INTRODUCTION
In the U.S., obesity is prevalent in epidemic proportions, and rates continue to rise. Current data indicate that the combined prevalence of overweight and obese adults in the U.S. is 69.2%.1 The prevalence of obesity alone accounts for 35.7% of adults in the U.S., with men and women equally affected.2 In the U.S., approximately 18.4% of adolescents 12 to 19 years of age are obese.1
Obesity is defined as a body mass index (BMI) of 30 kg/m2 or more. Over-weight is defined as a BMI of 25 to 29.9 kg/m2.3 Obesity is a significant risk factor contributing to the development of hypertension, type-2 diabetes mellitus, hyperlipidemia, coronary heart disease, stroke, metabolic syndrome, sleep apnea, osteoarthritis, gallbladder disease, fatty liver disease, pregnancy complications, and some cancers.4 Obesity is also a major cause of preventable death in the U.S.5 Increased health care costs are also associated with obesity and its related comorbidities. Obesity-related health costs in the U.S. are estimated at $147 billion annually.6
The exact cause of obesity is unknown; the condition develops from multiple genetic, behavioral, physiological, social, and metabolic factors.7 Goals of treatment include a reduction in baseline body weight, dietary changes, increased physical activity, and behavioral modifications to address the complexity of the condition.7 In clinically severe obesity, defined as a BMI of 40 or greater, or a BMI of 35 or greater with comorbid conditions, weight-loss surgery may be indicated.7 Ultimately, a reduction in mortality rates and an increased quality of life are the aims of treatment.
To address the growing prevalence and the related comorbidities associated with obesity, researchers have explored alternative therapies to behavior, diet, exercise, and surgery to promote weight loss, including pharmacological treatments.7 Earlier and current weight-loss medications have been designed to reduce appetite, increase satiety, and decrease the absorption of nutrients. Thyroid hormone, amphetamines, diethylpropion (e.g., Tenuate, formerly Sanofi Aventis), fenfluramine (Pondimin, American Home Products/ Wyeth), dexfenfluramine (e.g., Redux, Wyeth), orlistat (Alli, GlaxoSmithKline; Xenical, Roche/Genentech), sibutramine (Meridia, Abbott), rimonabant (Acomplia, Sanofi), and phenylpropanolamine (now available only for dogs) have been investigated and used in the treatment of obesity. However, many of these medications have been withdrawn from the market because of their adverse-risk profiles and safety concerns.8
On June 27, 2012, the FDA approved lorcaserin (Belviq, Arena/Eisai) for the treatment of obesity. Formerly known as Lorqess, this small-molecule agonist of the serotonin 2C (5-HT2C) receptor is designed to promote weight loss in obese and overweight patients as an adjunct to a reduced-calorie diet and increased physical activity.9 The selectivity of lorcaserin at the 5-HT2C receptors is an advantage to both its efficacy and safety by exerting its appetite-suppressing effects while avoiding cardiovascular effects that occurred in previous nonselective serotonergic weight-loss medications.8 Three phase 3 clinical trials have demonstrated safety and efficacy for lorcaserin (see pages 526–529).9–11
CHEMICAL PROPERTIES
Lorcaserin HCl is a 5-hydroxytryptamine (5-HT2C) agonist with a chemical formula of [(R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride hemihydrate].12 It is available as a blue, film-coated 10-mg round, biconvex tablet that is debossed with an “A” on one side and a “10” on the other. Inactive ingredients include silicified microcrystalline cellulose, hydroxypropyl cellulose NF, croscarmellose sodium NF, colloidal silicon dioxide NF, polyvinyl alcohol USP, polyethylene glycol NF, titanium dioxide USP, talc USP, FD&C Blue #2 aluminum lake, and magnesium stearate NF.12
The drug’s structural formula is illustrated in Figure 1.
Figure 1.
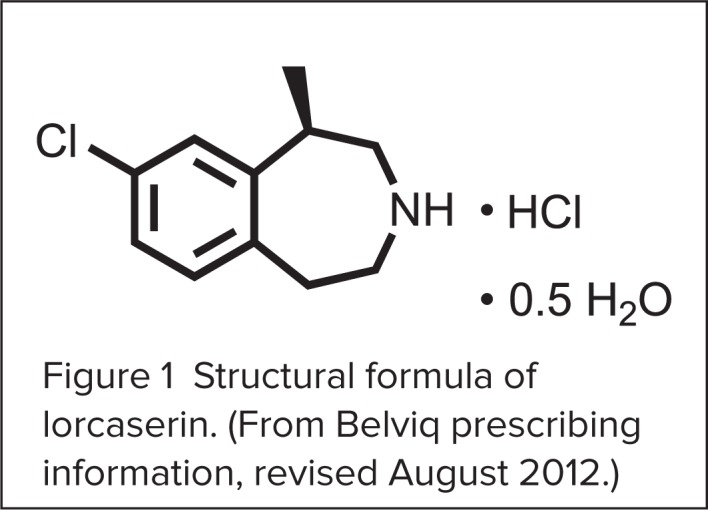
Structural formula of lorcaserin. (From Belviq prescribing information, revised August 2012.)
The delay in the drug’s availability stemmed from the FDA’s recommendation that lorcaserin be classified as a Schedule IV controlled substance.
MECHANISM OF ACTION
Lorcaserin acts at 5-HT2C receptors in the central nervous system (CNS), particularly the hypothalamus, to reduce appetite.12 The medication stimulates 5-HT2C receptors on the pro-opiomelanocortin (POMC) neurons in the arcuate nucleus; this causes the release of alpha-melanocortin-stimulating hormone (alpha-MSH), which acts on melanocortin-4 receptors in the paraventricular nucleus to suppress appetite.12–14
There are 14 subtypes of 5-HT receptors, each with different activity. 5-HT2C is closely related to 5-HT2A and 2B receptors.14–17 Previous weight-loss medications with a serotonin agonist mechanism of action, such as dexfenfluramine and fenfluramine, also stimulated 5-HT2B receptors, which led to pulmonary hypertension and heart-valve problems.
Lorcaserin’s affinity for the 5-HT2C receptor is approximately 15-fold and 100-fold greater than its affinity for 5-HT2A and 5-HT2B, respectively.13,18,19 Binding affinity is dose-dependent. When the maximum dose of 20 mg/day is exceeded, the binding becomes less selective for 5-HT2C and binds more to other serotonin receptors such as 5-HT2A.20 When this serotonin receptor is stimulated, hallucinations, euphoria, and altered mood may result.12,20,21–28
INDICATIONS AND DOSAGE
Lorcaserin is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in obese adults (a BMI of 30 kg/m2 or greater) or in overweight patients (BMI of 27 kg/m2 or greater) with at least one weight-related comorbid condition, such as hypertension, type-2 diabetes, or dyslipidemia.12
The recommended dose is 10 mg orally twice daily with or without food. If patients do not achieve a 5% reduction in weight by week 12, they should stop taking lorcaserin because it is unlikely that they will achieve any weight loss with the medication.12
Lorcaserin may be used in patients with mild-to-moderate hepatic impairment who require no dose adjustments; however, caution is recommended for patients with severe hepatic impairment, because the effects in this population have not been studied.12 Although lorcaserin may be used in patients with mild renal impairment, caution is advised for those with moderate renal impairment. The use of lorcaserin in patients with severe renal impairment or end-stage renal failure is not recommended.12
The effect of lorcaserin in geriatric populations is unknown, as most patients in the three published clinical trials were 65 years of age or younger.12 No dose adjustments should be made based solely on age. Safety and efficacy studies for pediatric patients younger than 18 years of age have not been established, and lorcaserin is not recommended for this age group.12
Lorcaserin is contraindicated in pregnant women, and it is listed as a Pregnancy Category X medication. It was found to offer no known benefit in pregnant women, because it promotes weight loss.12 There are no data supporting the use of the medication in nursing mothers.
DRUG INTERACTIONS
As a result of lorcaserin’s mechanism of action as a serotonergic agonist, potential interactions may occur with medications that affect serotonergic pathways. The risk of serotonin syndrome and neuroleptic malignant syndrome (NMS)–like reactions can occur if lorcaserin is used in combination with other serotonergic agents, although these effects have not been evaluated.
Medications to be avoided include serotonin–norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), bupropion (Wellbutrin, GlaxoSmithKline), triptans, St. John’s wort, tryptophan, monoamine oxidase (MAO) inhibitors, dextromethorphan, lithium (e.g., Eskalith, GlaxoSmithKline), tramadol (Ultram, Janssen), antipsychotic agents, and dopamine agonists.12
Multiple enzymatic pathways in the liver, including cytochrome P450 (CYP) 1A2, 2A6, 2B6, 2C19, 2D6, and 3A4, play a role in the metabolism of lorcaserin.22 In one study, it was observed that the enzymatic pathways predicted possible drug interactions that could occur with lorcaserin.22 A potent inhibitor of the CYP2D6 isoenzyme, quinidine, demonstrated that the CYP2D6 pathway plays a major role in the formation of the 7-hydroxylorcaserin metabolite. In addition, ketoconazole (Nizoral, Janssen), a potent inhibitor of CYP3A4, significantly inhibited the formation of 1-hydroxylorcaserin.22 However, because lorcaserin is metabolized by multiple enzymes and does not depend on a single enzymatic pathway, the probability of CYP drug interactions with concomitant medications via these pathways is low.22
PHARMACOKINETICS
Following oral administration, lorcaserin is rapidly absorbed from the gastro-intestinal (GI) tract and exhibits peak plasma concentrations (Tmax) in 1.5 to 2 hours.12 The exact bioavailability of the drug has not been determined.
Taking lorcaserin following a meal has no significant effect on peak concentrations (Cmax). The effect of food on the drug’s absorption was shown in an evaluation of 12 adult volunteers (six men and six women) who received a single lorcaserin 10-mg dose in a fasted state and after eating a high-fat meal. The Cmax and the area-under-the-curve (AUC) concentration were increased by 9% and 5%, respectively. The Tmax was delayed by only 1 hour when the participant was in the fed state. Therefore, no significant differences were seen between administration with and without food.12
Lorcaserin is distributed to the cerebrospinal fluid and the CNS in humans and is approximately 70% bound to plasma proteins.12 The drug’s metabolism includes multiple enzymatic pathways and is not dependent on a single isoenzyme. Numerous human isoenzymes, such as CYP1A1, CYP1A2, CYP2A6, CYP2B6,CYP2C19, CYP2D6, CYP2E1, and CYP3A4, as well as the human flavin-containing mono-oxygenase 1 (FMO1) enzyme, are responsible for metabolism.4
One study that evaluated 16 individual human liver microsomal preparations led to the identification of four major metabolites—N-hydroxylorcaserin, 7-hydroxylorcaserin, 5-hydroxylorcaserin, and 1-hydroxylorcaserin.15 These metabolites do not appear to exert any pharmacological activity at the serotonin receptors. The major circulating metabolite after oral administration is lorcaserin sulfamate, which has a plasma Cmax that exceeds lorcaserin by one-fold to five-fold.
Because lorcaserin is metabolized by multiple enzymes and is not dependent on one single enzymatic pathway, there is a low probability of CYP drug interactions with concomitant medications.22 The half-life of lorcaserin is approximately 11 hours.23
Lorcaserin is eliminated primarily in the urine (92%) and in the feces (2.2%) (Table 1).12 The major metabolite in urine is N-carbamoyl glucuronide lorcaserin.
Table 1.
Pharmacokinetic Profile of Lorcaserin
| Absorption | |
| • Oral bioavailability | Unknown |
| • Tmax | 1.5 2 hours |
|
| |
| Metabolism | |
| • Pathway | CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C19, CYP2D6, CYP2E1, CYP3A4, human FMO1 |
| • Half-life | Approximately 11 hours |
| • Protein binding | 70% |
| • Elimination | Approximately 92.3% urine; 2.2% feces |
EFFICACY IN CLINICAL TRIALS
The safety and efficacy of lorcaserin were assessed in three published clinical placebo-controlled trials:9–11
Behavior Modification and Lorcaserin for Overweight and Obesity Management (BLOOM)
Behavioral Modification and Lorcaserin Second Study of Obesity Management (BLOSSOM)
Behavior Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus (BLOOM-DM)
BLOOM was a 2-year randomized controlled trial; BLOSSOM and BLOOM-DM were 1-year trials. BLOOM and BLOSSOM were conducted to evaluate lorcaserin in adults with obesity and obesity-related comorbidities. BLOOM-DM was designed to evaluate lorcaserin specifically in obese adults with type-2 diabetes.
Smith et al. (BLOOM)9
A 2-year, multicenter, double-blind, placebo-controlled study was conducted to assess the efficacy and safety of lorcaserin in obese and overweight adults as an adjunct to diet and exercise. This trial began with 3,182 obese or overweight adults with a mean BMI of 36.2 kg/m2.
To be enrolled, patients had to be 18 to 65 years of age and had to have a BMI of 30 to 45 kg/m2 or a BMI of 27 to 45 kg/m2 with at least one obesity-related comorbid condition (hypertension, dyslipidemia, cardiovascular disease, impaired glucose tolerance, or sleep apnea). Patients were excluded from the study if they had moderate-to-severe mitral regurgitation, mild-to-severe aortic regurgitation (i.e., valvulopathy), diabetes, systolic blood pressure (BP) greater than 140 mm Hg, diastolic BP above 90 mm Hg, depression, or another major psychiatric disease within 2 years before randomization that necessitated treatment with prescription medication. Pregnant women and lactating mothers were also excluded.
A total of 3,182 patients received either lorcaserin (n = 1,595) or placebo (n = 1,587). They were assigned, in a 1:1 ratio, to receive either lorcaserin 10 mg twice daily or placebo twice daily before breakfast and before dinner. Patients were instructed to return to their research site 2 and 4 weeks after randomization and then monthly for evaluation and lifestyle management, which included dietary and exercise modifications.
After the first year, patients who remained in the trial were eligible to continue. For the second year, patients receiving placebo continued to receive placebo, whereas lorcaserin patients were reassigned, in a 2:1 ratio, to either receive lorcaserin or to start receiving placebo.
For the first year, the co-primary endpoints were a weight loss of 5% from baseline at 1 year, the proportion of patients with a reduced baseline body weight of 5% or more at 1 year, a change in weight between baseline and the end of year 1, and the proportion of patients with a reduced baseline body weight of 10% or more at 1 year.
For the second year, the primary endpoint was the proportion of patients who had a reduction in baseline body weight of 5% or more at the end of year 1 and maintained the weight reduction at year 2.
For the primary efficacy endpoints, an intent-to-treat (ITT) population and last observation carried forward (LOCF) imputation was used to perform the statistical analyses.
In the ITT/LOCF analysis, at the end of year 1 (52 weeks), 47.5% of patients receiving lorcaserin lost 5% or more of their baseline body weight, compared with 20.3% of patients receiving placebo (P < 0.001). Of these patients, 22.6% in the lorcaserin group and 7.7% of the placebo group lost more than 10% of their body weight (P < 0.001). Patients in the lorcaserin group lost an average 5.81% of their baseline body weight compared with 2.16% in the placebo group (P < 0.001). The results were statistically significant.
At the end of year 2, more patients who continued with lorcaserin (67.9%) maintained their weight loss compared with placebo patients (50.3%) (P < 0.001). Although the results were not statistically significant, mean body weight in the lorcaserin patients during both years of the trial was lower than in the placebo patients and in those receiving lorcaserin during year 1 and placebo in year 2.
Co-primary endpoints included waist circumference; BP; BMI; and levels of insulin, lipids, and C-reactive protein. Lorcaserin brought about a significant reduction in waist circumference—an average loss of 6.8 cm, compared with 3.9 cm for placebo after year 1, and an average loss of 8.1 cm with lorcaserin compared with 4.3 cm for placebo after year 2, respectively (P < 0.001).
After the first year, fasting glucose levels, insulin levels, glycosylated hemoglobin (HbA1c), and BMI were all significantly reduced with lorcaserin compared with placebo. Lipid levels, including total cholesterol, low-density lipoprotein-cholesterol (LDL-C), and triglycerides, were reduced at the end of year 1 for lorcaserin compared with placebo but were increased for both groups by the end of year 2. At the end of year 1, C-reactive protein levels and systolic and diastolic BP were significantly reduced with lorcaserin compared with placebo.
Adverse drug events (ADEs) reported for lorcaserin included headache, upper respiratory infections, nasopharyngitis, dizziness, and nausea. The occurrence of neuropsychiatric events was similar among the lorcaserin and the placebo groups, including rates of depression and anxiety at years 1 and 2. The incidence of valvular heart disease did not differ between the groups after year 2.
Fidler et al. (BLOSSOM)10
Following the BLOOM trial, a 1-year randomized, placebo-controlled, double-blind, parallel-arm trial was conducted at 97 academic and private research centers in the U.S. to assess the efficacy of lorcaserin in addition to a nutritional and physical exercise program in obese and at-risk overweight patients.
The study involved 4,008 patients; they had to be 18 to 65 years of age and had to have a BMI of 30 to 45 kg/m2 or a BMI of 27 to 29.9 kg/m2 with at least one comorbidity (hypertension, dyslipidemia, cardiovascular disease, impaired glucose tolerance, or sleep apnea). They also had to be participating in an exercise program of moderate intensity. Most patients (79.8%) were women.
Exclusion criteria included recent cardiovascular events, diabetes mellitus, major surgeries, medical conditions that would prevent participation in a nutritional and physical exercise program, a systolic BP of 150 mm Hg or higher or a diastolic BP of 95 mm Hg or higher, triglyceride levels exceeding 499 mg/ dL, use of SSRIs within the previous year, recent use of weight-loss drugs (within 1 month for over-the-counter agents, 3 months for prescription drugs), a very-low-calorie diet, or a change in weight of at least 5 kg within 3 months.
Primary endpoints were a 5% reduction in body weight measured at 1 year, mean change in body weight, and a 10% reduction in body weight at 1 year. Secondary endpoints included lipids, BP, body composition (total body fat and lean body mass), and quality of life. The modified ITT/LOCF approach was used to analyze primary and secondary efficacy endpoints.
Patients were randomly assigned, in a 2:1:2 ratio, to receive lorcaserin 10 mg twice daily, lorcaserin 10 mg once daily, or placebo in addition to diet and exercise counseling. They were assessed for follow-up at weeks 2, 4, and monthly thereafter to week 52. Patients were requested to reduce caloric intake to 600 kilocalories (kcal) below the World Health Organization equations, with a fixed activity factor of 1.3, and to exercise moderately for 30 minutes each day.
A total of 2,224 patients completed the study: 917 patients received twice-daily lorcaserin, 473 received once-daily lorcaserin, and 834 received placebo.
Patients treated with lorcaserin 10 mg twice daily and once daily were more likely to have a 5% reduction in baseline body weight at 1 year (47.2% and 40.2%, respectively [modified ITT/LOCF population]), compared with placebo patients (25%; P < 0.0001). According to the least-squares method, lorcaserin 10 mg twice daily and 10 mg once daily, compared with placebo, brought about mean weight losses of 5.8%, 4.7%, and 2.8%, respectively, from baseline (modified ITT population/ LOCF). Patients in the lorcaserin twice-daily and once-daily groups were more likely to achieve more than a 10% weight loss compared with those in the placebo group (22.6%, 17.4%, and 9.7%, respectively; P < 0.001).
Secondary measures (e.g., waist circumference and BMI) decreased significantly with lorcaserin 10 mg twice daily and 10 mg once daily in comparison with placebo (modified ITT population/ LOCF). Changes in lipid levels—total cholesterol, LDL-C, and high-density lipoprotein-cholesterol (HDL-C) were not consistently significant between the lorcaserin and placebo groups.
Discontinuation rates occurred mainly as a result of patient loss to follow-up and patients’ decisions to drop out. Because of ADEs, more patients withdrew from the lorcaserin 10-mg twice-daily and 10-mg once-daily groups compared with placebo patients (7.2%, 6.2%, and 4.6%, respectively).10 ADEs included headache, upper respiratory infection, nausea, dizziness, and fatigue. Serious ADEs attributed to the investigational drug occurred more often with lorcaserin 10 mg twice daily than with placebo.
Efficacy results were consistent with those in the BLOOM trial and showed both a clinically and statistically significant dose-dependent relationship for lorcaserin when used as an adjunct to diet and exercise in obese and overweight patients. The average weight reduction was 5.8% in patients using lorcaserin twice daily, correlating with the BLOOM results (modified ITT population/LOCF). The per-protocol population in BLOSSOM also experienced a dose-dependent relationship for lorcaserin 10 mg twice daily, resulting in a weight loss of 7.9% compared with 4.0% for placebo. Weight loss was similar for men and women; however, the loss was greater in Caucasian patients than in African-American and Hispanic patients. Dose concentrations did not differ among these groups.10
O’Neil et al. (BLOOM-DM)11
A 1-year trial was conducted to evaluate the efficacy and safety of lorcaserin for weight loss in patients with type-2 diabetes. Patients were eligible for enrollment if they had type-2 diabetes mellitus; were currently taking metformin (Glucophage, Bristol-Myers Squibb), a sulfonylurea, or both; had a HbA1c value between 7% and 10%; were 18 to 65 years of age; and had a BMI between 27 and 45 kg/m2.
Patients were excluded from the study if they were using insulin, exenatide (Byetta, Amylin/Eli Lilly), or pramlintide (Symlin, Amylin); had undergone bariatric surgery; experienced a change in weight of 5 kg or more within 3 months; had a score above 17 on the Binge Eating Scale or a score of 20 or above on the Beck Depression Inventory-II; started or stopped smoking cigarettes within the previous 3 months; had a malignancy within the previous 5 years; had recent surgery; had a history of seizure disorder within 5 years; experienced depression or other major psychiatric disease requiring a prescription medication within the year; had experienced suicidal thoughts; or were pregnant or lactating.
The primary endpoints were a greater than 5% reduction in baseline body weight after 1 year, weight change from baseline, and more than a 10% reduction in baseline body weight. Secondary endpoints included changes in glycemic control indices from baseline.
A total of 604 patients were enrolled and were randomly assigned, in a 1:1:1 ratio, to receive lorcaserin 10 mg twice daily, lorcaserin 10 mg once daily (lorcaserin 10 mg each morning and placebo each evening), and placebo twice daily. Data for 603 patients were analyzed; one patient withdrew consent before receiving the intervention. Stratification depended on the oral use of metformin or a sulfonylurea.
After 52 weeks, the study drug was found to significantly increase the proportion of patients achieving a 5% reduction in weight from baseline as follows: lorcaserin twice daily, 37.5%; lorcaserin once daily, 44.7%; and placebo, 16.1% (P < 0.001). Weight reductions of 10% or more were achieved by 16.3% of the lorcaserin twice-daily patients, by 18.1% of the lorcaserin once-daily patients, and by 4.4% of the placebo patients (P < 0.001). The once-daily patients lost an average of 5.0% of their baseline body weight, compared with losses of 4.5% in the twice-daily group and 1.5% in the placebo group (P < 0.001).
The effects of lorcaserin were not dose-dependent; efficacy was equivalent in the twice-daily and once-daily groups. The twice-daily dose was not significantly more efficacious than the once-daily dose, and vice versa. These findings are in contrast to those from the BLOSSOM trial, which demonstrated a dose-dependent relationship in lorcaserin’s effect on weight loss.11
Clinically and statistically significant improvements in HbA1c and fasting glucose levels were also observed with lorcaserin. Mean HbA1c decreased to 7% or less at week 52 in 50.4% of patients receiving lorcaserin twice daily, in 52.2% of those receiving the once-daily dose, and in 26.3% of those in the placebo group.
In addition, fewer antidiabetic medications were needed by 17.1% of the lorcaserin twice-daily group, by 23.4% of the lorcaserin once-daily group, and by 11.7% of the placebo group (P = 0.087). Fewer lorcaserin patients also required the addition of antidiabetic drugs (13.5%, 11.7%, and 22.2%, respectively; P = 0.011).
At week 52, waist and hip circumference were significantly decreased with lorcaserin compared with placebo.
More discontinuations were attributed to ADEs with lorcaserin twice daily (8.6%) and once daily (6.3%) than with placebo (4.3%). ADEs in the lorcaserin patients included headache, back pain, nasopharyngitis, nausea, urinary tract infections, cough, fatigue, and dizziness. Hypoglycemia occurred more frequently in the treated groups than in those receiving placebo, but there was no significant difference in the occurrence of severe hypoglycemia. None of the patients withdrew from any treatment group because of hypoglycemia.
ADVERSE DRUG REACTIONS
The most common ADEs that occurred in more than 5% of patients, and more commonly than with placebo, during the BLOOM and BLOSSOM trials were headache (in 14.5% to 16.8% of patients), dizziness (in 7.0% to 8.5%), fatigue (in 7.2% to 7.4%), nausea (in 8.3% to 9.4%), and dry mouth (in 5.3%). These events were mild to moderate in intensity.9,10,12 Other common ADEs included nasopharyngitis (in 11.3% to 13.1%) and upper respiratory infections (in 13.7%).9,10,12 Because of decreases in plasma glucose levels that occurred when diabetic patients lose weight, hypoglycemia was more common in these patients (29.3%).11,12 Serious ADEs were infrequent and did not differ significantly in the placebo group.9–12
CONTRAINDICATIONS
Lorcaserin is a Pregnancy Category X drug. It is recommended that pregnant women, even those who are overweight or obese, gain a minimum amount of weight with no weight loss. In a study conducted in rats, all doses of lorcaserin led to lower weights of offspring from birth to adulthood.12 Exposure to lorcaserin in late pregnancy did not result in teratogenicity; at higher doses (up to 44 times that of human exposure), however, stillbirths and reduced viability of the pups was observed.23 It is unknown whether lorcaserin is excreted in breast milk. Because several drugs are excreted in human milk, lorcaserin should be discontinued during breast-feeding or nursing.12
WARNINGS AND PRECAUTIONS
Serotonin syndrome and NMS-like reactions. Lorcaserin is a serotonergic drug that carries a risk of serotonin syndrome or NMS, especially when it is combined with other serotonergic drugs, including SSRIs; SNRIs such as venlafaxine (Effexor, Wyeth/Pfizer), duloxetine (Cymbalta, Eli Lilly), desvenlafaxine (Pristiq, Wyeth/Pfizer); the older tricyclic antidepressants, which resemble SNRIs in their mechanism of action; and MAO inhibitors. The safety of lorcaserin, when given concomitantly with these agents, has not been established. If the use of these antidepressants is warranted, they should be prescribed with extreme caution. Patients should be regularly monitored, especially during initial treatment and during dose increases.12,24
Valvular heart disease. Valvular heart disease has been associated with agents that have agonistic activity at the 5-HT2B receptor. At common therapeutic levels, lorcaserin is selective for the 5-HT2C receptor. In clinical trials, no significant increase in valvular disease was observed for lorcaserin when compared with placebo. Lorcaserin has not been studied in patients with congestive heart failure (CHF). It is suggested that 5-HT2B receptors might be overexpressed in patients with CHF;26 therefore, lorcaserin should be used with caution in this patient population.12 It should not be used in patients who are taking medications with 5-HT2B receptor agonist activity, such as cabergoline (e.g., Dostinex, Pfizer) and methylergonovine (Methergine, Novartis). Signs and symptoms of valvular heart disease include CHF, dyspnea, dependent edema, and a new cardiac murmur. If these symptoms develop, patients should be evaluated and discontinuation of therapy should be considered.12
Cognitive impairment. Impairments in attention and memory were associated with 1.9% of patients who received lorcaserin compared with 0.5% of patients receiving placebo during clinical trials lasting 1 year or more. Confusion, fatigue, and somnolence were also reported. Patients taking lorcaserin should use caution when operating hazardous machinery until they know that they will not be cognitively impaired.12
Psychiatric disorders. Lorcaserin is selective for the 5-HT2C receptor at therapeutic levels. However, if the dose exceeds 20 mg/day, the drug can begin to exert agonist activity at the 5-HT2A receptor.13,27,28 Subjects displayed euphoria, hallucinations, and dissociation in short-term studies with doses higher than 10 mg twice daily. Doses should not exceed 20 mg/day.12
Medications that interfere with CNS function have been known to cause depression and suicidal ideation As a result of depression, mood disorders, or suicidal ideation, 1.3% of patients receiving lorcaserin in clinical trials discontinued therapy compared with 0.6% of placebo patients.12
Hypoglycemia in type-2 diabetes. In clinical trials, lorcaserin was associated with hypoglycemia. It is important to monitor blood glucose during therapy, and lower doses of antidiabetic medications should be considered. Lorcaserin has not been studied in combination with insulin.12
Priapism. Priapism is a possible ADE after lorcaserin therapy. In rodent studies, agonist activity at the 5-HT2C receptor produced sexual responses, including penile erections.20 Lorcaserin should be used in caution in men with a predisposition for priapism (e.g., in those with sickle cell anemia, multiple myeloma, or leukemia) or in men with a penile anatomical deformation. Caution is advised for men using medications for erectile dysfunction. If an erection lasts longer than 4 hours, lorcaserin should be immediately discontinued and medical attention should be sought.12
Decreased heart rate. In clinical trials lasting 1 year or longer, bradycardia occurred in 0.3% of patients receiving lorcaserin compared with 0.1% of those receiving placebo. Caution is recommended in patients with a history of heart block greater than first-degree and in those with pre-existing bradycardia.12
Hematological changes. Low white blood cell counts occurred in 0.4% of patients receiving lorcaserin versus 0.2% of placebo-treated patients. Periodic complete blood cell monitoring should be considered.12
Elevated prolactin levels. Lorcaserin has been shown to increase prolactin levels. Serotonin can increase prolactin release from the pituitary gland when it stimulates 5-HT2A and 5-HT2C receptors.20 Prolactin levels above the upper limit of normal (ULN) were observed in 6.7% of treated subjects compared with 4.8% of placebo subjects. If symptoms of hyper-prolactinemia occur, such as gynecomastia and galactorrhea, prolactin levels should be measured.12
Pulmonary hypertension. A rare but lethal disease, pulmonary hypertension (PAH) has been observed in association with systemic serotonin weight-loss agents. PAH is clinically defined as a mean pulmonary arterial pressure exceeding 25 mm Hg at rest or exceeding 30 mm Hg during activity with an accompanying increase of pulmonary vascular resistance.25 Because of the low incidence of this disease, results from clinical trials have been inconclusive in determining whether lorcaserin increases the risk of PAH. Nevertheless, caution is warranted and patients should be instructed to seek a medical evaluation if they experience shortness of breath, fatigue, or fainting.12
CONCLUSION
The launch of lorcaserin was originally delayed. On May 7, 2013, Arena announced that the Office of the Federal Register filed for public inspection the U.S. Drug Enforcement Administration’s (DEA) final rule placing the drug into Schedule IV of the Controlled Substances Act. Lorcaserin became available to patients in the U.S. by prescription in June.
In three clinical trials, lorcaserin was found to be efficacious for adjunctive therapy and maintenance of weight loss. When taken as directed, it causes a lowto-moderate incidence of adverse drug events compared with other weight-loss agents. Many drug companies are pursuing pharmacological treatments because of the rising incidence of obesity, which affects more than 35% of U.S. women and men.5 At the time of the launch, the FDA had not approved a weight-loss agent in more than 13 years, and within a few weeks, in 2012, two new weight-loss medications (Belviq and Qsymia) were approved. Until recently, most medical researchers thought of weight-loss drugs as vanity or lifestyle products for which the risks were not worth the benefits. Because obesity is so prevalent and because of its association with several comorbid conditions, it is considered by many to be a disease and thus has become a renewed target for pharmaceutical development. Comparative studies have not been performed with lorcaserin and other established or newer agents.
The cost of lorcaserin is predicted to be $200 to $220 per month.29
Although the effect of lorcaserin on morbidity and mortality has not been established, this agent offers promise in the ongoing societal and medical fight against obesity and overweight-related conditions. As a result of its established efficacy in clinical trials and its safety profile, lorcaserin may be considered a possible alternative among the available weight-loss agents.
Footnotes
Disclosure: The authors report no commercial or financial relationships in regard to this article.
REFERENCES
- 1.Department of Health and Human Services Health, United States, 2011: With Special Feature on Socioeconomic Status. Table 69, page 242: Selected health conditions and risk factors. Hyattsville, Md.: Available at: www.cdc.gov/nchs/data/hus/hus11.pdf#069. Accessed October 7, 2012.
- 2.Centers for Disease Control and Prevention (CDC) Prevalence of Obesity in the United States, 2009–2010. National Center for Health Statistics (NCHS) Data Brief, No. 82, January 2012 Available at: www.cdc.gov/nchs/data/databriefs/db82.pdf. Accessed October 7, 2012.
- 3.Centers for Disease Control and Prevention (CDC) Defining Overweight and Obesity. Updated April 27, 2012 Available at: www.cdc.gov/obesity/adult/defining.html. Accessed October 7, 2012.
- 4.National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health. Weight-control Information Network (WIN) Do You Know Some of the Risks of Being Overweight? NIH Pub. No. 07-4098. November 2004; updated December 2012. Available at: www.win.niddk.nih.gov/publications/health_risks.htm. Accessed July 29, 2013.
- 5.Centers for Disease Control and Prevention (CDC) Chronic Diseases and Health Promotion. August 13, 2012. Available at: www.cdc.gov/chronicdisease/overview/index.htm#1. Accessed October 7, 2012.
- 6.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff (Millwood) 2009;28(5):w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 7.National Heart Lung, and Blood Institute/National Institutes of Health . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Sep, 1998. Genetic influence in the development of overweight and obesity. NIH Pub. No 98-4083 pp xx, 27, 53 Available at: www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf. Accessed October 7, 2012. [Google Scholar]
- 8.Ioannides-Demos LL, Piccenna L, McNeil JJ. Pharmacotherapies for obesity: Past, current, and future therapies. J Obes. 2011;2011:179674. doi: 10.1155/2011/179674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SR, Weisserman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 10.Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: The BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- 11.O’Neil PM, Smith SR, Weisserman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: The BLOOM-DM Study. Obesity. 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 12.FDA Center for Drug Evaluation and Research Belviq NDA 022529 drug label, June 27, 2012. Available at: www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed July 29, 2013. [Google Scholar]
- 13.Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine-2C agonist: In vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- 14.Heisler LK, Jobst EE, Sutton GM, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Heisler LK, Cowley MA, Tecott LH, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 16.Heisler LK, Cowley MA, Kishi T, et al. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann NY Acad Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- 17.Morton GJ, Cummings DE, Baskin DG, et al. Central nervous system control. [DOI] [PubMed]
- 18.Sachdev M, Miller WC, Ryan T, et al. Effect of fenfluramine-derivative diet pills on cardiac valves: A meta-analysis of observational studies. Am Heart J. 2002;144:1065–1073. doi: 10.1067/mhj.2002.126733. [DOI] [PubMed] [Google Scholar]
- 19.Loke YK, Derry S, Pritchard-Copley A. Appetite suppressants and valvular heart disease: A systematic review. BMC Clin Pharmacol. 2002;2:6. doi: 10.1186/1472-6904-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillip M, Bader M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep. 2009;61:701–777. doi: 10.1016/s1734-1140(09)70132-x. [DOI] [PubMed] [Google Scholar]
- 21.Miller KJ. Serotonin 5-HT-2C receptor agonists: Potential for the treatment of obesity. Mol Interv. 2005;5:282–291. doi: 10.1124/mi.5.5.8. [DOI] [PubMed] [Google Scholar]
- 22.Usmani KA, Chen WG, Sadeque AJ. Identification of human cytochrome P450 and flavin-containing monooxygenase enzymes involved in the metabolism of lorcaserin, a novel selective human 5-hydroxytryptamine 2C agonist. Drug Metab Dispos J. 2012;40:761–771. doi: 10.1124/dmd.111.043414. [DOI] [PubMed] [Google Scholar]
- 23.Clinical Pharmacology. Pharmacokinetics of lorcaserin. Available at: http://clinicalpharmacology-ip.com/Forms/Monograph/monograph.aspx?cpnum=3771&sec=monphar&t=0. Accessed August 19, 2013.
- 24.Boyer E, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 25.Schannwell CM, Steiner S, Strauer B-E. Diagnostics in pulmonary hypertension. J Physiol Pharmacol. 2007;58(Suppl 5):591–602. [PubMed] [Google Scholar]
- 26.Jaffré F, Bonnin P, Callebert J, et al. Serotonin and angiotensin receptors in cardiac fibroblasts co-regulate adrenergic-dependent cardiac hypertrophy. Circ Res. 2009;104:113–123. doi: 10.1161/CIRCRESAHA.108.180976. [DOI] [PubMed] [Google Scholar]
- 27.Smith BM, Smith JM, Tsai JH, et al. Discovery and structure–activity relationship of (1r)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1h-3-benzazepine (lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem. 2008;51:305–313. doi: 10.1021/jm0709034. [DOI] [PubMed] [Google Scholar]
- 28.Geyer MA, Vollenweider FX. Serotonin research: Contributions to understanding psychosis. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Goodrx.com. Belviq. Available at www.goodrx.com/belviq/price. Accessed August 19, 2013.


