, 5 weeks after myocardial infarction (MI). Double-staining of myocardial tissue was performed as described in Figs 1 and 3. Double positive cells are indicated by arrows. (A) Histochemical staining for hPLAP using NBT/BCIP as substrate reveals a large number of bone marrow (BM)-derived cells in the infarcted heart. (B and C) No differentiation of BM-derived cells into cardiomyocytes is observed at 5 weeks after MI as shown by lack of double staining for hPLAP and connexin 43 (B) or αSA (C). (D–F) Presence of hPLAP-vimentin double positive endothelial cells and fibroblasts (D and E), and of more rare hPLAP-SMA double positive myofibroblast-like cells (F) in the scar. (E) is an enlargement of the boxed area in (D). (G and H) SMA- (G) and vimentin-positive (H), hPLAP-negative, newly formed cardiomyocytes (arrowheads) of non-BM-origin in peri-infarct regions. Five-μm-thick paraffin sections of 40% ethanol-fixed hearts. Bar = 50 μm.
Fig. 5.
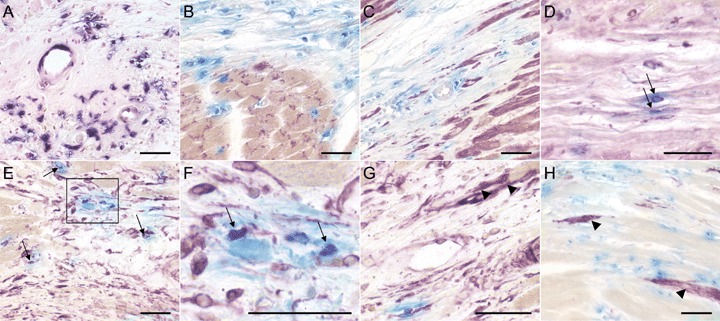
Double staining of the cellular infiltrate in hPLAP-BMT rats, 17 weeks after myocardial infarction (MI). Double staining of myocardial tissue was performed as described in Figs 1 and 3. Double positive cells are indicated by arrows. (A) hPLAP histochemistry using NBT/BCIP as substrate shows persistence of a much lower number of bone marrow (BM)-derived cells, 17 weeks after MI, compared to the earlier time points. (B and C) No evidence for differentiation of BMC into cardiomyocytes is seen, as shown by lack of double staining for hPLAP and connexin 43 (B) or αSA (C). (D–F) Only few BM-derived endothelial and fibroblastic cells remain in the scar as shown by double staining for hPLAP and tomato lectin (D) or anti-vimentin (E, F). (F) is an enlargement of the boxed area in (E). (G and H) hPLAP-negative cardiomyocytes staining positive for vimentin (G) and SMA (D) in peri-infarct regions (arrowheads). Five-μm-thick paraffin sections of 40% ethanol-fixed hearts. Bar = 50 μm.
Our data are at variance with the study by Yano et al. [18] in athymic rats transplanted with BM of GFP transgenic mice. The latter authors reported that myofibroblasts are exclusively of local origin, 1 week after MI. However, because T cells may be involved in the recruitment of BM-derived myofibroblasts to the infarct site, the use of athymic rats lacking T cells could be a possible explanation for the lack of BM-derived myofibroblasts in Yano's study [18]. Similar to our data, Möllmann et al. [11] reported that more than 50% of myofibroblasts in the infarcted myocardium derived from BM, whereas the remaining myofibroblasts derived from local resident precursor cells, 1 week after MI. The proportion of BM-derived myofibroblast-like cells decreased over the time period from 2 to 17 weeks after MI in our study. Virag and Murry [19] showed that the peak of myofibroblast influx is 4 days after MI in mice. Therefore, our data support the notion that influx of myofibroblasts from BM occurs predominantly during the early phases of cardiac repair. At later phases, only few BM-derived cells are recruited to the infarct site or persist in the scar.
In the peri-infarct regions, the number of CD68-, vimentin- and SMA-positive cells was generally lower compared with the infarct area (Fig. 6E–G). However, the ratio of single positive to double positive cells and the decline over time after MI was similar in infarct and peri-infarct regions.
Generally, co-staining of hPLAP and connexin 43 (Figs. 3F and G, 4B, 5B) or αSA (Figs. 3H, 4C, 5C) as markers of mature cardiomyocytes was absent throughout the study. Only a single hPLAP and αSA-double positive cardiomyocyte-like cell could be found 17 weeks after MI induction in all sections analysed (data not shown). Interestingly, we found hPLAP-negative cells with a clear cardiomyocyte-like phenotype in peri-infarct areas staining strongly positive for vimentin (Figs. 4H and 5G) or SMA (Figs. 3D, 4G and 5H) especially at 5 and 17 weeks after MI. These cells may be early differentiated cardiomyocytes [16, 17]. Taken together, our findings suggest that local cardiac progenitor cells are the major source for regeneration of cardiomyocytes, not BM-derived cells. Although myofibroblast-like cells were recruited from BM during cardiac healing, differentiation of BM-derived cells into cardiomyocytes was an extremely rare event. In a similar fashion, Möllmann et al. [11] found very little evidence of cardiomyogenic differentiation of BMC at 3 weeks after MI. In contrast, some earlier studies reported cardiomyogenic differentiation of BMC after MI in rats and mice [8–10, 12, 20]. Some [9, 12, 20], but not all [8, 10], of these studies used growth factors for mobilization of BMC. The mobilization of BMC by growth factors may explain some of these controversies. In addition, it was suggested that differentiation of BMC into cardiomyocytes occurs through cell fusion [20]. However, the present study employing the robust marker hPLAP clearly demonstrated that neither cardiomyogenic differentiation of BM-derived cells nor cell fusion of cardiomyocytes with BMC play an important role in the physiological healing processes after MI.
In conclusion, the inflammation-induced influx of BM-derived cells into the infarction area in our model was restricted to leukocytes, endothelial cells, fibroblasts and myofibroblast-like cells. Our long-term analysis casts doubt on the hypothesis that circulating BM-derived mesenchymal precursor cells participate in cardiomyogenesis after MI. Rather, we found evidence of cardiomyogenic differentiation of local cardiac progenitor cells in the peri-infarct regions during the later phases of cardiac repair. It is clear that during physiological healing of myocardial lesions, the number of newly formed cardiomyocytes is insufficient for the repair of the infarcted myocardium. However, the cardiac regeneration potential of these local mesenchymal precursors might be exploited for therapeutic purposes in the future.
Acknowledgments
We are indebted to Magdalena Helmreich for excellent technical assistance. This research was supported by a grant from the University of Veterinary Medicine Vienna to RGE.
References
- 1.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 2.Glaser R, Lu MM, Narula N, Epstein JA. Smooth muscle cells, but not myocytes, of host origin in transplanted human hearts. Circulation. 2002;106:17–9. doi: 10.1161/01.cir.0000021923.58307.8f. [DOI] [PubMed] [Google Scholar]
- 3.Muller P, Pfeiffer P, Koglin J, Schafers HJ, Seeland U, Janzen I, Urbschat S, Bohm M. Cardiomyocytes of noncardiac origin in myocardial biopsies of human transplanted hearts. Circulation. 2002;106:31–5. doi: 10.1161/01.cir.0000022405.68464.ca. [DOI] [PubMed] [Google Scholar]
- 4.Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–9. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 5.Ausoni S, Zaglia T, Dedja A, Di Lisi R, Seveso M, Ancona E, Thiene G, Cozzi E, Schiaffino S. Host-derived circulating cells do not significantly contribute to cardiac regeneration in heterotopic rat heart transplants. Cardiovasc Res. 2005;68:394–404. doi: 10.1016/j.cardiores.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–9. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi M, Hakamata Y, Murakami T, Takeda S, Kaneko T, Takeuchi K, Takahashi R, Ueda M, Kobayashi E. Establishment of lacZ-transgenic rats: a tool for regenerative research in myocardi-um. Biochem Biophys Res Commun. 2003;305:904–8. doi: 10.1016/s0006-291x(03)00841-6. [DOI] [PubMed] [Google Scholar]
- 9.Fukuhara S, Tomita S, Nakatani T, Ohtsu Y, Ishida M, Yutani C, Kitamura S. G-CSF promotes bone marrow cells to migrate into infarcted mice heart, and differentiate into cardiomyocytes. Cell Transplant. 2004;13:741–8. doi: 10.3727/000000004783983486. [DOI] [PubMed] [Google Scholar]
- 10.Kuramochi Y, Fukazawa R, Migita M, Hayakawa J, Hayashida M, Uchikoba Y, Fukumi D, Shimada T, Ogawa S. Cardiomyocyte regeneration from circulating bone marrow cells in mice. Pediatr Res. 2003;54:319–25. doi: 10.1203/01.PDR.0000078275.14079.77. [DOI] [PubMed] [Google Scholar]
- 11.Möllmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–71. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zagzag D, Miller DC, Chiriboga L, Yee H, Newcomb EW. Green fluorescent protein immunohistochemistry as a novel experimental tool for the detection of glioma cell invasion in vivo. Brain Pathol. 2003;13:34–7. doi: 10.1111/j.1750-3639.2003.tb00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–38. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- 15.Odörfer KI, Unger NJ, Weber K, Sandgren EP, Erben RG. Marker tolerant, immuno-competent animals as a new tool for regenerative medicine and long-term cell tracking. BMC Biotechnol. 2007;7:30. doi: 10.1186/1472-6750-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Papakonstantinou C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact Cardiovasc Thorac Surg. 2007;6:593–7. doi: 10.1510/icvts.2007.157875. [DOI] [PubMed] [Google Scholar]
- 17.Babai F, Musevi-Aghdam J, Schurch W, Royal A, Gabbiani G. Coexpression of alpha-sarcomeric actin, alpha-smooth muscle actin and desmin during myogenesis in rat and mouse embryos I. Skeletal muscle. Differentiation. 1990;44:132–42. doi: 10.1111/j.1432-0436.1990.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 18.Yano T, Miura T, Ikeda Y, Matsuda E, Saito K, Miki T, Kobayashi H, Nishino Y, Ohtani S, Shimamoto K. Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol. 2005;14:241–6. doi: 10.1016/j.carpath.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Virag JI, Murry CE. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol. 2003;163:2433–40. doi: 10.1016/S0002-9440(10)63598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]


