Abstract
Background
Understanding and modelling early events of floral meristem patterning and floral development requires consideration of positional information regarding the organs surrounding the floral meristem, such as the flower-subtending bracts (FSBs) and floral prophylls (bracteoles). In common with models of regulation of floral patterning, the simplest models of phyllotaxy consider only unbranched uniaxial systems. Racemose inflorescences and thyrses offer a useful model system for investigating morphogenetic interactions between organs belonging to different axes.
Scope
This review considers (1) racemose inflorescences of early-divergent and lilioid monocots and their possible relationship with other inflorescence types, (2) hypotheses on the morphogenetic significance of phyllomes surrounding developing flowers, (3) patterns of FSB reduction and (4) vascular patterns in the primary inflorescence axis and lateral pedicels.
Conclusions
Racemose (partial) inflorescences represent the plesiomorphic condition in monocots. The presence or absence of a terminal flower or flower-like structure is labile among early-divergent monocots. In some Alismatales, a few-flowered racemose inflorescence can be entirely transformed into a terminal ‘flower’. The presence or absence and position of additional phyllomes on the lateral pedicels represent important taxonomic markers and key features in regulation of flower patterning. Racemose inflorescences with a single floral prophyll are closely related to thyrses. Floral patterning is either unidirectional or simultaneous in species that lack a floral prophyll or possess a single adaxial floral prophyll and usually spiral in the outer perianth whorl in species with a transversely oriented floral prophyll. Inhibitory fields of surrounding phyllomes are relevant but insufficient to explain these patterns; other important factors are meristem space economy and/or the inhibitory activity of the primary inflorescence axis. Two patterns of FSB reduction exist in basal monocots: (1) complete FSB suppression (cryptic flower-subtending bract) and (2) formation of a ‘hybrid’ organ by overlap of the developmental programmes of the FSB and the first abaxial organ formed on the floral pedicel. FSB reduction affects patterns of interaction between the conductive systems of the flower and the primary inflorescence axis.
Keywords: Bracteole, flower, flower-subtending bract, inflorescence, inhibitory field, pattern formation, prophyll, regulation of development, vasculature
INTRODUCTION
Flowers and inflorescences are two intimately linked components of the angiosperm reproductive system. Studies of inflorescence morphology and floral morphology have their own distinct technical and methodological approaches (e.g. Troll, 1964, 1969; Weberling, 1989; Endress, 1994; Weberling and Troll, 1998; Leins and Erbar, 2010). In the present paper, we follow Kirchoff (2000, 2003) in highlighting some areas of interaction between floral morphology and inflorescence morphology. We focus on monocots, especially early-divergent and lilioid monocots, because this group offers a suitable spectrum of flower and inflorescence diversity. In contrast to some other inflorescence types (e.g. panicles: Troll, 1964; Endress, 2010), racemose inflorescences (as well as thyrses: see below) are highly appropriate in investigating flower–inflorescence interactions because the flowers are lateral with respect to the primary axis, without additional phyllomes on a pedicel, or with a limited number of such phyllomes. Throughout, we use the term ‘additional pedicel phyllomes’ for floral prophylls (also termed bracteoles; for terminology see Prenner et al., 2009; Endress, 2010) and/or calyculus phyllomes on a lateral pedicel. We use the term flower-subtending bract (FSB) as a synonym of floral pherophyll.
In common with mathematical models of the regulation of floral patterning, the simplest models of phyllotaxy consider only unbranched uniaxial systems (e.g. Skryabin et al., 2006; Smith et al., 2006). Racemose inflorescences and thyrses offer a useful model system for investigating the morphogenetic interaction of organs belonging to different axes, for example in assessing the correlations between phyllotaxic patterns on the primary inflorescence axis and floral prophyll position on the pedicel. This aspect of morphology was investigated in detail in the relatively derived monocot order Zingiberales, which bears thyrses with lateral cincinni (Kirchoff, 2000, 2003). These studies emphasized the role of the prophyll in the positional control of inflorescence branching and development of individual flowers. However, factors influencing the spatial arrangement of the prophyll itself require further investigation (e.g. Rüter, 1918; Choob and Mavrodiev, 2001; Choob, 2002).
In this paper, we review the racemose inflorescences of early-divergent and lilioid monocots, or partial inflorescences in cases of complex inflorescences, with the aim of investigating flower–inflorescence interactions. We discuss hypotheses on the morphogenetic significance of phyllomes surrounding developing flowers, such as FSBs and floral prophylls. We also consider the complex broader aspects of the positional information of these non-floral organs. This review provides a basis for studies of molecular and physiological aspects of floral patterning in monocots. In the two final sections, we review the patterns of FSB reduction and the vascular patterns in the primary inflorescence axis and lateral pedicels. These two topics are closely related because the evolutionary loss of FSBs typically results in considerable modification of the vascular system. The classical interpretation of stelar structure in seed plants regards the procambial strands developing at the bases of the leaf primordia as crucial for pattern formation in a stele, although the direction of procambium initiation is disputable and could differ between taxa (Esau, 1965; Sharman and Hitch, 1967; Hitch and Sharman, 1968; Larson, 1975; Dengler and Kang, 2001; Timonin, 2007). In a typical situation in seed plants in which shoot branching is axillary, the vascular pattern of the lateral bud is superimposed onto the developing stelar vasculature whose pattern is linked to phyllotaxy. Racemose inflorescences that lack well-developed FSBs provide a system in which the primary shoot apparently does not produce leaf primordia. Thus, in this respect, early-divergent monocots with bractless racemose inflorescences are morphologically similar to the (phylogenetically distantly related) eudicot model angiosperm Arabidopsis and other Brassicaceae that possess at least superficially bractless inflorescences (e.g. Hagemann, 1963; Baum and Day, 2004; Kwiatkowska, 2005, 2008; Penin, 2008).
RACEMOSE INFLORESCENCES OF EARLY-DIVERGENT AND LILIOID MONOCOTS
We follow most authors (e.g. Endress, 2010) in defining racemose inflorescences as those with a primary axis and lateral floral pedicels (see also Fig. 1 regarding terminology). In a racemose pattern, the primary axis can either be terminated by a flower (closed inflorescence) or not (open inflorescence) (Endress, 2010). The presence or absence of a terminal flower can be caused by different factors in different angiosperm families (Bull-Hereñu and Claßen-Bockhoff, 2011a, b).
Fig. 1.
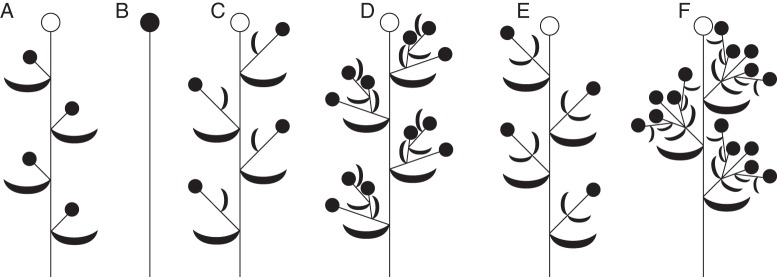
Schematic drawings of selected patterns of flower arrangement to explain the terminology used in the text (see also Endress, 2010). All drawings show the distal part of a shoot; the proximal part (developing during the same season or during the previous season or seasons) contains vegetative leaves, lateral renovation bud(s) and sometimes reduced leaves. (A) Bracteate raceme/botryoid without floral prophylls; (B) single terminal flower; (C) bracteate raceme/botryoid with a single floral prophyll per pedicel; (D) bracteate thyrse/thyrsoid with lateral monochasia; (E) bracteate raceme/botryoid with two floral prophylls per pedicel; (F) bracteate thyrse/thyrsoid with lateral dichasia. Closed circles indicate flowers that are invariably present; open circles represent terminal flowers that can be either present (botryoids, thyrsoids) or absent (racemes, thyrses).
Analysis of inflorescence diversity in a molecular phylogenetic context suggests that racemose (partial) inflorescences (racemes, spikes, spadices) represent the ancestral condition in monocots. Among early-divergent monocot lineages (e.g. Chase et al., 2006; Iles et al., 2013), racemose inflorescences are characteristic of Acorus (Acorales: e.g. Buzgo and Endress, 2000), the putative sister to all other monocots. Racemose inflorescences also characterize the order that is sister to all monocots except Acorales and Alismatales, Petrosaviales (Chase et al., 2006; Graham et al., 2006), including both genera, Japonolirion and (in most cases) Petrosavia (Remizowa et al., 2006a; M. V. Remizowa, unpubl. data). In Alismatales, racemose inflorescences are uniformly present in Aponogetonaceae, Araceae, Juncaginaceae, Posidoniaceae, Scheuchzeriaceae and Zosteraceae, and also in Potamogeton sensu lato (s.l.) (Potamogetonaceae) and Tofieldia (Tofieldiaceae) (e.g. Tomlinson, 1982; Dahlgren et al., 1985; Mayo et al., 1997; Remizowa et al., 2006a). Racemose partial inflorescences are also characteristic of some Liliales and Dioscoreales, and several species-rich derived monocot clades, including orchids (e.g. Takhtajan, 2009), sedges (e.g. Vrijdaghs et al., 2010) and grasses (e.g. Vegetti and Anton, 2000). Despite the absence of a single clear monocot outgroup, the occurrence of racemose inflorescences in several early-divergent monocot lineages strongly suggests that this condition is ancestral in monocots. However, performing formal character mapping is difficult because racemose and cymose branching patterns represent two extreme forms (Endress, 2010); scoring all monocots that do not possess strictly racemose inflorescences as cymose would be highly misleading.
Racemose inflorescences of early-divergent monocots (Acorales and Alismatales) show a range of variation in inflorescence tip structure (Lehmann and Sattler, 1992; Buzgo and Endress, 2000; Buzgo et al., 2004, 2006; Sokoloff et al., 2006; Lock et al., 2009). In addition to typical open and closed conditions, they include a condition with terminal flower-like structure of unstable morphology that could be variously interpreted in terms of disturbance of the flower-forming programme or amalgamation of the uppermost lateral flowers. More than one type of inflorescence tip morphology can be observed in some species, even in different inflorescences of a given individual plant. In some cases, a few-flowered racemose inflorescence can be entirely replaced by a terminal flower-like structure, so that free lateral flowers do not exist. In Ruppia maritima (Ruppiaceae: Alismatales), the condition with amalgamated flowers co-occurs with the more frequent condition of a two-flowered open racemose partial inflorescence (Sokoloff et al., 2006; Lock et al., 2011). We interpret the ‘flowers’ of Cymodoceaceae and Zannichelliaceae (Alismatales) as racemose partial inflorescences that are completely (and consistently) replaced by a terminal flower-like structure (Sokoloff et al., 2006; Remizowa et al., 2012b). Preliminary (poorly supported) molecular phylogenetic data indicate that Cymodoceaceae are paraphyletic with respect to Ruppia (Iles et al., 2013). If this relationship proves to be correct, then the consistent replacement of racemose partial inflorescences by terminal flower-like structures has occurred at least three times in Alismatales (twice in Cymodoceaceae, and independently in Zannichelliaceae). This phenomenon, termed ‘terminalization’, can result in an evolutionary shift from polytelic to monetelic synflorescences (Sokoloff et al., 2006). Terminalization often co-occurs with drastic reduction in the flowers of these submerged aquatics, including the gain of unisexuality. Another example of terminalization was described by Endress (1970, 1978, 1990) in the eudicot family Hamamelidaceae (Saxifragales), where it was accompanied by the loss of a perianth in flowers. In the vast majority of monocots, alternate (spiral or distichous) phyllotaxy is characteristic of vegetative shoots, probably due to the typically broad leaf base that characterizes monocots in general (e.g. Arber, 1925). In contrast, monocot flowers are almost always whorled rather than spiral (e.g. Endress, 1995; Remizowa et al., 2010b). FSB and lateral flower arrangement is most commonly alternate in monocot racemose inflorescences. Whorled patterns (sometimes termed pseudo-whorled in Alismataceae: Posluszny and Charlton, 1993) are reported in inflorescences of some Alismatales (e.g. Potamogeton spp.; Alismataceae) and Pandanales (Cyclanthaceae). Interestingly, Alismatales and Pandanales represent the two monocot orders in which the flower–inflorescence boundary is often regarded as obscure, and the diversity of the flower groundplan is considerable compared with other monocot groups (e.g. Uhl, 1947; Posluszny and Charlton, 1993; Posluszny et al., 2000; Rudall, 2003, 2008; Rudall and Bateman, 2006).
Lateral flowers of monocot racemose inflorescences can either possess or lack a floral prophyll, or rarely two floral prophylls, at least in some flowers, as in Tricyrtis (Liliaceae: M. V. Remizowa, unpubl. data), Triantha (Tofieldiaceae: M. V. Remizowa, unpubl. data) and Butomus (Butomaceae: e.g. Tomlinson, 1982). In our experience, the presence or absence of a floral prophyll is a character that is stable at a specific level and often seems to characterize large clades. Unfortunately, descriptive taxonomic literature does not always pay sufficient attention to the occurrence of a floral prophyll.
When phyllotaxy is distichous on the primary inflorescence axis, the floral prophyll is usually adaxial on the pedicel (e.g. Iridaceae). When phyllotaxy is spiral on the primary inflorescence axis, the position of the floral prophyll on the pedicel is more or less pronouncedly transverse (e.g. Petrosaviaceae, Liliaceae, Nartheciaceae, Dioscoreaceae).
The occurrence of a floral prophyll offers the possibility of further branching in its axil. Iterative branching leads to formation of a monochasium instead of a solitary flower in the axil of an FSB on the primary inflorescence axis. The entire inflorescence can be therefore described as a thyrse. In groups such as lilioid monocots, racemose inflorescences with floral prophylls (Fig. 1C) are closely related to thyrses (Fig. 1D) because the occurrence of the next-order flower in the axil of the floral prophyll represents a labile feature. The lability can even be seen at an infraspecific level, for example in Petrosavia stellaris (Petrosaviaceae). Both conditions are found in different species of Nartheciaceae. In Dioscorea (Dioscoreaceae), the female flowers are often in spikes with FSBs and floral prophylls, and the male flowers are arranged in thyrses (Remizowa et al., 2010a). An evolutionary transition from a raceme to a thyrse (and vice versa) is possible, but the presence of prophylls on the floral pedicels represents a key condition for such a transition (except in Tofieldiaceae: see below).
Endress and Doyle (2009) highlighted a close relationship between thyrses and racemose inflorescences in early-divergent angiosperms. For example, Hedyosmum (Chloranthaceae) has thyrses of female flowers and spikes of male flowers (Endress, 1987). For the purpose of scoring this character in a morphological cladistic analysis of early-divergent angiosperms, Endress and Doyle (2009) distinguished (as cladistic character states) between two major inflorescence types: those with a terminal flower, such as panicles, thyrsoids and botryoids (in a broad sense, including stachyoids; Endress, 2010), and those lacking a terminal flower, such as racemes (in a broad sense) and thyrses.
We argue that, at least for monocots, a different distinction could be more useful for evolutionary (including cladistic) assessments, because the presence or absence of a terminal flower is labile in early-divergent monocots. Thus, we distinguish between inflorescences in which the lateral flowers possess floral prophylls (including thyrses and some racemose inflorescences; Fig. 1C) and those in which the lateral flowers lack floral prophylls (Fig. 1A). Regardless of character coding, it is clear that the degree of variation in inflorescence morphology varies greatly between monocot clades. For example, the species-rich family Araceae (Alismatales) consistently possesses the same type of highly characteristic racemose inflorescence (spadix), despite considerable variation in flower groundplan. In contrast, inflorescence morphology exhibits a broad range of variation in Tofieldiaceae, a relatively species-poor, early-divergent family of Alismatales (Fig. 2). In Isidrogalvia (incl. Harperocallis), the inflorescence is a botryoid or a solitary terminal flower (Remizowa et al., 2011). In Tofieldia and Triantha, which represent sister genera (Azuma and Tobe, 2010), the inflorescences are open racemes and thyrsoids, respectively (M. V. Remizowa, unpubl. data).
Fig. 2.
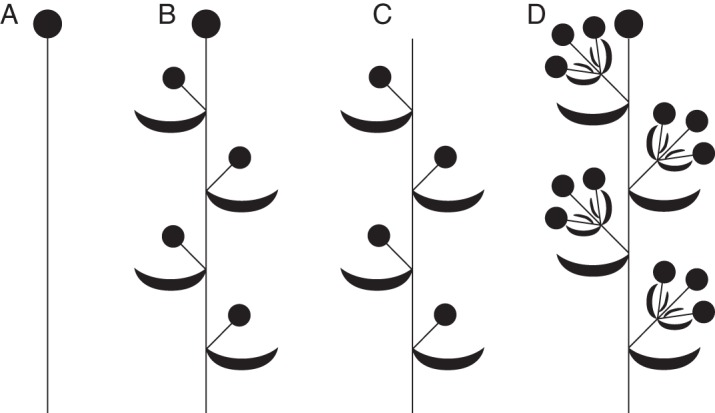
Inflorescences of Tofieldiaceae. (A) Single terminal flower (Harperocallis); (B) botryoid (Isidrogalvia); (C) raceme (Tofieldia); (D) thyrsoid (Triantha).
PATTERNING AND PRE-PATTERNING OF SHOOT AND FLOWER APICAL MERISTERM
Investigations of both the regulation of flower development (i.e. the factors that govern the establishment of the floral groundplan) and the underlying gene networks are among the most intriguing fields of botanical research, especially when they are addressed together in a phylogenetic context. It has become widely accepted that the precise location of incipient floral organs on the floral apex results from processes taking place in the floral meristem even before the organ primordia are visible. This phenomenon is known as pre-patterning (or pattern formation) of the floral meristem (e.g. Parcy et al., 2002; Choob and Penin, 2004; Skryabin et al., 2006; Souer et al., 2008; Rudall, 2010). Examination of developing flowers, as well as patterns of variation in the flower groundplan, can provide some post-factum evidence of regulation of floral pre-patterning.
Hofmeister's Rule (Leins and Erbar, 1997; Kirchoff, 2000, 2003) is an empirical heuristic that helps to understand and predict sites of initiation of new lateral organs on the shoot apical meristem. Kirchoff (2000, 2003) successfully used Hofmeister's Rule in interpretation of sequential development of floral organs in various taxa, especially in Zingiberales. Hofmeister's Rule postulates that each new organ is initiated as far as possible from the organs already present on the apex. In a strict sense, this concept does not consider patterning of the first primordium on a shoot, which is crucial in the development of spatial control of all subsequent organs. Kirchoff (2000, 2003) highlighted the shape of the apical meristem as a second important factor. Even with such a modification, Hofmeister's Rule fails to explain all existing phyllotaxic patterns (Kirchoff, 2000, 2003). One of the most enigmatic examples is Costus (Costaceae), where the angle of leaf divergence decreases to 45 ° or even less (Snow, 1952; Kirchoff and Rutishauser, 1990), and the position of primordia does not follow Hofmeister's Rule.
In vegetative lateral shoots of Tofieldiaceae, the first-formed leaf (prophyll) and the second-formed leaf are both adaxial, i.e. located on nearly the same radius (Remizowa et al., 2005, 2011). Unidirectional floral development is another problematic phenomenon. For example, in water-lily flower development, after the first sepal initiation (which is abaxial) one could expect the appearance of the subsequent sepal primordium on the opposite side of the floral meristem. In reality, two lateral sepals develop prior to the adaxial one (Schneider et al., 2003; Hu et al., 2009). With respect to inflorescences, the FSB has a major influence on the patterning of the lateral shoot because it creates space available to a meristem located in its axil (Kirchoff, 2003).
Based on the Reaction Model (Wardlaw, 1957), recent progress in computer simulation of plant development brings forward the concept of inhibitory fields (Skryabin et al., 2006; Smith et al., 2006; Choob, 2010). According to this concept, inhibitory zones can arise across any primordium on the meristem. Although the authors hypothesized a possible physiological context, the precise nature of the inhibitory zones (which is beyond the scope of the present review) is less important than their actual presence. New organs must be initiated in the regions of the meristem peripheral zone that are free from the inhibitory influences of primordia that are already formed. Instead of Hofmeister's Rule, a principle of meristem space economy has been postulated (Skryabin et al., 2006; Choob, 2010) in which a new primordium arises at a minimum possible distance from the existing primordia, conditioned by their inhibitory fields (Choob, 2010). Hofmeister's Rule may be regarded as a particular case of a long-distant inhibitory field of older primordia, but not as a general rule. Considering the inhibitory field concept, we speculate that the sequence of floral organ initiation (especially the outer tepals) in monocots is a consequence of inhibitory influences from structures that surround the floral meristem. We postulate that the initiation of incipient floral primordia is guided by inhibitory zones from previously initiated or patterned organs. The inhibitory zone gradually decreases during the course of primordium formation and growth, so that the newly patterned organs have the strongest inhibitory fields, while the inhibitory activity of older organs gradually decreases. Another point to be emphasized is that inhibitory fields of different organ types can differ in size, but the final type and shape of organ depends on the particular expression of the organ identity genes (Skryabin et al., 2006; Choob, 2010).
Even using mathematical simulation models of phyllotaxy, it is difficult to build a system of postulates that explains both spiral and whorled arrangements. Smith et al. (2006) created a useful model for spiral patterns, based on a single inhibitor. However, for whorled patterns they invented a second inhibitor. The model of Skryabin et al. (2006, see also Choob, 2010) provides an adequate simulation of whorled arrangements based on a single inhibitor postulation, but gives restricted results for spiral patterns. The results for 1/2 and 2/5 phyllotaxies appear useful, but for other types of phyllotaxy the model needs some additional postulations (Skryabin et al., 2006; Choob, 2010).
The possible physiological nature of the inhibitory effect of a newly initiated primordium has been extensively investigated (reviewed by Berleth et al., 2007; Kramer, 2008; Choob, 2010; Choob and Sinyushin, 2012; van Mourik et al., 2012). The inhibition seems to be based primarily on the self-organization process of polar auxin transport, although other factors are also involved (e.g. Kierzkowski et al., 2012; see also Choob and Sinyushin, 2012). At the site of the midvein of the future phyllome, auxin transport is re-directed from the upper cell layer of the tunica (L1) to the corpus. Thus, across any primordium, a drainage basin of auxin arises in the L1-cells. Within this basin, all the auxin moves towards the centre of the primordium, and its high concentration stimulates differential gene expression, further organ growth and initiation of vasculature. Any new drainage basin (large enough to collect the concentration of auxin that is required for new organ initiation) can arise only outside existing ones.
NON-FLORAL ORGANS INFLUENCE FLOWER DEVELOPMENT IN MONOCOTS
In the following paragraphs, we discuss examples of monocot species with trimerous flowers that are arranged in racemose inflorescences or open thyrses. We consider the possible influence that structures surrounding a flower can have on the location and sequence of floral organ initiation. In species with racemose inflorescences lacking floral prophylls (Figs 3A and 4A), the floral meristem starts to develop organ primordia in a close relationship with an FSB. We hypothesize that the FSB (or its primordium) establishes an inhibitory zone with a gradient towards the inflorescence axis so that its inhibitory influence is high in the close vicinity of the FSB and decreases towards the adaxial side. In terms of geometry, the inhibitory influence of the FSB expands along the median plane of the floral meristem. In species where flowers are initiated in the axils of already well-developed FSBs, the presence of a large FSB results in transversely elongated floral primordia. The larger the FSB, the more prominent is the transverse elongation of the floral meristem. Under the conditions established by the FSB, it follows that the first organs are initiated at a minimum distance from the FSB in the area that is not covered by the inhibitory zone. This area is the transverse–adaxial side of the floral meristem, where the first two lateral outer tepals are initiated (Fig. 4A), while the median abaxial tepal is delayed in development. Examples are Veratrum [Melanthiaceae sensu stricto (s.s.), Liliales] and Bulbine (Asphodelaceae, Asparagales) (Endress 1995).
Fig. 3.
Orientation of lateral flower in monocots shown on diagrams of anthetic flowers. (A) Bracteate flower without floral prophyll (e.g. Melanthiaceae s.s.); (B) non-bracteate flower (e.g. Acorus); (C) bracteate flower with a single lateral floral prophyll – all variants can be found within the same inflorescence (e.g. Nartheciaceae, Petrosaviaceae, some Liliaceae); (D) bracteate flower with a single adaxial floral prophyll (Iridaceae); (E) bracteate flower with two lateral floral prophylls (e.g. Tricyrtis hirta); (F) flower with three calycular phyllomes on the pedicel (Tofieldiaceae); (G) non-bracteate flower (some basal Araceae). Closed circles indicate inflorescence axis, black arcs represent the flower-subtending bract (FSB) and pedicel phyllome(s), dark grey arcs represent outer tepals and light grey arcs represent inner tepals.
Fig. 4.
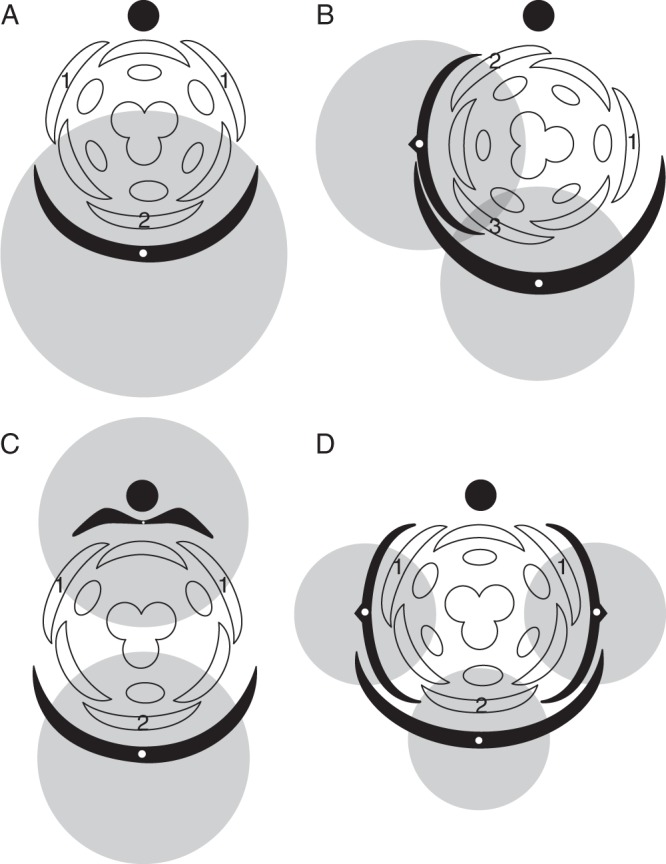
Patterns of floral orientation and development established by inhibitory fields of FSBs and floral prophyll(s). (A) Bracteate flower without floral prophyll; (B) bracteate flower with a single lateral floral prophyll; (C) bracteate flower with a single adaxial floral prophyll; (D) bracteate flower with two lateral floral prophylls. Closed circles indicate inflorescence axis, black arcs represent the FSB and pedicel phyllome(s). Grey areas represent inhibitory zones. Numbers indicate the sequence of initiation of outer tepals.
After this stage, there are three inhibitory zones occupying the floral meristem – one from the FSB and two from the outer tepals. In most cases, the inhibitory influence of the FSB ceases at this point, making it possible to insert the third outer tepal in a median abaxial position on the same radius as the FSB. However, in some cases, the inhibitory influence of the FSB is sufficient to arrest organ appearance in its close vicinity, even after initiation of the lateral tepals and the third tepal (the inner median tepal) is initiated abaxially between the lateral outer tepals. In both cases of delayed organ initiation and/or development on the abaxial side of the floral meristem, floral development is unidirectional. Unidirectional floral development is present even in some species that lack visible FSBs, due either to the formation of a ‘hybrid’ tepal–FSB structure or to the occurrence of a cryptic FSB (see below).
In species that possess both an FSB and a floral prophyll (species possessing open thyrses meet the same criteria as species with racemose inflorescences), the appearance of the floral prophyll establishes its own inhibitory zone that combines with the inhibitory zone of the FSB. Most monocots that possess floral prophylls usually develop only one (Fig. 3C). In taxa with racemose inflorescences and spiral flower arrangement, the floral prophyll is inserted transversely or nearly transversely but never occupies the median-abaxial position (Fig. 5D). In this case, the floral meristem is under the influence of two inhibitory zones: a median-abaxial zone established by the FSB, and a left- or right-transverse zone established by the floral prophyll (Fig. 4B). This arrangement results in spiral initiation of the outer tepals. The first outer tepal is initiated strictly opposite the floral prophyll in the area that is free from inhibitory zones (Fig. 4B). The second and third outer tepals are initiated between the inflorescence axis and the floral prophyll and between the FSB and floral prophyll, with the sequence of initiation depending on the inhibitory influence of the FSB. Examples of the pattern with a single transverse floral prophyll and sequential outer-whorl tepal initiation can be found in members of Petrosaviales (both genera: Remizowa et al., 2006a; M. V. Remizowa, unpubl. data), Liliales (e.g. Lilium: Greller and Matzke, 1970), Dioscoreales (Dioscorea: Remizowa et al., 2010a; Nartheciaceae: Remizowa et al., 2006a, 2008) and Asparagales (e.g. Dianella, Hemerocallidaceae: Eichler, 1875; Engler, 1888).
Fig. 5.
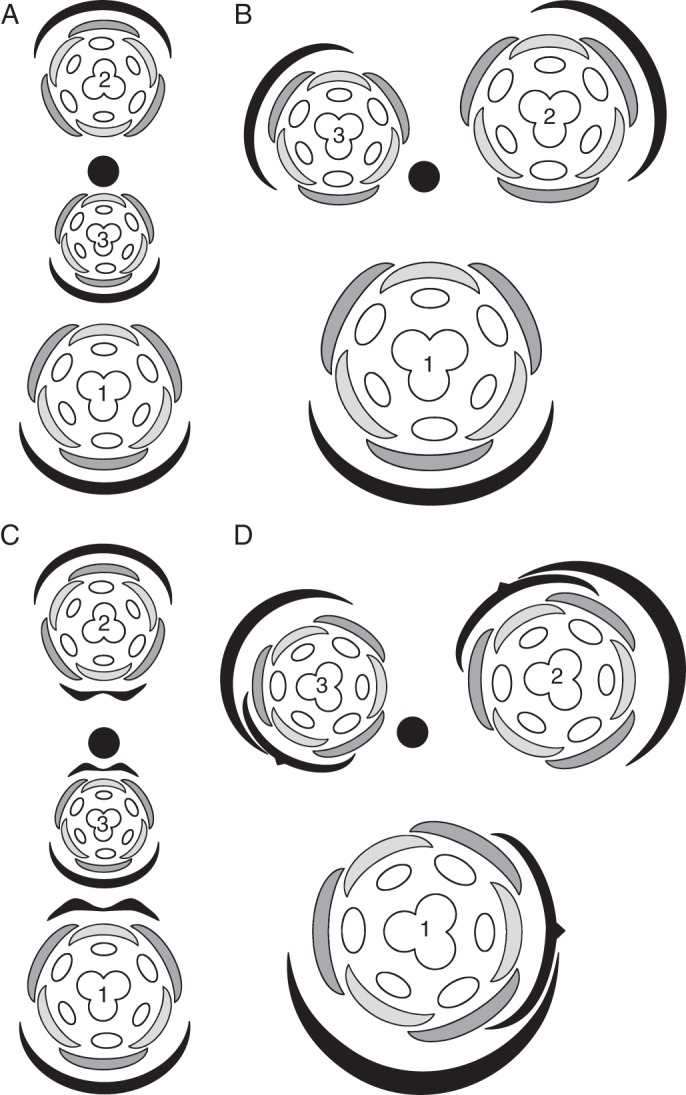
Orientation of lateral flowers in racemose monocot inflorescences with distichous and spiral phyllotaxy shown. (A) Bracteate inflorescence with distichous phyllotaxy, floral prophylls absent; (B) bracteate inflorescence with spiral phyllotaxy, floral prophylls absent; (C) bracteate inflorescence with distichous phyllotaxy and adaxial floral prophyll; (D) bracteate inflorescence with spiral phyllotaxy and lateral floral prophyll. Closed circles indicate inflorescence axis, black arcs show the FSB and pedicel phyllome(s), dark grey arcs represent outer tepals and light grey arcs represent inner tepals. Numbers indicate relative position of the flower within the inflorescence and sequence of their initiation and opening.
Because of the similar geometry of flower arrangement, our observations are comparable with data on the development of the first flower of the cincinnus in Zingiberales (Kirchoff, 2000, 2003), where the type of positional control is closely similar. Despite the accepted theoretical framework, the positions of the floral prophyll and/or its axillary bud appear to be among the guiding factors in the particular placement of the first perianth organ.
In species with distichous flower arrangement, the floral prophyll, when present, is inserted in a median-adaxial position and often possesses two keels (Fig. 5). Iridaceae offer a good example of this pattern (e.g. Payer, 1857; Fukai and Goi, 1998). In this case, the floral meristem is covered by both abaxial and adaxial inhibitory zones, with a similar effect as in the situation when only an FSB is present. In these cases, even though a floral prophyll is initiated later (or its future position is determined later), the inhibitory influence of the FSB is dominant over that of the floral prophyll. This arrangement can result in unidirectional tepal initiation with a delay on the abaxial side of the floral meristem. Alternatively, tepals can be initiated simultaneously within a whorl but in the same positions as above, with the median outer tepal being abaxial and the other two tepals being transverse-adaxial.
The correlation between the phyllotaxy of the organs of the primary axis and the position of the floral prophyll (Fig. 5) is important in the framework of comparing Hofmeister's Rule and the space economy concept. Distichous phyllotaxy on the primary axis occurs in cases of strong inhibitory activity of phyllome (FSB) sites (e.g. Choob, 2010). In this case, the strong inhibitory activity of the FSB dictates the insertion of the floral prophyll at the maximum possible distance from the FSB, i.e. adaxially. Spiral phyllotaxy on the primary axis occurs when the inhibitory zones of the FSB sites are narrower than in the previous case. This creates the possibility for location of the floral prophyll closer to the FSB (not at the maximum possible distance), i.e. in a transverse position. Depending on the FSB inhibitory zone, the floral prophyll can occupy either a transverse position or a transverse-abaxial position or a transverse-adaxial position (and all possible intermediate positions) and can be situated either on the left or on the right side of the flower. Compared with distichous phyllotaxy, the position of the floral prophyll is more or less variable. Within the concept of space economy, in species with spiral phyllotaxy, a strong inhibitory activity of the floral prophyll is hypothesized. Otherwise, it is difficult to explain why the first tepal is inserted on the radius opposite the floral prophyll. It is intriguing that in monocots that lack floral prophylls, the first tepal is very rarely inserted at the maximum distance from the non-reduced FSB (possibly Eriocaulaceae in Poales; Hamann, 1964).
The presence of two floral prophylls is a rare condition in monocots. At least in Tricyrtis, Triantha and Butomus, the two floral prophylls of the first flowers in lateral cymes are symmetrically inserted in a nearly transverse position (Figs 3E, F and 4D). By analogy with the case of a single transverse floral prophyll, it is logical to suppose a narrow inhibitory zone of the FSB. This scenario appears to contradict the observation that FSBs are distichous on the primary axis in Trianthia (and nearly so in Tricyrtis).
All Tofieldiaceae (Alismatales) possess an unusual structure termed a calyculus (Eichler, 1875; Zomlefer, 1997; Remizowa and Sokoloff, 2003; Remizowa et al., 2006a, 2010c; Takhtajan, 2009). This structure consists of three free or connate phyllomes alternating with the outer tepals. As a consequence of the presence of a calyculus, Tofieldiaceae differ strongly in floral orientation from most other monocots that possess bracteate racemose inflorescences, because the median outer tepal is adaxial (Fig. 3F). In many respects, the calyculus phyllomes behave like an additional perianth whorl and floral development resembles that of species with racemose inflorescences lacking floral prophylls. The initial stages of floral development can be described in the same terms, with the calyculus phyllomes playing the role of the outer tepals (Remizowa et al., 2006a). The FSB (or its primordium) establishes an inhibitory zone with a gradient towards the inflorescence axis. According to the principle of space economy, the first pair of organs are initiated at the border of the FSB inhibitory zone; thus, the two lateral calyculus phyllomes arise on the transverse-adaxial side of the floral meristem. After this stage, there are three inhibitory zones occupying the floral meristem, one from the FSB and two from the lateral calyculus phyllomes. Depending on the inhibitory influence (and size) of the FSB, either the inhibitory influence of the FSB ceases, allowing insertion of the median calyculus phyllome on the same radius as the FSB, or it is maintained and the median calyculus scale is initiated after the inception of the outer tepals. Unidirectional floral development is more prominent in cases with larger FSB primordia, whose size can vary even within the same inflorescence.
All species of Araceae (Alismatales) develop non-bracteate inflorescences, in which trimerous flowers are oriented as in Tofieldiaceae (Fig. 3F, G), i.e. with the median outer tepal in an adaxial position, if a trimeous perianth is present (Buzgo, 2001). In perianth-bearing species, all tepals within a whorl are initiated simultaneously (Buzgo, 2001). It is unclear whether the unusual flower orientation of Araceae results from FSB deletion and complete loss of positional information of the FSB, or both FSB and floral prophyll(s) are suppressed, with retained positional information due to residual inhibitory activity. It is intriguing that Araceae are phylogenetically closely related (though not sister) to Tofieldiaceae (e.g. Iles et al., 2013); this close relationship prompts us to speculate about the possible occurrence in Araceae of not only a cryptic FSB but also a cryptic calyculus.
FLOWER-SUBTENDING BRACT REDUCTION IN RACEMOSE INFLORESCENCES OF EARLY-DIVERGENT MONOCOTS
Racemose inflorescences in which the FSBs are either absent or inconspicuous are especially common among early-divergent monocots. Species of Alismatales with racemose inflorescences form two distinct morphological groups: those with well-developed FSBs and those that lack FSBs, at least superficially (Fig. 6). These crucial features of inflorescence architecture are sometimes not consistent, even at the generic level. Scheuchzeria (Scheuchzeriaceae), Posidonia (Posidoniaceae), most Tofieldia species (Tofieldiaceae) and some Potamogeton species (Potamogetonaceae) all belong to the group with consistently bracteate inflorescences (Sattler, 1965; Posluszny and Sattler 1974; Posluszny, 1981, 1983; Tomlinson, 1982; Sun et al., 2000; Remizowa et al., 2006a, 2013; Sokoloff et al., 2006; Lock et al., 2009). Tofieldia pusilla, Araceae, Aponogetonaceae, Juncaginaceae, Ruppiaceae, some Potamogetonaceae and possibly Zosteraceae (Alismatales) as well as Acorus (Acorales) are characterized by non-bracteate inflorescences, or at least the FSBs are not visible as separate organs (Uhl, 1947; Posluszny and Sattler, 1973, 1974; Singh and Sattler, 1977; Lieu, 1979; Soros-Pottruff and Posluszny, 1995a, b; Mayo et al., 1997; Buzgo and Endress, 2000; Buzgo, 2001; Barabé et al., 2002, 2011; Remizowa and Sokoloff, 2003; Buzgo et al., 2004, 2006; Remizowa et al., 2006a, 2013; Lock et al., 2011; Lock, 2012).
Fig. 6.
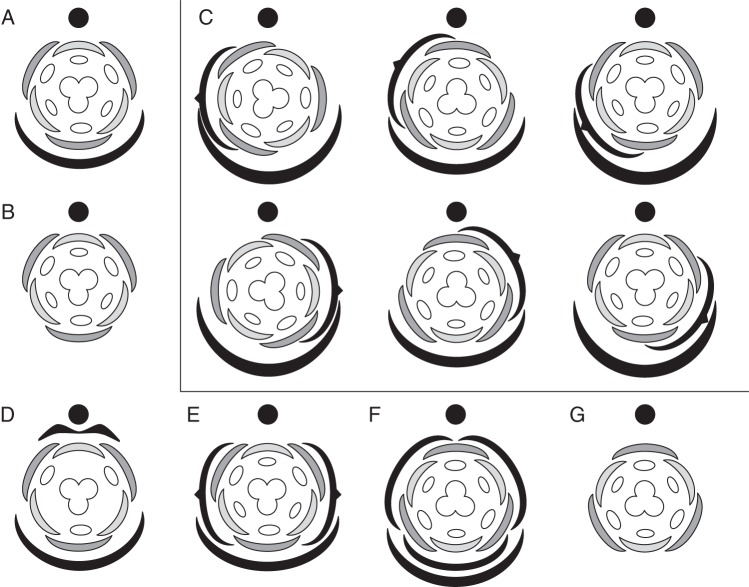
Patterns of flower-subtending bract (FSB) reduction in the order Alismatales. (A) Species of Potamogeton with bracteate inflorescences; (B) species of Potamogeton with non-bracteate inflorescences where FSBs are suppressed; (C) Potamogeton densus with ‘hybrid’ organ formed by the FSB and outer tepal; (D) species of Triglochin with non-bracteate inflorescences where FSBs are suppressed; (E) Triglochin maritima with ‘hybrid’ organ formed by the FSB and outer tepal; (F) species of Tofieldia with bracteate inflorescences; (G) Tofieldia pusilla with ‘hybrid’ organ formed by the FSB and calyculus phyllome. Closed circles indicate inflorescence axis, black arcs show morphologically expressed FSBs, dashed-lined arcs indicate suppressed FSBs and grey arcs show ‘hybrid’ organs. Numbers indicate the sequence of organ initiation.
In most angiosperms, floral primordia arise in the axils of FSBs that are already initiated, for example in Scheuchzeria (Scheuchzeriaceae, Alismatales: Posluszny, 1983), Metanarthecium (Nartheciaceae, Dioscoreales: Remizowa et al., 2008) or Xyris (Xyridaceae, Poales: Remizowa et al., 2012a). In contrast, in some members of Alismatales with racemose inflorescences, the flowers and their FSBs appear simultaneously and in some cases they even arise from a common meristem; this feature is not always consistent at the family and genus levels (reviewed by Remizowa et al., 2013).
The presence or absence of an FSB does not affect tepal position in species of Potamogeton (Posluszny and Sattler, 1974; Posluszny, 1981; Sun et al., 2000). This phenomenon suggests that in taxa without a morphologically expressed FSB, the cryptic FSB can still extend an inhibitory influence. In other words, the FSB is completely suppressed but its positional information is not lost.
A similar phenomenon occurs in Arabidopsis thaliana, which lacks FSBs in the wild type, at least superficially. The organ position in lateral flowers was not affected in bractea mutants, which possess FSBs (Penin et al., 2005). Consequently, the positional signal of the FSB is similar in the bractea mutant and the wild type, in spite of FSB reduction. Moreover, an extensive study of floral primordium shape in the wild type at the earliest developmental stages led to the conclusion that the cryptic FSB and the flower appear as a common primordium, and the entire incipient FSB is included in the flower (Kwiatkowska, 2005; see also Hagemann, 1963).
There is a strong tendency for reduction of FSBs to be accompanied by loss of their vasculature, e.g. in Potamogeton. Even in species of Potamogeton possessing bracteate inflorescences, FSBs are not vascularized (e.g. Uhl, 1947); either the FSB is initiated separately or from a common FSB-floral meristem. The same is true for Posidonia (Remizowa et al., 2012b), in which the flowers and their FSBs are initiated separately. In both Potamogeton and Posidonia, the FSBs are delayed in development and become clearly visible only at anthesis.
In Triglochin (Juncaginaceae), which lacks visible FSBs, species differ in patterns of perianth initiation and FSB reduction (reviewed by Remizowa et al., 2013). In T. maritima, the first organ produced on the floral apex is the median outer tepal, occupying a strictly abaxial position and demonstrating some FSB-like features during early developmental stages (Buzgo et al., 2006; Remizowa et al., 2013). It could be hypothesized that the median abaxial organ in the flower of T. maritima is a ‘hybrid’ organ in which the developmental programmes of the tepal and FSB overlap. A similar organ was found in T. striata (see Lieu, 1979). In contrast to T. maritima and T. striata, ‘hybrid’ organs are absent from three other species of Triglochin (T. barrelieri, T. bulbosa, T. palustris). In these species, FSBs are not detectable either in anthetic or in developing inflorescences, and perianth initiation and development are slightly delayed on the abaxial side of the young flower, suggesting the presence of a cryptic FSB (Remizowa et al., 2013). Here, in contrast to T. maritima and T. striata, the FSB primordia are absent, rather than amalgamated with the outer median tepal primordium.
The formation of a ‘hybrid’ organ that combines characters of the FSB and the first median abaxial phyllome on the pedicel is a feature common to a range of basal monocots with racemose inflorescences (Remizowa et al., 2013). Besides the above mentioned species Triglochin maritima and T. striata, this feature is present in Potamogeton densus (see Posluszny and Sattler, 1973), Acorus (Buzgo and Endress, 2000) and Tofieldia pusilla (Remizowa et al., 2006a). The formation of similar FSB-like organs could represent a homoplastic tendency among basal monocots (Alismatales and Acorales). In Acorus, Triglochin maritima, T. striata and Potamogeton densus the abaxial median outer tepal exhibits FSB-like features, whereas in Tofieldia pusilla this is the abaxial median calyculus phyllome. In these taxa, ‘hybrid’ organs are initiated in the same way as an FSB (i.e. they are initiated before other floral parts and require more extensive growth to cover the floral apex). It is worth mentioning that FSB features are more pronounced than calyculus features in the ‘hybrid’ organ of Tofieldia pusilla, especially in the vasculature (Remizowa et al., 2010c). In contrast, tepal features are more pronounced than FSB features in the ‘hybrid’ structures of Triglochin, Potamogeton and Acorus. Importantly, vascularization of the ‘hybrid’ structures in these early-divergent monocots is the same as in normal tepals of the same species, and not what could be expected from an FSB (Uhl, 1947; Buzgo and Endress, 2000; Buzgo et al., 2006; Remizowa et al., 2013).
To conclude, early-divergent monocots with racemose inflorescences have a general (homoplastic) tendency to FSB reduction. In this grade, the FSBs can be lost in two different ways – via morphological reduction (suppression) or via formation of putative ‘hybrid’ organs. Both morphological reduction (suppression) and formation of ‘hybrid’ organs can occur within the same family and even within the same genus. In alismatids, different non-bracteate conditions co-occur with cases of the presence of a true FSB, which can be initiated either before or simultaneously with the flower, either as a separate primordium or from a common meristem with the flower (reviewed by Remizowa et al., 2013). It would be interesting to explore whether different patterns of bract reduction and different temporal relations between the FSB and flower formation are linked with differences in meristem qualities, as discussed by Bull-Hereñu and Claßen-Bockhoff (2011a, b).
The ‘hybrid organ’ hypothesis is based on the concept of ‘hybridization of developmental pathways’ (Lodkina, 1983; Sattler, 1988; Weston, 2000; Remizowa et al., 2006a, 2013), or ‘amalgamation of developmental pathways’ (Rutishauser and Isler, 2001), resulting in ‘developmental mosaics’ between ‘organs’ that are normally assumed to have different ‘identities’ (Rutishauser and Isler, 2001; Rutishauser and Moline, 2005). The ‘hybrid organ’ hypothesis could be tested by data on gene expression patterns. However, the genes (or their precise combinations) that are specifically expressed in the FSB (but not in vegetative leaves or floral organs) have not hitherto been identified. Buzgo et al. (2006) reported a complex expression pattern of a putative orthologue of the B-class gene APETALA3/DEFICIENS in Triglochin maritima, but unfortunately these data do not provide sufficient evidence on the homologies of the abaxial phyllome. The absence of knowledge about FSB-specific regulatory genes also reflects the fact that the concept of the FSB is based on organ position (as subtending phyllome, or pherophyll, for a flower) rather than on its specific morphology. The most important feature of ‘hybrid’ structures in basal monocots is that they appear to belong simultaneously to shoots of different orders, i.e. to both the primary inflorescence axis and the lateral, flower-bearing axis. These ‘hybrid’ structures cannot be precisely morphologically classified.
VASCULATURE OF RACEMOSE INFLORESCENCES
The majority of investigations of reproductive vasculature have focused on flowers (the course of the vascular bundles in the receptacle and floral organs), while relatively few studies have traced the course of the vascular bundles through the inflorescence axis into the pedicel (e.g. Uhl, 1947; Singh, 1965; Tomlinson, 1982). We believe that deep functional, structural and morphogenetic correlations between the inflorescence parts require examination of inflorescence vasculature as a whole, without establishing artificial boundaries. Below, we review inflorescence vasculature in a range of early-divergent and lilioid monocots, based primarily on Remizowa and Lock (2012).
In many general aspects, the vascular systems of racemose inflorescences are alike among different taxa (Figs 7 and 8). This similarity is dictated by similar inflorescence architecture (i.e. the primary axis and lateral flowers). In monocots with spiral flower arrangement (Tofieldia and Isidrogalvia: Tofieldiaceae, Alismatales; Triglochin: Juncaginaceae, Alismatales; Japonolirion and Petrosavia: Petrosaviaceae, Petrosaviales; Narthecium and Matanarthecium: Nartheciaceae, Dioscoreales), more or less numerous vascular bundles form a ring in transverse sections. The number of bundles within a ring decreases toward the inflorescence tip. In Potamogeton, which is characterized by a whorled flower arrangement, the number of bundles within each internode corresponds to the number of flowers in a whorl (Remizowa and Lock, 2012).
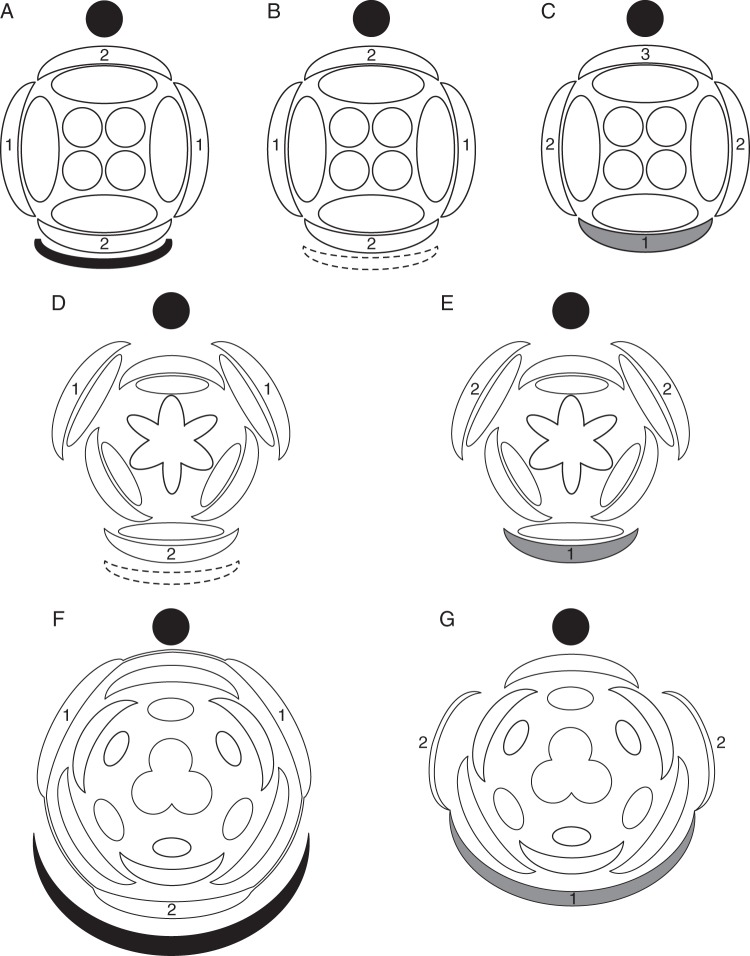
Fig. 7.
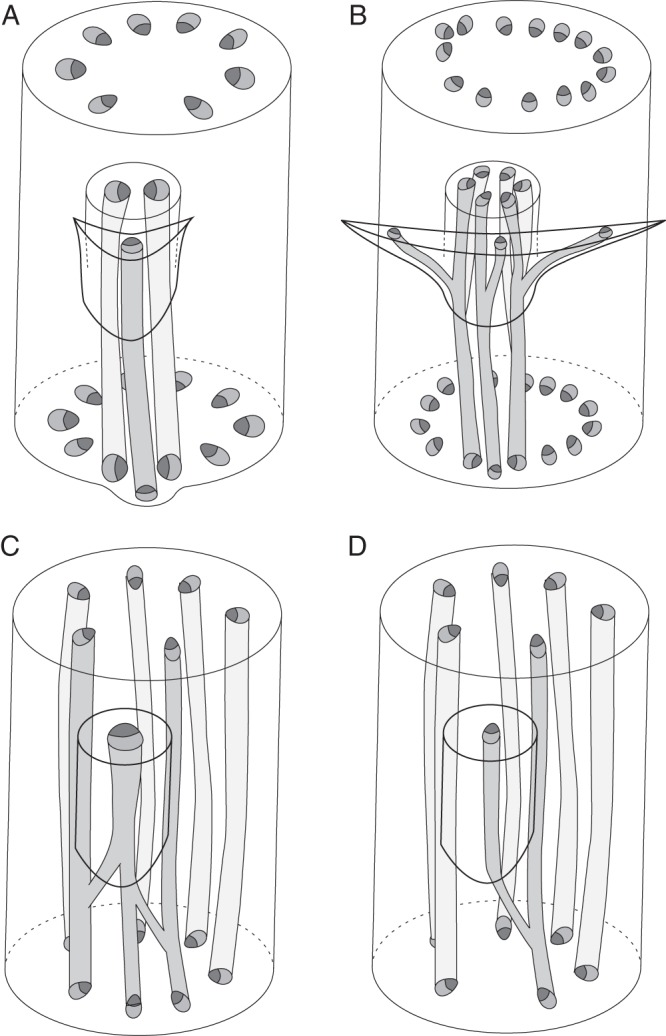
Three-dimensional diagrams of nodal vasculature in monocot inflorescences lacking floral prophylls. (A) Tofieldia cernua, bracteate raceme; (B) Isidrogalvia robustior, bracteate botryoid; (C) Triglochin maritima, non-bracteate botryoid; (D) Triglochin palustre, non-bracteate botryoid.
Fig. 8.
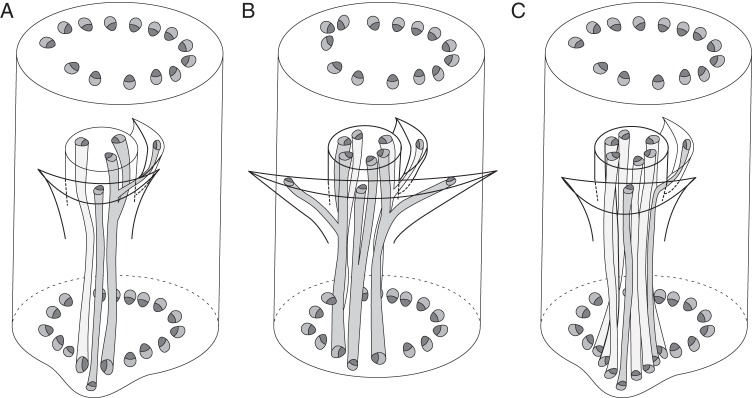
Three-dimensional diagrams of nodal vasculature in monocot inflorescences possessing floral prophylls. (A) Metanarthecium luteo-viride; (B) Narthecium ossifragum; (C) Petrosavia stellaris.
The differences between various monocots lie mainly in the number of vascular bundles entering the inflorescence axis from the FSB and pedicel and patterns of their interaction in a node. Generally, the larger the organ base, the greater the number of veins that supply it. Thus, an FSB with a broad base receives more vascular bundles than one with a narrow base. Similarly, thick pedicels contain more veins than thinner ones. The relative sizes of the FSB and its axillary pedicel are also essential. In taxa in which the width of the FSB and the diameter of the pedicel are comparable, their traces form a common gap while entering the vascular system of the inflorescence axis. In Sсheuchzeria, FSBs of which are much wider than the pedicel diameter (Posluszny, 1983), the traces of the FSB and pedicel enter the inflorescence stele independently, forming two separate gaps (Remizowa and Lock, 2012).
Several monocots that are not closely related possess flowers that are superficially very similar. Tofieldia and Metanarthecium, which belong in different monocot orders, Alismatales and Dioscoreales, respectively (Azuma and Tobe, 2010; Zhao et al., 2012), both possess small flowers on thin pedicels and narrow short FSBs (Anderson, 1940; Utech, 1978; Remizowa et al., 2006a, 2008); their pedicel traces consist of two bundles which are oriented transversely (Figs 7A and 8A). Japonolirion, which belongs in yet another order, Petrosaviales (Chase et al., 2006), is another small-flowered taxon with narrow FSBs (Utech, 1984; Remizowa et al., 2006a). In this genus, the pedicel trace consists of three bundles (one median adaxial and two transverse) but the median bundle fuses in the node with one of the two lateral bundles (Remizowa and Lock, 2012). In all three unrelated genera, the FSBs are single-traced and the two pedicel bundles and the FSB bundle form a compact group of three bundles in the node.
Taxa with larger flowers on thicker pedicels, such as Isidrogalvia (Tofieldiaceae, Alismatales; Remizowa et al., 2011) and Narthecium (Nartheciaceae, Dioscoreales; Remizowa et al., 2006b), possess six vascular bundles in the pedicel (Figs 7B and 8B), arranged in three transverse pairs, a pattern that correlates with a three-bundled FSB trace (Remizowa and Lock, 2012). In the node, the bundles of the FSB trace join the pedicel bundles and fuse to form three bundles in the underlying internode. This group of three bundles corresponds to the vascular arrangement in species with smaller flowers.
Petrosavia, which is the sister genus to Japonolirion (Cameron et al., 2003; Chase et al., 2006), possesses narrow FSBs accompanied by relatively thick pedicels. The pedicels are vascularized by seven bundles, while the FSB is supplied by a single bundle (Fig. 8C). In the nodes, the pedicel bundles form an arc, and the FSB trace is incorporated between them (Remizowa and Lock, 2012). All the bundles enter the stele of the inflorescence axis via the same gap.
In species with a single transverse floral prophyll, its single bundle enters the pedicel and either joins with a pedicel bundle nearest to the point of bracteole attachment (Metanarthecium, Narthecium, Japonolirion) or extends as a distinct bundle of the pedicel stele (Petrosavia). Although flower orientation and the sequence of tepal initiation are both governed by the position of the floral prophyll (see above), pedicel vasculature is not affected by the occurrence of a floral prophyll and the pedicel bundles form one to three transverse pairs according to pedicel diameter. This apparent non-correlation could be explained by the timing of procambium initiation (Remizowa and Lock, 2012). The inhibitory activity of the floral prophyll must be strong at the earliest stages of development, but at subsequent stages, its activity as a patterning centre for the procambial system is relatively weak.
Flowers of Acorus and some Alismatales (Aponogetonaceae, Araceae, Juncaginaceae, Posidoniaceae, Potamogetonaceae, Ruppiaceae) are supplied by a single vascular strand despite differences in flower arrangement between these taxa (Uhl, 1947; Singh, 1965; Eyde et al., 1967; Gamerro, 1968; Singh and Sattler, 1977; Buzgo and Endress, 2000; Buzgo, 2001; Lock et al., 2011; Lock, 2012; Remizowa et al., 2012b). This condition is correlated with the absence of an FSB trace, even if the FSB is present (Posidonia, Potamogeton spp.).
Remizowa and Lock (2012) highlighted the differences in inflorescence vasculature between species of Triglochin (Juncaginaceae) that possess flowers supplied by a single vascular bundle. In T. palustris (Fig. 7D), the pedicel trace passes the node and joins the nearest vascular bundle of the inflorescence axis. Due to this feature, the number of vascular bundles within the inflorescence axis is more or less constant along the inflorescence. In T. maritima (Fig. 7C), the single bundle of the pedicel trace splits into two or three branches directly in the node or below it. Further down, these branches fuse (often at different levels) with the stele bundles. In cases where the pedicel trace divides into three branches, they form a group of three bundles and the situation resembles that described above for inflorescences with vascularized FSBs. The differences in vasculature between T. palustris and T. maritima could be due to different ways of FSB reduction. In T. palustris, the FSB is presumably suppressed, while a ‘hybrid’ organ is developed in T. maritima. We speculate that, in the case of T. maritima, the ‘FSB presence’ remains evident not only in the peculiar flower development (discussed above) but also in the splitting of the pedicel trace into three bundles, where the middle bundle corresponds to the bundle of the FSB.
In Potamogeton species that differ from Triglochin in possessing a whorled rather than spiral flower arrangement (see Charlton, 1980; Lock et al., 2009), the pedicel bundle splits in the node of the inflorescence axis or slightly below it to form two branches that fuse with the nearest bundles of the inflorescence axis. Taxa with distichous phyllotaxy (Potamogeton densus, Ruppia and Posidonia) demonstrate an unusual pattern of vasculature of the inflorescence axis that involves fusion between the pedicel trace and stem bundles in a radial rather than tangential plane. The inflorescence axis of Potamogeton densus and Posidonia contains two bundles situated in the plane of distichy. At the node, the pedicel trace joins one of the two bundles of the inflorescence axis (i.e. the closest one) (Posluszny and Sattler, 1973; Remizowa et al., 2012b). In Ruppia, the inflorescence axis is very thin and contains a single bundle, to which the single flower trace fuses directly in a radial longitudinal plane, i.e. in the median plane of the flower (Singh, 1965; Gamerro, 1968; Lock et al., 2011; Lock, 2012).
A single-bundled pedicel trace was also reported for the model angiosperm Arabidopsis (Kang et al., 2003; Aloni et al., 2006; Dengler, 2006), whose inflorescence lacks FSBs. Presumably, innervation of the flower by a single strand is a common feature for taxa where the FSB supply has somehow been lost.
In summary, at least in early-divergent monocots, patterns of inflorescence vasculature appear to correspond to patterns of floral development in that they relate mainly to changes in morphology, specifically in relative organ sizes, and do not reflect phylogenetic relationships (Remizowa et al., 2006a; Remizowa and Lock, 2012).
OUTLOOK
This review of monocot flowers and inflorescences highlights the means by which organs surrounding flowers can influence floral patterning. Floral patterning is apparently affected not only by organs belonging to the same axis as the flower but also by an FSB that belongs to a different axis. However, the inhibitory activity of surrounding phyllomes cannot entirely explain the details of early flower development. The shape of the floral apex is also a factor (Kirchoff, 2000, 2003). The meristem space economy concept helps to explain some aspects of patterning, such as why the first organ(s) formed on the lateral pedicel is often not inserted at the maximum possible distance from the subsequently formed phyllome (the FSB). Another factor that could explain the non-adaxial position of the first organ is the inhibitory activity of the primary inflorescence axis. Absence of a visible FSB does not always mean the absence of its morphogenetic influence. Two patterns of FSB reduction are recognized that differ in their impact on early floral development. The presence or absence of a vascularized FSB is important for organization of the interaction between the vascular systems of the lateral flower and the inflorescence axis. It appears that the presence or absence and abundance of the vascular supply are dependent on the sizes of FSBs and floral prophylls at a critical developmental stage. As this event takes place much later than the patterning of organ positions, structures that appear to have strong inhibitory fields at an early stage may have relatively little morphogenetic activity at subsequent stages and thus receive only limited vascular supply, or may even be non-vascularized.
An intriguing fact is the great stability of organ number and position in the vast majority of lilioid monocots (e.g. Endress, 1995; Remizowa, 2010b), despite strong differences in patterns of arrangement of organs surrounding the flower and patterns of early flower development. Although surrounding structures are crucial in establishing floral orientation and sequence of tepal initiation, the resulting relative arrangement of floral organs remains highly stable, a phenomenon that could be viewed in terms of equifinality of flower development (see also Mavrodiev and Sokoloff, 1998).
ACKNOWLEDGEMENTS
We are grateful to Peter Endress, Regine Classen-Bockhoff and an anonymous reviewer for their comments and suggestions. This work was supported by an International Joint Project grant from the Royal Society (to D.D.S., P.J.R.) and grants (no. 12-04-01-070 and 12-04-33050) from the Russian Foundation for Basic Research (to M.V.R., D.D.S.).
LITERATURE CITED
- Aloni R, Aloni E, Langhans M, Ullrich CI. Role of auxin in regulating Arabidopsis flower development. Planta. 2006;223:315–328. doi: 10.1007/s00425-005-0088-9. [DOI] [PubMed] [Google Scholar]
- Anderson CE. Some studies on the floral anatomy of the Liliales. Cornell University, Ithaca: NY; 1940. PhD Thesis. [Google Scholar]
- Arber A. Monocotyledons. A morphological study. Cambridge: Cambridge University Press; 1925. [Google Scholar]
- Azuma H, Tobe H. Molecular phylogenetic analyses of Tofieldiaceae (Alismatales): family circumscription and intergeneric relationships. Journal of Plant Research. 2010;124:349–357. doi: 10.1007/s10265-010-0387-5. [DOI] [PubMed] [Google Scholar]
- Barabé D, Bruneau A, Forest F, Lacroix C. The correlation between development of atypical bisexual flowers and phylogeny in the Aroideae (Araceae) Plant Systematics and Evolution. 2002;232:1–19. [Google Scholar]
- Barabé D, Lacroix C, Gibernau M. Floral development of Urospatha: merosity and phylogeny in the Lasioideae (Araceae) Plant Systematics and Evolution. 2011;296:41–50. [Google Scholar]
- Baum DA, Day CD. Cryptic bracts exposed. Insights into the regulation of leaf expansion. Developmental Cell. 2004;6:318–319. doi: 10.1016/s1534-5807(04)00071-1. [DOI] [PubMed] [Google Scholar]
- Berleth T, Scarpella E, Prusinkiewicz P. Towards the systems biology of auxin-transport-mediated patterning. Trends in Plant Science. 2007;12:151–159. doi: 10.1016/j.tplants.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Bull-Hereñu K, Claßen-Bockhoff R. Open and closed inflorescences: more than simple opposites. Journal of Experimental Botany. 2011a;62:79–88. doi: 10.1093/jxb/erq262. [DOI] [PubMed] [Google Scholar]
- Bull-Hereñu K, Claßen-Bockhoff R. Ontogenetic course and spatial constraints in the appearance and disappearance of the terminal flower in inflorescences. International Journal of Plant Sciences. 2011b;172:471–498. [Google Scholar]
- Buzgo M. Flower structure and development of Araceae compared with alismatids and Acoraceae. Botanical Journal of the Linnean Society. 2001;136:393–425. [Google Scholar]
- Buzgo M, Endress PK. Floral structure and development of Acoraceae and its systematic relationships with basal angiosperms. International Journal of Plant Sciences. 2000;161:23–41. doi: 10.1086/314241. [DOI] [PubMed] [Google Scholar]
- Buzgo M, Soltis DE, Soltis PS, Ma H. Towards a comprehensive integration of morphological and genetic studies of floral development. Trends in Plant Science. 2004;9:164–173. doi: 10.1016/j.tplants.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Buzgo M, Soltis DE, Soltis PS, et al. Perianth development in the basal monocot Triglochin maritima. Aliso. 2006;22:107–127. [Google Scholar]
- Cameron KM, Chase MW, Rudall PJ. Recircumscription of the monocotyledonous family Petrosaviaceae to include Japonolirion. Brittonia. 2003;55:214–225. [Google Scholar]
- Charlton WA. Features of inflorescences of Triglochin maritima. Canadian Journal of Botany. 1980;59:2108–2115. [Google Scholar]
- Chase MW, Fay MF, Devey DS, et al. Multi-gene analyses of monocot relationships: a summary. Aliso. 2006;22:63–75. [Google Scholar]
- Choob VV. On the problem of homologization of prophylls and cotyledons. Wulfenia. 2002;9:73–76. [Google Scholar]
- Choob VV. The impact of the positional information in regulation of development of floral organs and leaf series of shoots. Moscow: Binom: 2010. [Google Scholar]
- Choob VV, Mavrodiev EV. Morphological characters of the leaf series in the Commelinaceae family with special emphasis on the number of prophylls and their homology in monocots. Botanichesky Zhurnal. 2001;86:1–11. [Google Scholar]
- Choob VV, Penin AA. Structure of flower in Arabidopsis thaliana: spatial pattern formation. Russian Journal of Developmental Biology. 2004;35:224–228. [Google Scholar]
- Choob VV, Sinyushin AA. Flower and shoot fasciation: from phenomenology to the construction of models of apical meristem transformations. Russian Journal of Plant Physiology. 2012;59:530–545. [Google Scholar]
- Dahlgren RMT, Clifford HT, Yeo PF. The families of the Monocotyledons. Berlin: Springer; 1985. [Google Scholar]
- Dengler N. The shoot apical meristem and development of vascular architecture. Canadian Journal of Botany. 2006;84:1660–1671. [Google Scholar]
- Dengler N, Kang J. Vascular patterning and leaf shape. Current Opinion in Plant Biology. 2001;4:50–56. doi: 10.1016/s1369-5266(00)00135-7. [DOI] [PubMed] [Google Scholar]
- Eichler AW. Blüthendiagramme. Leipzig: W. Engelmann; 1875. [Google Scholar]
- Endress PK. Die Infloreszenzen der apetalen Hamamelidaceen, ihre grundsätzliche morphologische und systematische Bedeutung. Botanische Jahrbücher für Systematik. 1970;90:1–54. [Google Scholar]
- Endress PK. Blütenontogenese, Blütenabgrenzung und systematische Stellung der perianthlosen Hamamelidoideae. Botanische Jahrbücher für Systematik. 1978;100:249–317. [Google Scholar]
- Endress PK. The Chloranthaceae: reproductive structures and phylogenetic position. Botanische Jahrbücher für Systematik. 1987;109:153–226. [Google Scholar]
- Endress PK. Patterns of floral construction in ontogeny and phylogeny. Biological Journal of the Linnean Society. 1990;39:153–175. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Endress PK. Major traits of monocot flowers. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, editors. Monocotyledons: systematics and evolution. Kew, London: Royal Botanic Gardens; 1995. pp. 43–79. [Google Scholar]
- Endress PK. Disentangling confusions in inflorescence morphology: patterns and diversity of reproductive shoot ramification in angiosperms. Journal of Systematics Evolution. 2010;48:225–239. [Google Scholar]
- Endress PK, Doyle JA. Reconstructing the ancestral angiosperm flower and its initial specializations. American Journal of Botany. 2009;96:22–66. doi: 10.3732/ajb.0800047. [DOI] [PubMed] [Google Scholar]
- Engler A. Liliaceae. In: Engler A, Prantl K, editors. Die Natürlichen Planzenfamilien. Leipzig: Engelmann; 1888. pp. 10–91. II, 5. [Google Scholar]
- Esau K. Plant anatomy. New York: Wiley; 1965. [Google Scholar]
- Eyde RH, Nicolson DH, Sherwis P. A survey of floral anatomy in Araceae. American Journal of Botany. 1967;51:478–497. [Google Scholar]
- Fukai S, Goi M. Floral initiation and development in Freesia. Technical Bulletin of the Faculty of Agriculture, Kagawa University. 1998;50:69–72. [Google Scholar]
- Gamerro JC. Observaciones sobre la biologia floral y morfologia de la Potamogetonacea “Ruppia cirrhosa” (Petag.) Grande (= “R. spiralis” L. ex Dum.) Darwiniana. 1968;14:575–607. [Google Scholar]
- Graham SW, Zgurski JM, McPherson MA, et al. Robust inference of monocot deep phylogeny using an expanded multigene plastid data set. Aliso. 2006;22:3–21. [Google Scholar]
- Greller AM, Matzke EB. Organogenesis, aestivation, and anthesis in the flower of Lilium tigrinum. Botanical Gazette. 1970;131:304–311. [Google Scholar]
- Hagemann W. Weitere Untersuchungen zur Organisation des Sprossscheitelmeristems; der Vegetationspunkt traubiger Floreszenzen. Botanische Jahrbücher für Systematik. 1963;82:273–315. [Google Scholar]
- Hamann U. Reihe Commelinales. In: Melchior H, editor. A. Engler's Syllabus der Pflanzenfamilien (edn 12). Berlin: Bornträger; 1964. pp. 549–561. Bd. 2. [Google Scholar]
- Hitch PA, Sharman BC. Initiation of procambial strands in leaf primordia of Dactylis glomerata L. as an example of a temperate herbage grass. Annals of Botany. 1968;32:153–164. [Google Scholar]
- Hu GW, Lei LG, Liu K, Long CL. Floral development in Nymphaea tetragona. Botanical Journal of the Linnean Society. 2009;159:211–221. [Google Scholar]
- Iles WJD, Smith SY, Graham SW. A well-supported phylogenetic framework for the monocot order Alismatales reveals multiple losses of the plastid NADH dehydrogenase complex and a strong long-branch effect. In: Wilkin P, Mayo S, editors. Early events in monocot evolution. Cambridge: Cambridge University Press, in press; 2013. [Google Scholar]
- Kang J, Tang J, Donnelly P, Dengler N. Primary vascular pattern and expression of ATHB-8 in shoots of Arabidopsis. New Phytologist. 2003;158:443–454. doi: 10.1046/j.1469-8137.2003.00769.x. [DOI] [PubMed] [Google Scholar]
- Kierzkowski D, Nakayama N, Routier-Kierzkowska A-L, et al. Elastic domains regulate growth and organogenesis in the plant shoot apical meristem. Science. 2012;335:1096–1099. doi: 10.1126/science.1213100. [DOI] [PubMed] [Google Scholar]
- Kirchoff BK. Hofmeister's Rule and primordium shape: influences on organ position in Hedychium coronarium (Zingiberaceae) In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Collingwood: CSIRO; 2000. pp. 75–83. [Google Scholar]
- Kirchoff BK. Shape matters: Hofmeister's Rule, primordium shape, and flower orientation. International Journal of Plant Sciences. 2003;164:505–517. [Google Scholar]
- Kirchoff BK, Rutishauser R. The phyllotaxy of Costus (Costaceae) Botanical Gazette. 1990;151:88–105. [Google Scholar]
- Kramer EM. Computer models of auxin transport: a review and commentary. Journal of Experimental Botany. 2008;59:45–53. doi: 10.1093/jxb/erm060. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska D. Flower primordium formation at the Arabidopsis shoot apex: quantitative analysis of surface geometry. Journal of Experimental Botany. 2005;57:571–580. doi: 10.1093/jxb/erj042. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska D. Flowering and apical meristem growth dynamics. Journal of Experimental Botany. 2008;59:187–201. doi: 10.1093/jxb/erm290. [DOI] [PubMed] [Google Scholar]
- Larson PR. Development and organization of the primary vascular system in Populus deltoides according to phyllotaxy. American Journal of Botany. 1975;62:1084–1099. [Google Scholar]
- Lehmann NL, Sattler R. Irregular floral development in Calla palustris (Araceae) and the concept of homeosis. American Journal of Botany. 1992;79:1145–1157. doi: 10.1002/j.1537-2197.1992.tb13710.x. [DOI] [PubMed] [Google Scholar]
- Leins P, Erbar C. Floral developmental studies: some old and new questions. International Journal of Plant Sciences. 1997;158 (Suppl.):S3–S12. [Google Scholar]
- Leins P, Erbar C. Flower and fruit. Morphology, ontogeny, phylogeny, function and ecology. Stuttgart: Schweizerbart; 2010. [Google Scholar]
- Lieu SM. Organogenesis in Triglochin striata. Canadian Journal of Botany. 1979;57:1418–1438. [Google Scholar]
- Lock IE. Anomalous inflorescences of Ruppia cirrhosa (Ruppiaceae): morphology, anatomy and morphogenesis. Botanichesky Zhurnal. 2012;97:41–52. [Google Scholar]
- Lock IE, Ashurkova LD, Belova OA, et al. A continuum between open and closed inflorescences? Inflorescence tip variation in Potamogeton (Potamogetonaceae: Alismatales) Wulfenia. 2009;16:33–50. [Google Scholar]
- Lock IE, Sokoloff DD, Remizowa MV. Morphogenetic lability of the Ruppia maritima (Ruppiaceae, Alismatales) reproductive organs: from two lateral flowers to a terminal flower. Russian Journal of Developmental Biology. 2011;42:247–260. [PubMed] [Google Scholar]
- Lodkina MM. Features of morphological evolution in plants conditioned by their ontogenesis. Journal of General Biology. 1983;44:239–253. [Google Scholar]
- Mavrodiev EV, Sokoloff DD. On morphology of European species of families Zannichelliaceae, Ruppiaceae, Potamogetonaceae and Zosteraceae. Bulletin of Moscow Society of Naturalists. Biological Series. 1998;103:49–60. [Google Scholar]
- Parcy F, Bomblies K, Weigel D. Interaction of LEAFY, AGAMOUS and TERMINAL FLOWER1 in maintaining floral meristem identity in Arabidopsis. Development. 2002;129:2519–2527. doi: 10.1242/dev.129.10.2519. [DOI] [PubMed] [Google Scholar]
- Payer JB. Traité d'organogénie comparée de la fleur. Paris: Masson; 1857. [Google Scholar]
- Penin AA, Choob VV, Ezhova TA. Basic principles of terminal flower formation. Russian Journal of Developmental Biology. 2005;36:65–69. [PubMed] [Google Scholar]
- Penin AA. Bract reduction in Cruciferae: possible genetic mechanisms and evolution. Wulfenia. 2008;15:63–73. [Google Scholar]
- Posluszny U. Unicarpellate floral development in Potamogeton zosteriformis. Canadian Journal of Botany. 1981;59:495–504. [Google Scholar]
- Posluszny U. Re-evaluation of certain critical relationships in the Alismatidae: floral organogenesis of Scheuchzeria palustris (Scheuchzeriaceae) American Journal of Botany. 1983;70:925–533. [Google Scholar]
- Posluszny U, Charlton WA. Evolution of the helobial flower. Aquatic Botany. 1993;44:303–324. [Google Scholar]
- Posluszny U, Sattler R. Floral development of Potamogeton densus. Canadian Journal of Botany. 1973;51:647–656. [Google Scholar]
- Posluszny U, Sattler R. Floral development of Potamogeton richardsonii. American Journal of Botany. 1974;61:209–216. [Google Scholar]
- Posluszny U, Charlton WA, Les DH. Modularity in helobial flowers. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Collingwood: CSIRO; 2000. pp. 63–74. [Google Scholar]
- Prenner G, Vergara-Silva F, Rudall PJ. The key role of morphology in modelling inflorescence architecture. Trends in Plant Science. 2009;14:302–309. doi: 10.1016/j.tplants.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Remizowa MV, Lock IE. Patterns of racemose inflorescence vasculature in phylogeny and ontogeny of early divergent monocots. Botanichesky Zhurnal. 2012;97:183–202. [Google Scholar]
- Remizowa MV, Sokoloff DD. Inflorescence and floral morphology in Tofieldia (Tofieldiaceae) compared with Araceae, Acoraceae and Alismatales s.str. Botanische Jahrbücher für Systematik. 2003;124:255–271. [Google Scholar]
- Remizowa MV, Sokoloff DD, Moskvicheva LA. Morphology and development of flower and shoot system in Tofieldia pusilla (Tofieldiaceae) Botanichesky Zhurnal. 2005;90:840–853. [Google Scholar]
- Remizowa MV, Sokoloff DD, Rudall PJ. Patterns of floral structure and orientation in Japonolirion, Narthecium, and Tofieldia. Aliso. 2006a;22:159–171. [Google Scholar]
- Remizowa MV, Sokoloff DD, Rudall PJ. Evolution of the monocot gynoecium: evidence from comparative morphology and development in Tofieldia, Japonolirion, Petrosavia and Narthecium. Plant Systematics and Evolution. 2006b;258:183–209. [Google Scholar]
- Remizowa MV, Sokoloff DD, Kondo K. Floral evolution in the monocot family Nartheciaceae (Dioscoreales): evidence from anatomy and development in Metanarthecium luteo-viride Maxim. Botanical Journal of the Linnean Society. 2008;158:1–18. [Google Scholar]
- Remizowa MV, Sokoloff DD, Kondo K. Early flower and inflorescence development in Dioscorea tokoro Makino (Dioscoreales): shoot chirality, handedness of cincinni and common tepal-stamen primordia. Wulfenia. 2010a;17:77–97. [Google Scholar]
- Remizowa MV, Sokoloff DD, Rudall PJ. Evolutionary history of the monocot flower. Annals of the Missouri Botanical Garden. 2010b;97:617–645. [Google Scholar]
- Remizowa MV, Sokoloff DD, Timonin AC, Rudall PJ. Floral vasculature in Tofieldia (Tofieldiaceae) is correlated with floral morphology and development. In: Seberg O, Petersen G, Barfod A, Davis JI, editors. Diversity, phylogeny, and evolution in the monocotyledons. Aarhus: Aarhus University Press; 2010c. pp. 81–99. [Google Scholar]
- Remizowa MV, Sokoloff DD, Campbell LM, Stevenson DW, Rudall PJ. Harperocallis is congeneric with Isidrogalvia (Tofieldiaceae, Alismatales): evidence from comparative floral morphology. Taxon. 2011;60:1076–1094. [Google Scholar]
- Remizowa MV, Kuznetsov AN, Kuznetsova SP, Rudall PJ, Nuraliev MS, Sokoloff DD. Flower development and vasculature in Xyris grandis (Xyridaceae, Poales); a case study for examining petal diversity in monocot flowers with a double perianth. Botanical Journal of the Linnean Society. 2012a;170:93–111. [Google Scholar]
- Remizowa MV, Sokoloff DD, Calvo S, Tomasello A, Rudall PJ. Flowers and inflorescences of the seagrass Posidonia (Posidoniaceae, Alismatales) American Journal of Botany. 2012b;99:1592–1608. doi: 10.3732/ajb.1200227. [DOI] [PubMed] [Google Scholar]
- Remizowa MV, Sokoloff DD, Rudall PJ. Patterns of bract reduction in racemose inflorescences of early-divergent monocots. In: Wilkin P, Mayo S, editors. Early events in monocot evolution. Cambridge: Cambridge University Press, in press; 2013. [Google Scholar]
- Rudall PJ. Monocot pseudanthia revisited: floral anatomy and systematics of the mycoheterotrophic family Triuridaceae. International Journal of Plant Sciences. 2003;164 (Suppl.):307–320. [Google Scholar]
- Rudall PJ. Fascicles and filamentous structures: comparative ontogeny of morphological novelties in the mycoheterotrophic family Triuridaceae. International Journal of Plant Sciences. 2008;169:1023–1037. [Google Scholar]
- Rudall PJ. All in a spin: centrifugal organ formation and floral patterning. Current Opinion in Plant Biology. 2010;13:108–114. doi: 10.1016/j.pbi.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Bateman RM. Morphological phylogenetic analysis of Pandanales: testing contrasting hypotheses of floral evolution. Systematic Botany. 2006;31:223–238. [Google Scholar]
- Rüter E. Über Vorblattbildung bei Monocotylen. Flora. 1918;110:193–261. [Google Scholar]
- Rutishauser R, Isler B. Developmental genetics and morphological evolution of flowering plants, especially bladderworts (Utricularia): fuzzy Arberian morphology complements classical morphology. Annals of Botany. 2001;88:1173–1202. [Google Scholar]
- Rutishauser R, Moline P. Evo-devo and the search for homology (“sameness”) in biological systems. Theory in Biosciences. 2005;124:213–241. doi: 10.1007/BF02814485. [DOI] [PubMed] [Google Scholar]
- Sattler R. Perianth development of Potamogeton richardsonii. American Journal of Botany. 1965;52:35–41. [Google Scholar]
- Sattler R. Homeosis in plants. American Journal of Botany. 1988;75:1606–1617. [Google Scholar]
- Schneider EL, Tucker SC, Williamson PS. Floral development in the Nymphaeales. International Journal of Plant Sciences. 2003;164 (Suppl.): S279–S292 [Google Scholar]
- Sharman BC, Hitch PA. Initiation of procambial strands in leaf primordia of bread wheat, Triticum aestivum L. Annals of Botany. 1967;31:229–243. [Google Scholar]
- Singh V. Morphological and anatomical studies in Helobiae. II. Vascular anatomy of the flower of Potamogetonaceae. Botanical Gazette. 1965;126:137–144. [Google Scholar]
- Singh V, Sattler R. Floral development of Aponogeton natans and A. undulatus. Canadian Journal of Botany. 1977;55:1106–1120. [Google Scholar]
- Skryabin K, Alekseev D, Ezhova N, et al. Type specification and spatial pattern formation of floral organs: a dynamic development model. Biology Bulletin. 2006;33:523–535. [Google Scholar]
- Smith RS, Kuhlemeier C, Prusinkiewicz P. Inhibition fields for phyllotactic pattern formation: a simulation study. Canadian Journal of Botany. 2006;84:1635–1649. [Google Scholar]
- Snow R. On the shoot apex and phyllotaxis in Costus. New Phytologist. 1952;51:359–363. [Google Scholar]
- Souer E, Rebocho AB, Bliek M, Kusters E, Bruin RAM, Koes R. Patterning of inflorescences and flowers by the F-box protein DOUBLE TOP and the LEAFY homolog ABERRANT LEAF AND FLOWER in Petunia. Plant Cell. 2008;20:2033–2048. doi: 10.1105/tpc.108.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff DD, Rudall PJ, Remizowa MV. Flower-like terminal structures in racemose inflorescences: a tool in morphogenetic and evolutionary research. Journal of Experimental Botany. 2006;57:3517–3530. doi: 10.1093/jxb/erl126. [DOI] [PubMed] [Google Scholar]
- Soros-Pottruff CL, Posluszny U. Developmental morphology of reproductive structures of Zostera and a reconsideration of Heterozostera (Zosteraceae) International Journal of Plant Sciences. 1995a;156:143–158. [Google Scholar]
- Soros-Pottruff CL, Posluszny U. Developmental morphology of reproductive structures of Phyllospadix (Zosteraceae) International Journal of Plant Sciences. 1995b;155:405–420. [Google Scholar]
- Sun K, Zhang Z-Y, Chen J-K. Floral organogenesis of Potamogeton distinctus A. Benn. (Potamogetonaceae) Acta Phytotaxonomica Sinica. 2000;38:528–531. [Google Scholar]
- Takhtajan A. Flowering plants, 2nd edn. New York: Springer; 2009. [Google Scholar]
- Timonin AC. Botany. Vol. 3. Higher plants. Moscow: Academia; 2007. [Google Scholar]
- Tomlinson PB. Helobiae (Alismatidae), including the seagrasses. Oxford: Clarendon Press; 1982. (Anatomy of Monocotyledons, vol. 3, Metcalfe CR. [Google Scholar]
- Troll W. Die Infloreszenzen. Typologie und Stellung im Aufbau des Vegetationskörpers. Jena: Fischer; 1964. Bd. 1. [Google Scholar]
- Troll W. Die Infloreszenzen. Typologie und Stellung im Aufbau des Vegetationskörpers. Stuttgart: Fischer; 1969. Bd. 2, 1. [Google Scholar]
- Uhl N. Studies in the floral anatomy and morphology of certain members of the Helobiae. Cornell University, Ithaca: NY; 1947. PhD Thesis. [Google Scholar]
- Utech FH. Floral vascular anatomy of the monotypic Japanese Metanarthecium luteoviride Maxim. (Liliaceae-Melanthioideae) Annals of Carnegie Museum of Natural History. 1978;47:455–477. [Google Scholar]
- Utech FH. Floral vascular anatomy of Japonolirion osense Nakai (Liliaceae) and its tribal relationship. Annals of Carnegie Museum of Natural History. 1984;53:447–461. [Google Scholar]
- van Mourik S, Kaufmann K, van Dijk ADJ, Angenent GC, Merks RMH, Molenaar J. Simulation of organ patterning on the floral meristem using a polar auxin transport model. PLoS ONE. 2012;7:Oe28762. doi: 10.1371/journal.pone.0028762. http://dx.doi.org/10.1371/journal.pone.0028762 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegetti C, Anton AM. The grass inflorescence. In: Jacobs SWL, Everett J, editors. Grasses: systematics and evolution. Melbourne: CSIRO; 2000. pp. 29–31. [Google Scholar]
- Vrijdaghs A, Reynders M, Larridon I, Muasya AM, Smets E, Goetghebeur P. Spikelet structure and development in Cyperoideae (Cyperaceae): a monopodial general model based on ontogenetic evidence. Annals of Botany. 2010;105:555–571. doi: 10.1093/aob/mcq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw CW. The floral meristem as a reaction system. Proceedings of the Royal Society of Edinburgh. 1957;66:394–408. [Google Scholar]
- Weberling F. Morphology of flowers and inflorescences. New York: Cambridge University Press; 1989. [Google Scholar]
- Weberling F, Troll W. Die Infloreszenzen. Typologie und Stellung im Aufbau des Vegetationskörpers. Stuttgart: Fischer; 1998. Bd. 2, 2. [Google Scholar]
- Weston PH. Process morphology from a cladistic perspective. In: Scotland R, Pennington RT, editors. Homology and systematics. London: Taylor & Francis; 2000. pp. 124–144. [Google Scholar]
- Zhao Y-M, Wang W, Zhang S-R. Delimitation and phylogeny of Aletris (Nartheciaceae) with implications for perianth evolution. Journal of Systematics and Evolution. 2012;50:135–145. [Google Scholar]
- Zomlefer WB. The genera of Tofieldiaceae in the southeastern United States. Harvard Papers in Botany. 1997;2:179–194. [Google Scholar]