Abstract
Background
Most angiosperms present flowers in inflorescences, which play roles in reproduction, primarily related to pollination, beyond those served by individual flowers alone. An inflorescence's overall reproductive contribution depends primarily on the three-dimensional arrangement of the floral canopy and its dynamics during its flowering period. These features depend in turn on characteristics of the underlying branching structure (scaffold) that supports and supplies water and nutrients to the floral canopy. This scaffold is produced by developmental algorithms that are genetically specified and hormonally mediated. Thus, the extensive inflorescence diversity evident among angiosperms evolves through changes in the developmental programmes that specify scaffold characteristics, which in turn modify canopy features that promote reproductive performance in a particular pollination and mating environment. Nevertheless, developmental and ecological aspects of inflorescences have typically been studied independently, limiting comprehensive understanding of the relations between inflorescence form, reproductive function, and evolution.
Scope
This review fosters an integrated perspective on inflorescences by summarizing aspects of their development and pollination function that enable and guide inflorescence evolution and diversification.
Conclusions
The architecture of flowering inflorescences comprises three related components: topology (branching patterns, flower number), geometry (phyllotaxis, internode and pedicel lengths, three-dimensional flower arrangement) and phenology (flower opening rate and longevity, dichogamy). Genetic and developmental evidence reveals that these components are largely subject to quantitative control. Consequently, inflorescence evolution proceeds along a multidimensional continuum. Nevertheless, some combinations of topology, geometry and phenology are represented more commonly than others, because they serve reproductive function particularly effectively. For wind-pollinated species, these combinations often represent compromise solutions to the conflicting physical influences on pollen removal, transport and deposition. For animal-pollinated species, dominant selective influences include the conflicting benefits of large displays for attracting pollinators and of small displays for limiting among-flower self-pollination. The variety of architectural components that comprise inflorescences enable diverse resolutions of these conflicts.
Keywords: Inflorescence, angiosperm, form and function, evolution, development, architecture, floral display, pollination, geitonogamy, heterochrony
INTRODUCTION
The diversity of floral morphology and inflorescence architecture within angiosperms illustrates the extreme evolutionary plasticity of reproductive structures. Strangely, although floral diversity has stimulated functional interpretations for over two centuries (Baker, 1983) and has featured as evidence of adaptation since Darwin (1862, 1877), inflorescence diversity has received much less attention from this perspective (although see Wyatt, 1982; Harder et al., 2004; Prusinkiewicz et al., 2007). This disparity is surprising, as plants typically produce and display flowers aggregated in inflorescences in which flowers seldom act independently during development, pollination, fruit development and seed dispersal. For example, plants that display many flowers simultaneously attract more pollinators than those with small displays (Ohashi and Yahara, 2001), but are also more likely to experience among-flower self-pollination (geitonogamy: Barrett et al., 1994; Karron et al., 2004) and an associated reduction in pollen export (pollen discounting) and siring success (Harder and Barrett, 1995; Karron and Mitchell, 2012). Such collective effects probably shape the evolution of the extensive inflorescence diversity that is evident within and among angiosperm clades (e.g. Fig. 1; Stebbins, 1974; Weberling, 1992; Claßen-Bockhoff, 2000; Bradford and Barnes, 2001; Doust and Kellogg, 2002; Evans et al., 2003; Weller et al., 2006; Tripp, 2007; Pozner et al., 2012).
Fig. 1.
Examples of architectural diversity caused by interspecific variation in branching geometry on an underlying cymose topology within South African Iridaceae, including: (A) Moraea tripetala, (B) Chasmanthe floribunda, (C) Crocosmia paniculata, (D) Dierama latifolium, (E) Freesia occidentalis, (F) Babiana villosa, (G) Hesperantha coccinea (with Prosoeca ganglbaueri) and (H) Babiana ringens. The inset phylogeny in (D) illustrates the relationships between the species as inferred by Goldblatt et al. (2008).
As for all aspects of morphology, inflorescence adaptation and diversification depend on both the functional consequences of alternative characteristics, and the evolutionary options and limits established by the generating developmental processes (cf. Brakefield, 2006; Breuker et al., 2006). Nevertheless, the bodies of literature that address inflorescence development and function rarely intersect: ontogenetic studies seldom consider consequences for plant performance, and adaptive hypotheses commonly ignore developmental possibilities and constraints. Here, we review this disparate literature in search of a unified perspective that recognizes the reciprocal relations through which inflorescence structure both influences and is evolutionarily influenced by reproductive function. After providing an ontogenetic definition of an inflorescence and identifying the essential components of its architecture, we review current understanding of the developmental controls on those components, with emphasis on architectural effects with identifiable ecological implications. We then examine the consequences of inflorescence architecture for reproduction, focusing on pollination, which is the essential function of inflorescences. Given the largely independent progress of studies of inflorescence development and function, our synthesis is unavoidably incomplete. In particular, we do not consider biomechanics, the flow of water, hormones and nutrients, or the developmental progression of inflorescences into infructescences and their role in seed dispersal. Nevertheless, new perspectives emerge, which may serve to motivate more integrated future studies.
INFLORESCENCES AND THEIR ARCHITECTURAL COMPONENTS
Flowering plants are modular organisms that develop as shoot apical meristems repeatedly produce metamers, each of which comprises a stem segment (internode), distal node, attached leaf and axillary bud(s) (Bell, 2008). Consequently, a plant's architecture grows as metamers are added, internodes elongate (or not) and axillary buds create branches (or not). This iterated pattern continues after a shoot apical meristem switches from vegetative mode to become an inflorescence meristem. In this reproductive mode, apical and lateral meristems may switch from producing inflorescence axes (shoot identity) to producing flowers (floral identity) (Bennett and Leyser, 2006). As a flower is a specialized, highly condensed reproductive branch, each axillary flower is subtended by a leaf, which may not develop, be rudimentary (bract) or be indistinguishable from the leaves that subtend vegetative buds. An inflorescence is thus recognized by the transition along an axis from the production of vegetative branches to flower production, not by the presence or absence of leaves (see Hempel and Feldman, 1994).
The form (architecture) of inflorescences can be considered from several intersecting perspectives. Within an inflorescence one can distinguish the branching scaffold (Fig. 2J) and the canopy of fully open flowers that it supports (Fig. 2K). The scaffold can be described based on connections between component axes and flowers (branching topology), or geometric aspects, such as the lengths of internodes and branching angles (branching geometry) (see, for example, Reinheimer et al., 2009; Prusinkiewicz and Runions, 2012). Likewise, the floral canopy can be viewed topologically or geometrically, by focusing respectively on the numbers of flowers, perhaps of different types and/or developmental stages, or on their spatial arrangement. From a functional perspective, scaffold structure provides physical support and a conduit for the flow of water, nutrients and hormones to flowers and fruits (Wyatt, 1982), whereas canopy structure determines interactions with pollen vectors, fruit/seed dispersers, floral herbivores and seed predators (see below). Thus, both structurally and functionally, the scaffold and canopy represent essential and complementary components of inflorescence architecture.
Fig. 2.
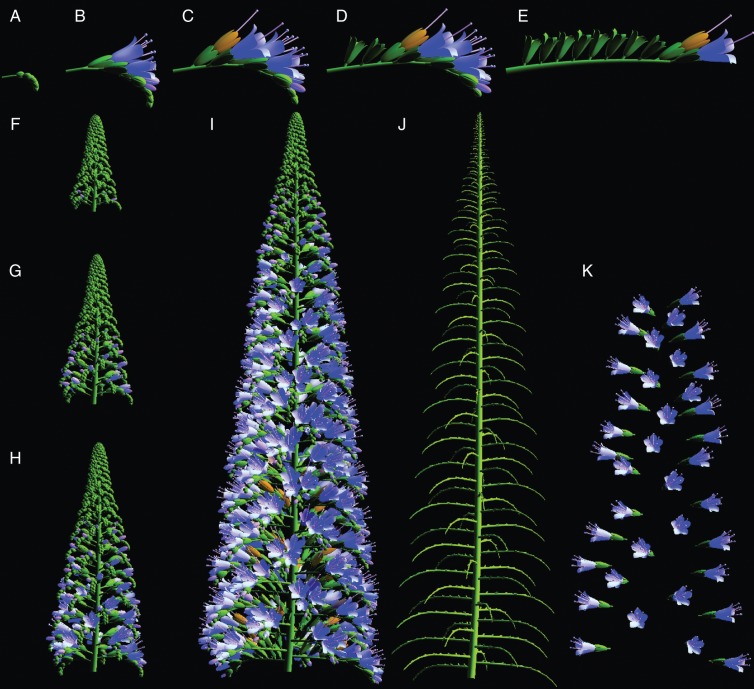
Simulated development of an Echium candicans thyrse, including (A–E) successive stages during the development of a single cymose inflorescence branch (all at the same scale), (F–I) successive stages during the development of an entire inflorescence (all at the same scale), and (J) the branch scaffold and (K) floral canopy of the inflorescence depicted in (I). The model was inspired by the exploration of inflorescence diversity by Prusinkiewicz et al. (2007) and implemented in the L-studio environment (Prusinkiewicz, 2004).
The form and function of an inflorescence can be considered statically, like a snapshot, or dynamically, like a movie (Fig. 2). The temporal co-ordination of developmental processes, such as the order of development along axes, has a paramount effect on inflorescence structure at any time during ontogeny, and creates opportunities for evolutionary diversification via heterochrony (e.g. Grimes, 1999; Li and Johnston, 2000; Park et al., 2012). Furthermore, the timing and duration of floral exposure (floral phenology), specifically the rate at which flowers open and their longevity, determine the presentation of the flower canopy (Harder and Johnson, 2005: Fig. 2A–I). This presentation typically changes over time, with the set of flowers open simultaneously referred to as the floral display (Harder and Barrett, 1996). Both static and dynamic aspects of inflorescence architecture are functionally relevant. Individual pollinators interact with architectural snapshots, generating pollination success when integrated over the entire architectural movie.
ARCHITECTURAL CONTROLS AND EVOLUTIONARY OPPORTUNITIES
The magnitude and scope of inflorescence evolution necessarily depend on the nature of the genetic control of the underlying developmental rules. As inflorescences are iterated structures, limited change in the rules that govern branching, the order of flower differentiation, internode elongation and phyllotaxis can induce cascading effects that alter inflorescence architecture extensively (Prusinkiewicz and Lindenmayer, 1990; Prusinkiewicz et al., 2007). Thus, although diverse genes control inflorescence development (Malcomber et al., 2006; Benlloch et al., 2007), relatively little genetic change can produce considerable diversity of inflorescence architectures (Doust and Kellogg, 2002; Prusinkiewicz et al., 2007; Bosch et al., 2008). Such changes could affect the developmental rules that apply either universally, altering overall architecture, or differentially within and among a plant's inflorescences, creating systematic continuous or discrete variation. We now review aspects of the current understanding of developmental processes that suggest predictive approaches to studying the architectural and functional diversity of inflorescences.
Inflorescence topology
The branching topology of inflorescences is established by the pattern of meristem initiation, which can assume one of two main forms (e.g. Prusinkiewicz and Lindenmayer, 1990; Endress 2010). With racemose initiation, the apical meristem repeatedly generates axillary meristems on its flank, so that development progresses along the main axis. The axillary meristems can differentiate into flowers or initiate axillary meristems to produce a branched inflorescence. In contrast, with cymose initiation, axillary meristems repeatedly assume the role of the apical meristem in producing additional axillary meristems, so development progresses along increasingly higher order branches. Whether cymose initiation yields extensive branching systems depends on whether the apical meristem generates more than one active axillary meristem (Park et al., 2012: compare Fig. 1A, B, E, G with 1C, D, F, H). Developmentally, panicles lie intermediate between racemes and cymes, with axillary meristems initiating along both the main and higher order axes. Thyrses arise by a switch from racemose initiation along the main axis to cymose initiation on higher order axes (Endress, 2010: Fig. 2).
Whether the apical meristem eventually differentiates into a flower (producing a ‘closed’ inflorescence) or not (‘open’ inflorescence) may largely be a separate matter from the pattern of meristem initiation. In particular, evidence from 19 species in four eudicot families indicates that the ultimate fate of the apical meristem depends on its ability to maintain its size as axillary meristems are initiated (Bull-Hereñu and Claßen-Bockhoff, 2011), which can even vary within and among plants of individual species (Bull-Hereñu and Claßen-Bockhoff, 2010). Obviously, differentiation of the apical meristem before it produces axillary meristems produces a single-flowered ‘inflorescence’.
Do different inflorescence types result from distinct regulatory mechanisms and so differ qualitatively, or do they represent landmarks in a continuum of possibilities generated by a common mechanism? Classic descriptions of inflorescences (e.g. Troll, 1964, 1969; Weberling, 1992) emphasize distinctions between inflorescence types and thus promote the first viewpoint, whereas subsequent conceptual and computational models (Schultz and Haughn, 1993; Kellogg, 2000; Prusinkiewicz et al., 2007) reflect a more continuous perspective. For example, Prusinkiewicz et al. (2007) modelled the fate of meristems as governed by a hypothetical variable, vegetativeness (veg), which decreases over time. As long as a meristem's veg level exceeds the flower identity threshold V, the meristem produces an inflorescence axis. In contrast, when veg < V, the meristem's identity switches and it produces a flower. Thus, during early stages of inflorescence development, meristems are more likely to produce new axes, whereas later they tend to produce flowers. A key component of this model is the possibility of a temporary difference Δveg between the veg levels in the apical meristem, vegA, and a newly initiated lateral meristem, vegL (Δveg = vegL – vegA). If Δveg = 0, veg drops below the flower identity threshold simultaneously in all meristems, converting them into flower buds and resulting in a panicle. If Δveg < 0, veg in lateral meristems falls below V sooner than in apical meristems. One or more axes supporting lateral flowers are then produced, yielding a (possibly compound) raceme. Conversely, if Δveg > 0, veg falls below V first in apical meristems, which produce flowers, while lateral meristems still produce branches, generating a cyme. This model, which is consistent with empirical results (Park et al., 2012), can thus simulate a continuum of inflorescences spanning racemes, panicles and cymes, with only quantitative changes of parameter values. Thyrses can be incorporated in this spectrum by further changing parameter values, or by differential control of the apex of the main axis and those of the lateral branches (the mechanism used to generate Fig. 2).
Of course, identification of a common mechanism that can generate diverse topologies is not evidence of common genetic control among species with contrasting architectures. Indeed, comparative studies of developmental genetics indicate heterogeneity in the genetic control of inflorescences among angiosperms. Although grasses (Poaceae) and Arabidopsis (Brassicaceae) both produce variations on panicles/racemes, they exhibit limited homology of inflorescence genes (Malcomber et al., 2006). Even comparisons within eudicots between Arabidopsis racemes and Petunia (Solanaceae) cymes indicate divergence in both the genes that control specific events and the expression patterns of homologous genes (Rebocho et al., 2008; Souer et al., 2008). Paniculate species, which bridge racemes and cymes (Prusinkiewicz et al., 2007), may thus represent a developmentally heterogeneous group. The genetic differences between species with racemes and cymes may impose constraints that render evolutionary transitions between racemes and cymes much less likely than between either racemes and panicles, or cymes and panicles, although racemes and cymes are known from individual genera (Cavalcantia and Rua, 2008), tribes (Tucker, 1998) or subfamilies (Reuther and Claßen-Bockhoff, 2010).
Two sequential processes establish the total number of flowers produced by an inflorescence: primordium initiation and activation. Primordium initiation usually continues along an axis until the apical meristem either converts into a floral primordium or ceases growth (Tucker and Grimes, 1999; Bull-Hereñu and Claßen-Bockhoff, 2011), establishing the maximum number of flowers that an inflorescence could produce. The realized total flower number then depends on the fraction of these primordia that activate and develop into flowers (see Brown et al., 2006). The capacity for continued initiation of floral primordia declines with both the initial size of the inflorescence meristem and the extent to which its size decreases as primordia initiate, both of which differ among species (Bull-Hereñu and Claßen-Bockhoff, 2011).
The order of flowering within inflorescences is closely coupled with inflorescence topology and is of key importance for pollination success in species with flowers that exhibit separate female and male phases (dichogamy: see ‘Animal pollination – behaviour of attracted pollinators’). Although floral primordia are typically initiated acropetally (i.e. from axis base to tip [but see Harris (1999) for examples of divergent initiation in Asteraceae capitula], flowers can open basipetally within axes (e.g. Aquilegia, Itagaki and Sakai, 2006; Iris, Wesselingh and Arnold, 2003). Furthermore, inflorescence branches may develop basipetally, and some species even exhibit divergent development, proceeding acropetally in upper branches and basipetally among lower ones (Sell, 1980; Janssen and Lindenmayer, 1987; Hempel and Feldman, 1994). Basipetal development requires a control mechanism regulating the interval between bud initiation and activation.
Several hypothetical mechanisms of basipetal development and flower opening were examined by Janssen and Lindenmayer (1987). According to their Model I, flowering order emerges from a race between the development of inflorescence axes and the propagation of a flower-inducing signal (florigen) in these axes. This signal is assumed to originate at the base of the plant and propagate acropetally with different speeds along axes of different order. Janssen and Lindenmayer's (1987) mathematical analysis and simulations of Model I showed that flowers can be induced in an acropetal sequence, basipetal sequence or simultaneously, depending on the rates of growth and signal propagation. The ability of this model to generate different flowering sequences is an attractive feature, as it suggests a mechanism for evolutionary transitions between sequences within clades (cf. Harder et al., 2004; Reinheimer et al., 2009). However, its biological plausibility is uncertain: although Arabidopsis FT protein and its orthologues trigger the switch from vegetative to inflorescence identity (Lifschitz et al., 2006; Zeevaart, 2008), whether they also induce the activation of floral buds and propagate at rates proposed by Janssen and Lindenmayer (1987) is not clear. An alternative or complementary mechanism for basipetal development involves apical dominance, whereby the actively developing apical meristem in the vegetative state delays development of more proximal buds on the same axis [Model II of Janssen and Lindenmayer (1987) and Models II and III of Prusinkiewicz and Lindenmayer (1990)]. Physiologically, apical dominance is likely to be effected by auxin, originating near apical meristems and propagating basipetally. Molecular-level hypotheses and models of the role of auxin in basipetal bud activation suggest control of bud activation by the concentration, transport or action of one or more secondary signals modulated by auxin (Dun et al., 2006, 2012) or by efflux of auxin (Prusinkiewicz et al., 2009; Domagalska and Leyser, 2011).
Both floral initiation and activation are probably subject to environmental effects on resource allocation to an inflorescence; however, these processes have not been distinguished in studies of either the heritability or phenotypic plasticity of total flower number. In a review of the quantitative genetics of flower production, Ashman and Majetic (2006) reported an average heritability for flower number of h2 = 0·34 (n = 63 estimates). Several lines of evidence illustrate that resource supply is a key environmental influence on phenotypic variation that cannot be attributed to additive genetic effects, including: reduced flower production in response to either defoliation (e.g. Brookes et al., 2008) or prolific fruit development by an inflorescence's early flowers (e.g. Carroll and Delph, 1996); and increased flower production following the addition of soil nutrients, especially phosphorus (e.g. Campbell and Halama, 1993). In addition, for plants that produce multiple inflorescences, flower production per inflorescence depends on the hierarchical allocation of resources among and within inflorescences (Schoen and Dubuc, 1990), which may vary among environments (Preston, 1999). An additional resource trade-off between flower number and size could also affect flower production; however, within-species phenotypic and genetic correlations are inconsistent, and often neutral or positive (Burd, 1999; Worley and Barrett, 2000; Ashman and Majetic, 2006), perhaps because of variation among individuals in resource allocation and/or hierarchical allocation among multiple inflorescences (Worley et al., 2003). Among angiosperms as a whole, flower size and number per inflorescence generally vary negatively, although the relation is less clear for some intrageneric comparisons (Sargent et al., 2007).
Inflorescence geometry
Inflorescence geometry emerges as development of the branching structure determines the three-dimensional arrangement of flowers over time. Figure 1 illustrates a sample of the extensive geometric variation evident among species in the Iridaceae of South Africa, all of which have cymose meristem initiation. The standard, planar zig-zag cyme (rhipidium) in the Iridaceae can be modified by corolla displacement to produce three-dimensional floral displays (Fig. 1B, C, G) or by altered orientation of consecutive branches to produce linear displays (Fig. 1E). Within branches, flowers can be closely clustered (Fig. 1B, F, H) or distinctly separated (Fig. 1A, E, G), depending on internode length. Flowers may mature acropetally (Fig. 1B–H) or basipetally (Fig. 1A), with the position of the flowering zones progressing correspondingly. Particularly unusual is the inflorescence of Babiana ringens (Fig. 1H), which produces a pair of branches at ground level on an otherwise naked main axis that rises above the ground to provide a perch for pollinating sunbirds (de Waal et al., 2012a). Interestingly, the phylogenetic relationships illustrated in Fig. 1 suggest that at least some aspects of this geometric variation have evolved several times, further illustrating the evolutionary flexibility of inflorescence architecture. As impressive as this variation is, it represents just a small sub-set of the geometric diversity among angiosperms as a whole. We now consider the main attributes of geometry that contribute to this diversity.
The divergence angle between successive leaf primordia is a component of phyllotaxis and determines the arrangement of next-order branches and/or flowers around their supporting axis. This process is mediated by auxin dynamics in the apical meristem (Reinhardt et al., 2003; Jönsson et al., 2006; Smith et al., 2006; but see Guenot et al., 2012), with primordia forming at the site of highest auxin concentration. As each primordium draws auxin from its vicinity, a new primordium forms at some distance from the existing ones, where auxin is now most concentrated. In their computer simulations, Smith et al. (2006) replicated these auxin dynamics and generated common phyllotactic patterns (spiral, distichous, decussate and tricussate) by altering the details of meristem geometry and auxin dynamics. This theoretical result is consistent with observed changes in meristem geometry and rate of primordium initiation, sometimes correlated with the changes in phyllotaxis, during transition from the vegetative to flowering state (Kwiatkowska, 2008). Whether a similar mechanism governs the angular disposition of successive axillary branches in cymose inflorescences remains to be examined.
As inflorescence development progresses, the occurrence and extent of internode and pedicel elongation determine the three-dimensional geometry of the floral canopy. For example, umbel-like inflorescences arise from lack of internode elongation if flowers are elevated on pedicels (e.g. Fig. 3F: Mann, 1959) and capitula arise from the lack of internode and pedicel elongation (e.g. Fig. 3D: Harris, 1999; Pozner et al., 2012). Similarly, an umbel in which pedicels of all flowers are the same length is either domed or spherical, depending on flower number and size (e.g. Asclepias), whereas variation in pedicel length creates a more linear inflorescence (e.g. Fig. 3C). In branched inflorescences, contrasting relative growth of the primary vs. secondary axes determines whether the inflorescence is long and narrow, or short and broad (Malcomber et al., 2006). Internode and/or pedicel elongation can continue after flowering, as in many Brassicaceae, in which case fruits are presented higher up and dispersed less densely within the infructescence than were the flowers that produced them in the inflorescence (Verbeek and Boasson, 1995).
Fig. 3.
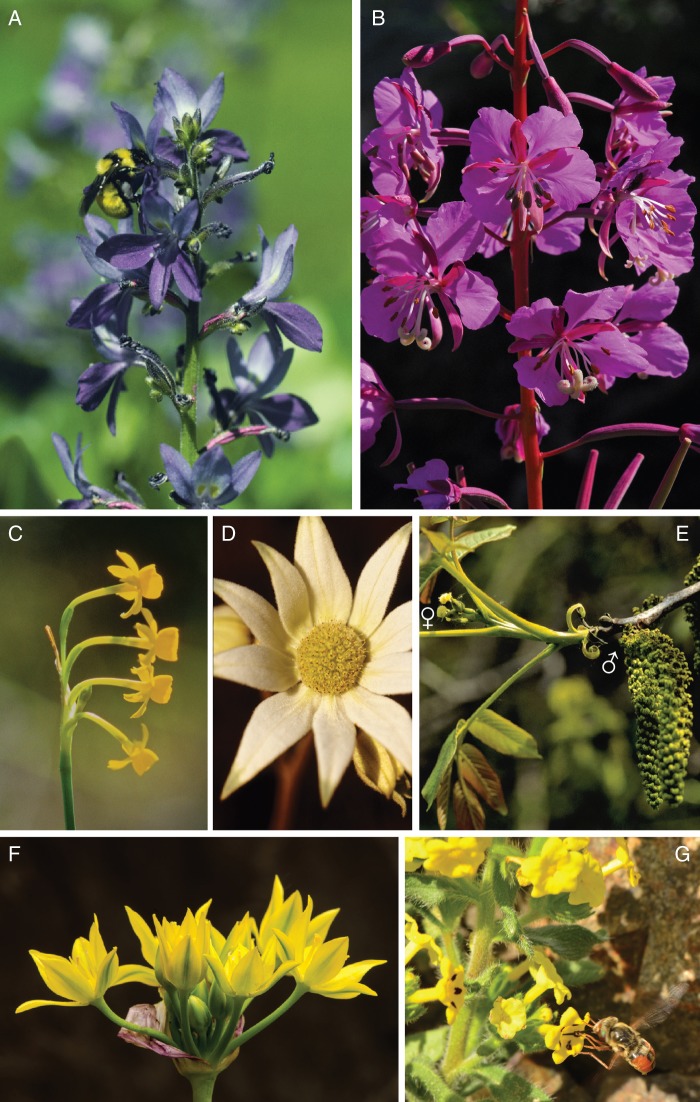
Examples of the influences of branching geometry and flowering phenology on static inflorescence architecture, including: (A) the branched inflorescence of Eichhornia paniculata (Pontederiaceae), which displays flowers as a simple raceme, because only one flower opens per branch at one time; (B) structured sexual segregation within a Chamerion angustifolium (Onagraceae) raceme, with older, female-phase flowers (note large cross-shaped stigma and splayed, depleted stamens) below younger, male-phase flowers (note erect stamens and apparent absence of stigma); (C) the umbel of Narcissus gaditanus (Amaryllidaceae), which presents a vertical, linear display, because of variation in pedicel length; (D) the umbel (and associated bracts)of Actinotus major (Apiaceae), which presents flowers in a capitulum, because of contracted pedicels; (E) the contrasting architectures of the female and male inflorescences of Juglans regia (Judlandaceae); (F) the umbel-like inflorescence of Allium moly (Amaryllidaceae) formed by lack of internode elongation during cymose initiation of floral primordia (Mann, 1959); and (G) the increased display size of an Arnebia guttata (Boraginaceae) inflorescence caused by the retention of flowers after their sexual roles are complete (unspotted flowers) to enhance long-distance attraction of pollinators, which then visit only the currently fertile, spotted flowers.
Internode and pedicel development are subject to strong genetic control. The available evidence, which involves wind-pollinated species, indicates stronger genetic determination of internode and pedicel lengths than of other inflorescence and floral characteristics (Brown et al., 2006; Weller et al., 2006). Genetic control provides ample opportunity for evolution of inflorescence geometry. For example, elongation of inflorescence internodes can be completely eliminated by mutation of individual genes (Goosey and Sharrock, 2001; Bosch et al., 2008). Thus, major events in the evolution of condensed inflorescences, such as capitula (Harris, 1999; Pozner et al., 2012), could involve relatively little genetic change. Interestingly, the heterochrony responsible for condensed inflorescences in related lineages need not involve the same genes, as is illustrated by the comparison of rosette flowering in the Brassicaceae by Bosch et al. (2008). Unlike typical species in this family, which produce elongate racemes, perhaps with basal racemose branches, the inflorescence internodes of rosette-flowering species do not elongate, and instead flowers are elevated individually on relatively long pedicels. Rosette flowering has evolved repeatedly within the Brassicaceae and occurs in at least 29 genera. Bosch et al. found that the apparent phenotypic similarity in rosette flowering for three species in different genera represents convergent, rather than parallel, evolution, because it evolved by different modifications of the same genetic programme controlling meristem identity.
Despite the iterative processes that generate inflorescences, flowers are seldom identical within an inflorescence. The most obvious examples involve species that produce both sterile and fertile flowers (e.g. some Hydrangea, Viburnum, Asteraceae, Hyacintheae), or flowers with contrasting sex phenotypes within inflorescences, such as species that produce both bisexual and male flowers (andromonoecy: e.g. some Solanum), both female and male flowers (monoecy: e.g. some Sagittaria and Dalechampia) or both female and bisexual flowers (gynomonoecy: e.g. many Asteraceae). Even within species with only bisexual flowers, Diggle (2003) found that all 42 species for which data were available exhibited systematic, within-inflorescence variation in floral traits, including measures of corolla, gynoecium and androecium size, and ovule and pollen production (also see Herrera, 2009). For all of these traits, size usually declined from proximal to distal flowers, although because pollen and ovule production typically decline at different rates the pollen:ovule ratio often increases distally. Most of the few cases of increases in non-ratio traits from proximal to distal flowers in Diggle's survey involved species in which distal flowers open first. Although gradients in floral traits could arise from resource competition between developing flowers (Bränn and Lehtilä, 2007) or hormonal control, with older flowers enjoying priority, position per se also influences development (Diggle, 2003). The developmental causes of these positional effects are not well understood, but limited evidence suggests that they may be genetically determined. Specifically, Elle (1998) found significant heritability for the proportion of male flowers produced distally in the andromonoecious inflorescences of Solanum carolinense. Systematic sex-allocation patterns within inflorescences are commonly consistent with adaptive expectations (Brunet and Charlesworth, 1995; Herrera, 2009) and can vary among populations in association with contrasting pollination environments (e.g. Kudo and Kasagi, 2004).
Flowering phenology
As described below, an inflorescence's role in pollination depends on its flowering duration, the number and arrangement of open flowers, and the distribution of sex roles, all of which are affected by aspects of intrainflorescence flowering phenology. These characteristics are emergent consequences of temporal properties manifest at the level of individual flowers, specifically the rate at which flowers open sequentially (anthesis rate), their longevity, and the extent to which receptive stigmas and viable pollen are presented asynchronously in bisexual flowers (dichogamy). Despite their pivotal influences on the flowering dynamics of inflorescences, studies of flower opening (van Doorn and van Meeteren, 2003; Reeves et al., 2012), longevity (Ashman and Schoen, 1996; Shahri and Tahir, 2011) and dichogamy (Bertin and Newman, 1993) have focused primarily on individual flowers, with limited exploration of their inflorescence consequences (although see Harder et al., 2000; Ishii and Sakai, 2001; Harder and Johnson, 2005).
A flower's opening culminates its growth with a relatively rapid phase of cell expansion in the perianth, gynoecium and/or androecium (Smyth et al., 1990; van Doorn and van Meeteren, 2003). In Arabidopsis thaliana flowers, these events are co-ordinated by a transcriptional network involving auxin, gibberellins and jasmonates, which regulates the expression of genes throughout the flower or only in specific organs (Reeves et al., 2012). In contrast, the mechanisms that co-ordinate flower opening within an inflorescence remain largely unexplored. The growth spurt that marks anthesis requires considerable energy, which is typically derived from carbohydrate metabolism (van Doorn and van Meeteren, 2003), so that initial anthesis must often cause a spike in a flower's strength as a resource sink, perhaps intensifying resource competition within the inflorescence. The rate at which flowers open within inflorescences ranges extensively among species. For example, within Delphinium, the anthesis rate ranges from one flower opening every 3 d, as in D. bicolor (Gallwey, 2011), to all flowers opening simultaneously, as in the large racemes of D. cardinale (Harder et al., 2004). Furthermore, the anthesis rate varies within individual inflorescences. For example, in a study of 11 racemose, herbaceous perennials, Gallwey (2011) found that the anthesis rate declined systematically during an inflorescence's flowering period for five species (e.g. Fig. 4B) and varied with temperature for nine species. Declining anthesis rate with increasing inflorescence age may reflect diminishing resource supply as the combined number of open flowers and developing fruits increases. Yakimowski et al. (2011) similarly reported that female flowers of monoecious Sagittaria latifolia plants open faster than male flowers, but whether this reflects a direct effect of floral sex type, flower size (female smaller than male) and/or inflorescence age (female flowers open first) is unclear. Whether anthesis rate varies with the incidence and rate of pollination also remains to be examined.
Fig. 4.
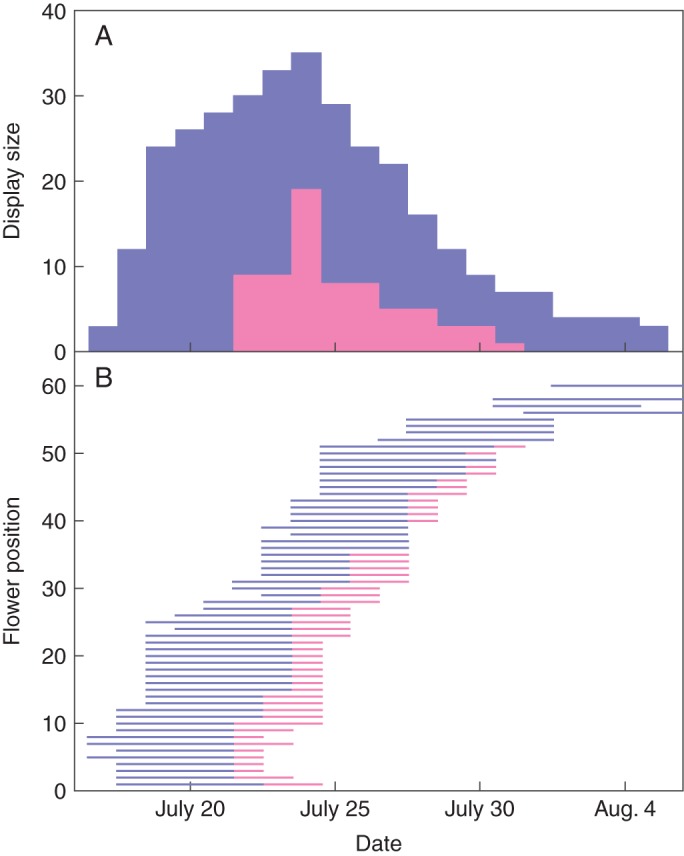
Phenology of a Delphinium glaucum inflorescence, including (A) the dynamics of display size and (B) the male-phase (blue lines) and female-phase (pink lines) periods of individual flowers (arranged from bottom to top according to position within the inflorescence). Later flowers did not produce gynoecia, so this plant was andromonoecious. Display size for a given date in (A) equals the sum of the open flowers (lines) in (B) on that date. In (A), the proportion of female flowers approximates the inflorescence's phenotypic gender, which changes during the flowering period. In (B), the declining slope of the left edge of the plot illustrates the slowing anthesis rate, and the lengths of the lines for an individual flowers depict their longevities.
The longevity of individual flowers allows an adequate period for pollen import and export within the resource constraints associated with flower maintenance (Ashman and Schoen, 1994; Schoen and Ashman, 1995). Thus, the flowers of infrequently pollinated species such as deceitful orchids can persist for weeks, whereas those of rapidly pollinated species such as grasses can last a few hours (Ashman and Schoen, 1996: see also Giblin, 2005 for an interpopulation example). Like flower opening, senescence is a developmental process, although it involves programmed cell death (Shahri and Tahir, 2011) rather than rapid growth. The timing and nature of senescence are under strong hormonal regulation: ethylene often plays a central role in species in which flowering terminates with perianth abscission, whereas other hormones, such as auxin, are key in species with perianth wilting (Shahri and Tahir, 2011). Several lines of evidence point to the resource dependence of floral longevity. Most importantly, flower senescence accompanies mobilization and redistribution of nutrients from organs with no further utility (Shahri and Tahir, 2011). Furthermore, removal of flower buds lengthens the longevity of the remaining flowers (Ashman and Schoen, 1996; Abdala-Roberts et al., 2007; Parra-Tabla et al., 2012), as expected from resource competition. [However, late flowers within inflorescences commonly live longer than early flowers (Gallwey, 2011; nine of 11 species), which seems inconsistent with a resource-based hypothesis.] Finally, for many species, pollen receipt stimulates ethylene-mediated floral senescence (van Doorn, 1997), which implements resource savings by curtailing maintenance of open flowers that have served their pollination function.
Together, anthesis rate and floral longevity determine the number of flowers open simultaneously within an inflorescence (Fig. 4, also see Fig. 2H, I), just as population size depends jointly on birth and death rates (Meagher and Delph, 2001; Harder and Johnson, 2005). Thus, display dynamics, rather than display size per se, characterizes flower presentation by individuals and species (Ishii and Sakai, 2001; Meagher and Delph, 2001; Gallwey, 2011). For species with multiday flowers, display size increases until initial flowers senesce, after which it varies according to the dynamics of anthesis and longevity until all flowers have opened (Fig. 4). Species in which the anthesis rate declines with inflorescence age can exhibit a ‘grand-opening sale’ pattern, whereby display size increases rapidly to a peak within a few days of the first flower opening and then declines slowly during the remainder of the flowering period (Fig. 4). In species with pollination-induced flower senescence, display size can vary negatively with pollinator abundance: large displays accumulate if pollinators visit infrequently, whereas frequent visits and the associated rapid pollination induce small displays (Harder and Johnson, 2005).
An additional facet of dynamic display architecture occurs in species with bisexual flowers that exhibit separate female and male phases (dichogamy), determining a plant's relative maternal and paternal contributions (gender) at any instant. Simultaneous anthesis within inflorescences of these species creates temporarily female and male plants (temporal dioecy: Cruden, 1988). More commonly, flowers open at different times within inflorescences, so dichogamy creates both spatial heterogeneity in floral sex phenotype (sexual segregation: Bertin and Newman, 1993) and continuous gender variation during a plant's flowering period (Fig. 4: Thomson and Barrett, 1981; Ishii and Harder, 2012). As pollinator visitation can shorten the durations of female and, less commonly, male phases (Devlin and Stephenson, 1984; Evanhoe and Galloway, 2002; Giblin, 2005), a plant's gender dynamics can be modified by its pollination environment. Depending on branching patterns and the order of flowering, sexual segregation can be highly structured within inflorescences. For example, acropetal anthesis of flowers with male phase preceding female phase generates lower female-phase flowers and upper male-phase flowers in vertical racemes (Figs 3B and 4B), or outer female-phase flowers and inner male-phase flowers in capitula (e.g. Lloyd and Webb, 1986; Harder et al., 2000).
Being an emergent property, display dynamics evolves as a consequence of genetically determined changes in anthesis rate, floral longevity and dichogamy. The regulatory networks involved in both anthesis (Reeves et al., 2012) and floral longevity (Shahri and Tahir, 2011) provide many opportunities for genetic modification of flowering dynamics. For example, changes in the co-ordination of gynoecium and androecium development could alter the occurrence and extent of dichogamy (Reeves et al., 2012). Correspondingly, studies of several species have detected significant genetic variation for aspects of dichogamy (Campbell, 1996; Vogler et al., 1999; Routley and Husband, 2005). This includes a genetic trade-off between the durations of male and female phases that does not affect floral longevity (Routley and Husband, 2005), allowing patterns of sexual segregation to evolve independently of floral display size. These inferences are all based on studies of individual flowers: the genetic control of flowering dynamics within inflorescences awaits direct analysis.
INFLORESCENCE ARCHITECTURE AND PLANT REPRODUCTION
The dynamic floral canopy created by inflorescence development both governs a plant's reproductive capacity, as determined by flower number, and establishes the surface with which pollen vectors, seed dispersers and herbivores interact to determine female and male mating success and reproductive output. We now briefly review this linkage between architecture and reproductive performance, which is essential for adaptive evolution of inflorescence characteristics via modification of the underlying developmental processes.
Flower production and maintenance
Inflorescence architecture contributes strongly to fitness by determining a plant's capacity to produce and maintain flowers. A recent review of phenotypic selection on floral and inflorescence traits found that reproductive output varied positively with flower production in 80 % of studies, compared with significant associations in only 30 % of studies of other floral or inflorescence traits, including floral longevity and display size (Harder and Johnson, 2009). Panicles may be particularly advantageous in this context, because inflorescence development proceeds along both main and axillary axes, maximizing total flower production, rather than being limited to just one or the other, as with racemes and cymes (see ‘Inflorescence topology’). This benefit is particularly relevant for species with predictable reproductive periods (Prusinkiewicz et al., 2007). However, flowering in panicles is delayed while branch development proceeds, so this architecture may be less advantageous in environments with more variable reproductive periods, for which more continual flower production ensures some reproduction during unpredictably brief seasons. In support of this hypothesis, Prusinkiewicz et al. (2007) found panicles to be relatively more common in tropical environments, whereas racemes (but not cymes) were relatively more common in temperate environments. Of course these associations are not strict, so that other influences must contribute to the relative merits of different architectures. For example, branch junctions may restrict the flows of water, nutrients and hormones, limiting the extent of branching in structures such as panicles (see Schulte and Brooks, 2003). Similarly, the repeated branching that characterizes cymose development may generate stronger inflorescences, which are better able to support extensive horizontal floral displays, than axes generated by racemose development. Unfortunately, reproduction has rarely been considered in studies of physiological ecology and biomechanics, so such effects remain poorly understood.
Self-pollination
Simultaneous display of multiple flowers creates opportunities for self-pollination between a plant's flowers (geitonogamy). This pollination mode commonly contributes most of the self-pollen on stigmas and an appreciable fraction of all received pollen (Table 1). Consequently, geitonogamy can significantly influence the evolution of inflorescence architecture, depending on its implications for siring success and seed production. We now outline these implications before considering the broader influences of inflorescence architecture on wind and animal pollination.
Table 1.
The contributions of geitonogamous self-pollination to overall mating and self-mating
| Species | Percentage of mating attributed to within-inflorescence geitonogamy | Percentage of selfing attributed to geitonogamy | Source |
|---|---|---|---|
| Sagittaria latifolia Alismataceae | 41 | 100* | Dorken et al. (2002)* |
| Bulbine vagans Asphodelaceae | 51 | 95 | Vaughton and Ramsey (2010)† |
| Carex arctata Cyperaceae | 35 | 100** | Friedman and Barrett (2009a)‡ |
| Carex hirtifolia Cyperaceae | 8 | 100** | Friedman and Barrett (2009a)‡ |
| Carex laxiflora Cyperaceae | 13 | 100** | Friedman and Barrett (2009a)‡ |
| Carex pedunculata Cyperaceae | 14 | 100** | Friedman and Barrett (2009a)‡ |
| Carex pensylvanica Cyperaceae | 33 | 100** | Friedman and Barrett (2009a)‡ |
| Carex plantaginea Cyperaceae | 14 | 100** | Friedman and Barrett (2009a)‡ |
| Carex scabrata Cyperaceae | 25 | 100** | Friedman and Barrett (2009a)‡ |
| Impatiens pallida Balsaminaceae | 40 | 91 | Schoen and Lloyd (1992)§ |
| Decodon verticilattus Lythraceae | 7·4 | 58 | Eckert (2000)§ |
| Disa cooperi Orchidaceae | 35 (2001) 28 (2002) | 67 (2001) 97 (2002) | Johnson et al. (2005)¶ |
| Satyrium longicauda Orchidaceae | 88 | Harder and Johnson (2005)¶ | |
| Mimulus guttatus Phrymaceae | 7·9 | 41 | Leclerc-Potvin and Ritland (1994)§ |
| Aquilegia coerulea Ranunculaceae | 51 | 86 | Brunet and Sweet (2006b)§ |
* Based on differences in the proportions of selfed seeds.
† Based on differences in fruit set between intact and emasculated flowers.
‡ Based on differences in pollen deposition between intact and emasculated flowers.
§ Based on differences in the proportions of selfed seeds between intact and emasculated flowers.
¶ Based on stained pollen recovered on stigmas.
** Monoecious, hence all selfing must involve geitonogamy.
Geitonogamy bears several negative consequences, depending on the pollination system (animal or wind pollinated) and the possibility of self-fertilization. Most pollen removed from flowers is lost during transport and fails to reach conspecific stigmas (Harder and Johnson, 2008), placing a premium on the relatively few grains that disperse successfully. For animal-pollinated species, the pollen involved in geitonogamy must have been suitably placed on a pollinator's body for deposition on stigmas, so if instead of moving among flowers within an inflorescence the pollinator had visited another plant, cross-pollination would probably have resulted. Thus, animal-mediated geitonogamy reduces opportunities for outcross siring success (pollen discounting: Harder and Barrett, 1995; Lau et al., 2008; Karron and Mitchell, 2012). Unlike animals, abiotic vectors do not move purposefully between conspecific plants, so pollen deposited on a plant's own stigma does not significantly reduce the amount of pollen available for export (Friedman and Barrett, 2009a). Regardless of the pollination system, geitonogamy can limit seed production if self-pollen disables ovules (ovule discounting: Sage et al., 1999, 2006), or self-fertilized embryos survive poorly compared with cross-fertilized embryos (inbreeding depression: Husband and Schemske, 1996).
Given these detrimental effects, selection on floral and inflorescence traits commonly acts to limit the incidence of geitonogamy. Not surprisingly, geitonogamy generally increases with floral display size for animal-pollinated species (Barrett et al., 1994; Karron et al., 2004; Brunet and Sweet, 2006a; Lau et al., 2008), because individual pollinators visit more flowers on large displays (Ohashi and Yahara, 2001). This behaviour favours restriction of the number of flowers displayed simultaneously, all else being equal. Alternatively, larger displays could be maintained if floral/inflorescence traits prevent or limit geitonogamy. Obviously, geitonogamy is impossible for dioecious plants (Dorken et al., 2002), or those that display only female- or male-phase flowers at one time (temporal dioecy) as a result of synchronous flowering and dichogamy (Bhardwaj and Eckert, 2001). The most common mechanism for limiting geitonogamy involves segregation of the sex roles among an inflorescence's flowers (monoecy or dichogamy), so that pollinators with stereotypic movement patterns encounter female(-phase) flowers before male(-phase) flowers (Fig. 3B: Harder et al., 2000; Jordan and Harder, 2006: see ‘Behaviour of attracted pollinators’). Less common mechanisms involve the production of distinct flower or plant types with anthers of one morph positioned to place pollen on pollinators where it is most accessible by stigmas of the other morph(s), and vice versa (heterostyly, Kohn and Barrett, 1992; enantiostyly, Jesson and Barrett, 2005; flexistyly, Sun et al., 2011). In such species, pollen dispersal occurs most effectively between floral morphs, rather than among flowers of the same morph, limiting pollen exchange within and among a plant's inflorescences.
Wind pollination
The central role of inflorescence architecture in wind-pollinated species is illustrated by the contrast between their remarkable inflorescence diversity and modest floral diversity. These species commonly have condensed inflorescences (Fig. 3E) or inflorescence sub-units (e.g. grass spikelets) arranged in a variety of architectures, whereas the flowers are greatly reduced, often comprising little more than pistils with comparatively large stigmas and long, lax stamens. Typically, the inflorescences of wind-pollinated species are borne on flexible, elastic stems and/or are elastic themselves, suggesting that material properties are an important component of inflorescence design. As wind pollination is a physical process governed largely by fluid dynamics, pollen removal and deposition occur through the interplay of inflorescences with airflows. Details of this interplay and its relevance to inflorescence evolution remain an essentially open question, as functional components of wind pollination have received limited attention (for recent reviews, see Friedman and Barrett, 2009b; Cresswell et al., 2010) compared with animal pollination.
Pollen removal benefits from fast airflow, whereas deposition benefits from slow airflow (Niklas, 1985), and these conflicting physical influences bear important consequences for the inflorescence architecture of wind-pollinated species. Removal requires mobilization of stationary pollen (at least with respect to the anther), despite its molecular adhesion to the anther and the stillness of air close to surfaces (boundary layer). Deposition requires that moving pollen grains have sufficiently low momentum to impact stigmas, rather than being swept past them. The evolutionary imprint of these contrasting requirements is obvious in monoecious and dioecious species, as their male inflorescences are often elongate, flexible and projected on peduncles, whereas their female inflorescences are usually compact, stiff and sessile (Fig. 3E; see also Friedman and Barrett, 2009b). Owing to this distinction, male inflorescences are exposed to relatively fast airflow and prone to shaking, which facilitate pollen removal, whereas female inflorescences are located in stiller air and are less mobile, promoting pollen deposition. Architectural specialization for female and male function may be the main factor maintaining monoecy in wind-pollinated species, as monoecy is only moderately effective in limiting geitonogamy (e.g. Eppley and Pannell, 2007; Friedman and Barrett, 2009a, b), which is the other main explanation for this sexual system (Friedman and Barrett, 2009a). Given the opportunity for architectural specialization, it is not surprising that wind pollination has evolved from animal pollination most often in monoecious or dioecious lineages, rather than in lineages of species with bisexual flowers (Friedman and Barrett, 2008). The striking sexual dimorphism evident in inflorescences of dioecious and monoecious species also suggests that the inflorescence architecture of species with bisexual flowers probably embodies compromise solutions.
The nature of these compromises is poorly understood, because both theoretical and experimental studies generally consider only pollen removal or deposition, with few measurements of geitonogamy and none of siring success. Most aerodynamic models of pollen removal and deposition have considered stationary flowers subject to wind of constant velocity, but predictions of these models are not entirely consistent with each other. For example, according to the model of Niklas (1985), most pollen is deposited by sedimentation on stigmas within leeward eddies, whereas according to the model and experimental results of Cresswell et al. (2010), pollen is deposited by windward impact. Urzay et al. (2009) adopted a different perspective in their theoretical study of pollen removal, recognizing that wind pollination occurs in gusty environments, which maintain flowers and inflorescences in constant motion, with frequent changes in direction and velocity. Interestingly, experimental immobilization of grass culms principally reduces pollen removal for species with compact spikes, whereas it reduces pollen receipt for species with diffuse panicles (Friedman and Harder, 2004). Friedman and Harder (2004) also found a higher proportion of removed pollen on stigmas for three grass species with compact spikes than for three species with open panicles, perhaps because the greater proximity of flowers within compact inflorescences allows more geitonogamy. These contrasts suggest that compact spikes and diffuse panicles represent alternative mechanisms for promoting pollination, with spikes emphasizing pollen removal and panicles limiting geitonogamy. Whatever the explanation, it is clear that inflorescence diversity in wind-pollinated species presents many opportunities for functional analysis.
Animal pollination: attraction of pollinators
Outcrossing animal-pollinated plants benefit from attracting many pollinators if the probability of a removed pollen grain being exported to another plant decreases as individual pollinators remove more pollen from the whole plant (Harder and Thomson, 1989; Harder and Wilson, 1994). In an inflorescence context, the negative relation between pollen removal and the probability of successful pollen export arises when geitonogamy uses pollen that would otherwise have been exported. In this situation, total pollen export is enhanced by restricting pollen removal by individual pollinators and attracting many pollinators to participate in pollen dispersal (Harder et al., 2001).
The most effective means of limiting pollen removal per pollinator involves pollen packaging, whereby only some of a plant's pollen is accessible during individual pollinator visits (Harder and Thomson, 1989). Such packaging is an obvious consequence of sequential flowering, as governed by inflorescence phenology. However, limited floral displays also reduce an inflorescence's attractiveness (Ohashi and Yahara, 2001), so the evolution of display size for animal-pollinated species balances the incremental benefits of large displays for pollinator attraction against the incremental costs associated with increased self-pollination and reduced pollen export (Klinkhamer and de Jong, 1993; Harder and Barrett, 1996). This balance shifts in favour of larger displays for species with mechanisms that limit geitonogamy (see ‘Self-pollination’: Harder et al., 2000). Alternatively, a variety of developmentally controlled mechanisms enhance the inflorescence display while limiting the number of open fertile flowers, including showy accessory bracts (Herrera, 1997; Sun et al., 2008; Vekemans et al., 2012), showy sterile flowers at the inflorescence periphery (Thomas et al., 2009; Morales et al., 2013) and retention of flowers that have ceased pollen exchange and contribute to long-distance display, but signal that they are not rewarding because they differ in colour from fertile flowers (Fig. 3G: Farzad et al., 2002; Kudo et al., 2007).
The optimal balance between attractiveness and limitation of geitonogamy depends on pollinator abundance: the benefits of attracting some visits dominate when pollinators are rare, whereas if pollinators are common the benefits of limiting geitonogamy take precedence (Harder and Barrett, 1996). However, pollinator abundance varies among populations and during flowering seasons (Aizen, 2001; Forrest and Thomson, 2009), favouring correspondingly different display sizes, depending on the pollination environment. Such variation is evident in two aspects of display-size dynamics. First, except for species flowering very late in seasonal environments, pollinator abundance commonly increases during flowering seasons as pollinators both increase in abundance and recruit onto a newly flowering species from previously visited species (Aizen, 2001; Forrest and Thomson, 2009). However, pollinators exhibit inertia (neophobia) in their plant preferences (Forrest and Thomson, 2009), promoting the ‘grand-opening sale’ flowering pattern produced by faster anthesis among early flowers on inflorescences than among late inflorescences (e.g. Fig. 4) to attract reticent pollinators that are visiting other species. Secondly, the dependence of inflorescence display size on anthesis rate and floral longevity combined with pollination-induced floral senescence allows facultative adjustment of display size in response to current pollinator abundance (Harder and Johnson, 2005). Specifically, when pollinators are rare, flowers persist longer, increasing display size; whereas when pollination occurs quickly, flowers senesce sooner, limiting display size and reducing geitonogamy (Harder and Johnson, 2005). This response provides the most explicit example of the interdependence between inflorescence development and function.
Like display size, inflorescence architecture can also influence pollinator attraction, although its role in this context has received much less attention. Insects detect objects visually if the viewed angle subtended by the object's edges exceeds a threshold (3–5 º for bees: Kapustjansky et al., 2010), so that larger objects, such as inflorescences, are perceived more readily than smaller ones, including isolated flowers. For example, Fishbein and Venable (1996) observed more visits to Asclepias tuberosa inflorescences aggregated into broad displays than to narrow displays, even though the latter displayed more flowers, with corresponding positive effects on pollen removal, but not pollen receipt. In accordance with this visual explanation, Ishii et al. (2008) found that an increase in display size of artificial inflorescences from seven to 13 flowers enhanced their attractiveness to bumble bees more for inflorescences with a dominant vertical (raceme) or horizontal (umbel) dimension (i.e. large difference in visual angle) than for dome-shaped panicles. In the only other study to assess the effects of inflorescence architecture on attractiveness, Iwata et al. (2012) demonstrated that increased divergence angle between adjacent flowers reduced bee visitation to the helical racemes of Spiranthes sinensis, with consequent reduction in pollen removal, but not fruit production. Together, these results illustrate that alternative three-dimensional arrangements of flowers within inflorescences differentially influence pollinator visitation, which seems primarily to affect male, rather than female, reproductive success.
Animal pollination: behaviour of attracted pollinators
Once a pollinator arrives at an inflorescence, the number and three-dimensional arrangement of its open flowers can influence both the number and sequence of flower visits, thereby affecting that pollinator's contribution to overall pollen removal and deposition, and the incidence and intensity of geitonogamy. Both within and among plant species, the number of flowers visited per pollinator generally increases with display size (Ohashi and Yahara, 2001; Harder et al., 2004), although this tendency differs among pollinators, being stronger for bees and flies than for wasps and butterflies (Glaettli and Barrett, 2008). Even if all flowers are equally rewarding, bumble bees visit only a fraction of available flowers (Jordan and Harder, 2006; Ishii et al., 2008), apparently because they retain limited memory of which flowers they have visited. This interpretation is supported by an increasing incidence of bees hovering briefly in front of flowers to inspect flowers without landing during visits to individual inflorescences (Ishii et al., 2008). As flight is energetically expensive (Heinrich, 1975), such inspection increases foraging costs, stimulating departure from an inflorescence, even though rewarding flowers remain unvisited. At least for bumble bees, this effect varies with inflorescence architecture, being stronger on inflorescences with a dominant horizontal dimension than on more vertical inflorescences (Ishii et al., 2008). Such interacting effects of display size and architecture on pollinator memory and foraging energetics should have correlated consequences for geitonogamy and cross-pollination (Jordan and Harder, 2006), although they remain to be assessed empirically.
The movement patterns of attracted pollinators among an inflorescence's flowers depend on both pollinator and inflorescence characteristics. Large-bodied bees and hawk moths typically move upward on vertical inflorescences, whereas flies commonly move downward, and hummingbirds move in either direction, depending on their arrival location (reviewed by Harder et al., 2001, 2004). In contrast, on horizontal inflorescences, most pollinators arrive at outer flowers and then move inward (Gross, 2003; Jordan and Harder, 2006). These innate tendencies strongly influence movement within inflorescences, as illustrated by two examples. First, in response to decreasing vertical nectar gradients within racemes, bumble bees start foraging at bottom flowers and leave after encountering flowers with limited nectar. In contrast, on racemes with increasing gradients, they adjust their starting position and leave from top flowers, rather than changing their foraging direction (Waddington and Heinrich, 1979). Secondly, an increased divergence angle between flowers on S. sinensis inflorescences caused bumble bees to skip adjacent flowers as they moved upward (Iwata et al., 2012). However, more complex, three-dimensional arrangements of flowers, such as those created by branched inflorescences with multiple open flowers per branch, provide less opportunity for the expression of such stereotypic behaviour, resulting in less consistent movement patterns within inflorescences (Jordan and Harder, 2006).
Movement consistency apparently governs selection of specific patterns of segregation of floral sex roles within inflorescences. For example, with upward-moving pollinators, presentation of male(-phase) flowers above female(-phase) flowers on vertical inflorescences promotes outcrossing by restricting geitonogamy and associated pollen discounting compared with either the opposite arrangement, or simultaneous female and male function within flowers (Harder et al., 2000; Jersáková and Johnson, 2007). Presumably as a result of such selection, the incidence of dichogamy varies among pollinator types (Bertin and Newman, 1993) that differ in the consistency of their movement on inflorescences. Similarly, the less consistent foraging patterns by large-bodied bees on inflorescences with a pronounced horizontal dimension may explain the prevalence of vertical inflorescences among plant species that they pollinate commonly (Jordan and Harder, 2006).
Although the pollination roles of inflorescences have been studied increasingly during the past two decades, understanding of their ecological functions remains too limited to explain the relative abundance of particular architectures thoroughly. Most studies have examined pollination by large-bodied bees, so the general applicability of their findings awaits comparison with the effects of other pollinators. Furthermore, field studies of phenotypic selection on reproductive traits (Harder and Johnson, 2009) and phylogenetic studies of the consequences of pollinator shifts rarely consider inflorescence traits (although see Bruneau, 1997; Weller et al., 2006). Indeed, a recent review of pollination syndromes (Fenster et al., 2004) mentioned inflorescences (specifically height) only once. More generally, other influences of inflorescences on plant function, including resource distribution (Wyatt, 1982), mechanical support for flowers and fruits, the susceptibility and response to herbivory (Toräng et al., 2008; Bertin et al., 2010; de Waal et al., 2012b) and seed dispersal, remain largely open topics.
CONCLUDING DISCUSSION
The modular structure of plant bodies underlies their vegetative and reproductive function. To the extent that modules interact to determine plant performance, the operation of individual modules, such as shoot internodes, leaves and flowers, provides a limited perspective on an angiosperm's growth, survival and reproduction. Specifically, the pollination roles of inflorescences illustrate that angiosperm reproduction depends on the integrated functioning of all of a plant's flowers.
Few species either produce single large flowers or display multiple flowers individually. Instead, the widespread production of inflorescences suggests general benefits of modularized reproduction that is nevertheless consolidated into larger units, despite the cost of producing a specialized flower-supporting structure. As discussed above, these benefits include enhanced pollinator attraction, facilitation of wind pollination and configuration of interfloral sex roles in accordance with the movement patterns of pollen vectors. Additional benefits may involve resource economies associated with the production and maintenance of flowers and fruits.
No single inflorescence architecture is appropriate for all reproductive environments, as is implied by the extensive diversity of structure and dynamics, even within clades (e.g. Fig. 1: Claßen-Bockhoff, 2000; Bradford and Barnes, 2001; Doust and Kellogg, 2002; Evans et al., 2003; Weller et al., 2006; Tripp, 2007; Pozner et al., 2012). The inflorescence modification responsible for this diversity probably represents adaptive responses to changes in environmental features, such as population density, the timing and duration of suitable flowering conditions, the types and abundances of pollen vectors, seed dispersers, floral herbivores and seed predators, and resource availability. Such responses to environmental change are clearly illustrated by the domestication of cereals, which variously altered branch and flower number, internode length, apical dominance, and phyllotaxis (Harlan et al., 1973; Doebley, 2004). Most of these changes involved shifts in quantitative traits controlled by multiple genes, rather than allelic replacement for genes of large effect and underlying developmental revolutions (e.g. Doebley, 2004). Furthermore, the genetic diversity that enabled morphological shifts may have been largely present in the ancestral populations, rather than created by mutation during domestication (Doebley, 2004). These examples suggest considerable capacity for inflorescence evolution within plant populations, allowing adaptive responses to altered reproductive environments.
This quantitative evolution of cereal inflorescences is consistent with the interpretation that inflorescence diversity represents a multidimensional continuum (Schultz and Haughn, 1993; Kellogg, 2000; Prusinkiewicz et al., 2007), rather than a collection of discrete types. Multidimensionality arises because inflorescence architecture incorporates topological, geometric and phenological components, which increase the architectural degrees of freedom for evolutionary response. These components are subject to somewhat independent genetic control, which can be regulated differentially (Malcomber et al., 2006), enabling heterochronic adjustment of inflorescence morphology (Li and Johnston, 2000; Park et al., 2012). Such modification generates the continuity that links contrasting inflorescence architectures within lineages.
Two architectural properties probably expedite inflorescence evolution. First, developmental constraints on inflorescence diversification may be relatively weak, because events that occur early during development of the inflorescence scaffold, such as establishment of the branching pattern, need not predispose a particular canopy structure (e.g. the cases depicted in Fig. 3C, D and F). Secondly, inflorescence evolution may also be subject to weak functional constraints, because the reproductive functions of inflorescences depend primarily on the three-dimensional arrangement of the floral canopy and its dynamics, rather than on the details of the underlying scaffold. Together, these features allow many developmental patterns to produce functionally similar inflorescences [compare the capitulate inflorescence of Actinotus in the Apiaceae (Fig. 3D) with those of the Asteraceae] and, conversely, diverse canopy architectures can be generated by similar scaffold topologies and geometries (see examples in Fig. 1). Nevertheless, the various components of inflorescence architecture are probably not equally amenable to diversification. For example, Bradford and Barnes (2001) found fewer changes in flowering order within the Cunoniaceae than in branching pattern, suggesting either that order is more genetically constrained or that ecological shifts that favour transitions between alternative orders occur less frequently than those that affect selection on branching patterns.
Despite the extensive range of architectural options defined by all possible combinations of topologies, geometries and phenologies, some combinations occur disproportionately, as reflected in the typological tradition of inflorescence taxonomy (e.g. Troll, 1964, 1969; Weberling, 1992). In contrast, other combinations are rarely, if ever, observed (see Prusinkiewicz et al., 2007). If inflorescence diversification is subject to only weak developmental and functional constraints, this heterogeneous representation of architectures probably reflects the biased proliferation of adaptive combinations, rather than options that are developmentally inaccessible. According to this interpretation, the remarkable diversity of angiosperm inflorescences is brewed non-randomly in the crucible formed by the interplay between development and ecology.
ACKNOWLEDGEMENTS
We thank Regine Claßen-Bockhoff and Bruce Kirchoff for the opportunity to contribute to this issue. Janice Gallwey provided the data illustrated in Fig. 4. The Natural Sciences and Engineering Research Council of Canada funded this research through Discovery Grants to L.D.H. and P.P.
LITERATURE CITED
- Abdala-Roberts L, Parra-Tabla V, Navarro J. Is floral longevity influenced by reproductive costs and pollination success in Cohniella ascendens (Orchidaceae)? Annals of Botany. 2007;100:1367–1371. doi: 10.1093/aob/mcm219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizen MA. Flower sex ratio, pollinator abundance, and the seasonal pollination dynamics of a protandrous plant. Ecology. 2001;82:127–144. [Google Scholar]
- Ashman T-L, Majetic CJ. Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity. 2006;96:343–352. doi: 10.1038/sj.hdy.6800815. [DOI] [PubMed] [Google Scholar]
- Ashman T-L, Schoen DJ. How long should flowers live? Nature. 1994;371:788–791. [Google Scholar]
- Ashman T-L, Schoen DJ. Floral longevity: fitness consequences and resource costs. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman and Hall; 1996. pp. 112–139. [Google Scholar]
- Baker HG. An outline of the history of anthecology, or pollination biology. In: Real LA, editor. Pollination biology. Orlando, FL: Academic Press; 1983. pp. 7–28. [Google Scholar]
- Barrett SCH, Harder LD, Cole WW. Effects of flower number and position on self-fertilization in experimental populations of Eichhornia paniculata (Pontederiaceae) Functional Ecology. 1994;8:526–535. [Google Scholar]
- Bell A. Plant form: an illustrated guide to plant morphology. Portland, OR: Timber Press; 2008. [Google Scholar]
- Benlloch R, Berbel A, Serrano-Mislata A, Madueño F. Floral initiation and inflorescence architecture: a comparative view. Annals of Botany. 2007;100:659–676. doi: 10.1093/aob/mcm146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Leyser O. Something on the side: axillary meristems and plant development. Plant Molecular Biology. 2006;60:843–854. doi: 10.1007/s11103-005-2763-4. [DOI] [PubMed] [Google Scholar]
- Bertin RI, Newman CM. Dichogamy in angiosperms. Botanical Review. 1993;59:112–152. [Google Scholar]
- Bertin RI, Connors DB, Kleinman HM. Differential herbivory on disk and ray flowers of gynomonoecious asters and goldenrods (Asteraceae) Biological Journal of the Linnean Society. 2010;101:544–552. [Google Scholar]
- Bhardwaj M, Eckert CG. Functional analysis of synchronous dichogamy in flowering rush, Butomus umbellatus (Butomaceae) American Journal of Botany. 2001;88:2204–2213. [PubMed] [Google Scholar]
- Bosch JA, Heo K, Sliwinski MK, Baum DA. An exploration of LEAFY expression in independent evolutionary origins of rosette flowering in Brassicaceae. American Journal of Botany. 2008;95:286–293. doi: 10.3732/ajb.95.3.286. [DOI] [PubMed] [Google Scholar]
- Bradford JC, Barnes RW. Phylogenetics and classification of Cunoniaceae (Oxalidales) using chloroplast DNA sequences and morphology. Systematic Botany. 2001;26:354–385. [Google Scholar]
- Brakefield PM. Evo-devo and constraints on selection. Trends in Ecology and Evolution. 2006;21:362–368. doi: 10.1016/j.tree.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bränn KH, Lehtilä K. Maternal plant responses to high pollen loads. International Journal of Plant Sciences. 2007;168:1013–1019. [Google Scholar]
- Breuker CJ, Debat V, Klingenberg CP. Functional evo-devo. Trends in Ecology and Evolution. 2006;21:488–492. doi: 10.1016/j.tree.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Brookes RH, Jesson LK, Burd M. A test of simultaneous resource and pollen limitation in Stylidium armeria. New Phytologist. 2008;179:557–565. doi: 10.1111/j.1469-8137.2008.02453.x. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Klein PE, Bortiri E, Acharya CB, Rooney WL, Kresovich S. Inheritance of inflorescence architecture in sorghum. Theoretical and Applied Genetics. 2006;113:931–942. doi: 10.1007/s00122-006-0352-9. [DOI] [PubMed] [Google Scholar]
- Bruneau A. Evolution and homology of bird pollination syndromes in Erythrina (Leguminosae) American Journal of Botany. 1997;84:54–71. [Google Scholar]
- Brunet J, Charlesworth D. Floral sex allocation in sequentially blooming plants. Evolution. 1995;49:70–79. doi: 10.1111/j.1558-5646.1995.tb05959.x. [DOI] [PubMed] [Google Scholar]
- Brunet J, Sweet HR. Impact of insect pollinator group and floral display size on outcrossing rates. Evolution. 2006a;60:234–246. [PubMed] [Google Scholar]
- Brunet J, Sweet HR. The maintenance of selfing in a population of the Rocky Mountain columbine. International Journal of Plant Sciences. 2006b;167:213–219. [Google Scholar]
- Bull-Hereñu K, Claßen-Bockhoff R. Developmental conditions for terminal flower production in apioid umbellets. Plant Diversity and Evolution. 2010;128:221–232. [Google Scholar]
- Bull-Hereñu K, Claßen-Bockhoff R. Ontogenetic course and spatial constraints in the appearance and disappearance of the terminal flower in inflorescences. International Journal of Plant Sciences. 2011;172:471–498. [Google Scholar]
- Burd M. Flower number and floral components in ten angiosperm species: an examination of assumptions about trade-offs in reproductive evolution. Biological Journal of the Linnean Society. 1999;68:579–592. [Google Scholar]
- Campbell DR. Evolution of floral traits in a hermaphroditic plant: field measurements of heritabilities and genetic correlations. Evolution. 1996;50:1442–1453. doi: 10.1111/j.1558-5646.1996.tb03918.x. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Halama KJ. Resource and pollen limitations to lifetime seed production in a natural plant population. Ecology. 1993;74:1043–1051. [Google Scholar]
- Carroll SB, Delph LF. The effects of gender and plant architecture on allocation to flowers in dioecious Silene latifolia (Caryophyllaceae) International Journal of Plant Sciences. 1996;157:493–500. [Google Scholar]
- Cavalcantia TB, Rua GH. Inflorescence patterns in the woody Brazilian genus Diplusodon (Lythraceae) Flora. 2008;203:261–271. [Google Scholar]
- Claßen-Bockhoff R. Inflorescences in Bruniaceae. Opera Botanica Belgica. 2000;12:5–310. [Google Scholar]
- Cresswell JE, Krick J, Patrick MA, Lahoubi M. The aerodynamics and efficiency of wind pollination in grasses. Functional Ecology. 2010;24:706–713. [Google Scholar]
- Cruden RW. Temporal dioecism: systematic breadth, associated traits, and temporal patterns. Botanical Gazette. 1988;149:1–15. [Google Scholar]
- Darwin CR. On the various contrivances by which British and foreign orchids are fertilised by insects. London: John Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- Darwin CR. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- Devlin B, Stephenson AG. Factors that influence the duration of the staminate and pistillate phases of Lobelia cardinalis flowers. Botanical Gazette. 1984;145:323–328. [Google Scholar]
- Diggle PK. Architectural effects on floral form and function: a review. In: Stuessy T, Hörandl E, Mayer V, editors. Deep morphology: toward a renaissance of morphology in plant systematics. Germany: Koeltz; 2003. pp. 63–80. Königstein. [Google Scholar]
- Doebley J. The genetics of maize evolution. Annual Review of Genetics. 2004;38:37–59. doi: 10.1146/annurev.genet.38.072902.092425. [DOI] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nature Reviews Molecular Cell Biology. 2011;12:211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- van Doorn WG. Effects of pollination on floral attraction and longevity. Journal of Experimental Botany. 1997;48:1615–1622. [Google Scholar]
- van Doorn WG, van Meeteren U. Flower opening and closure: a review. Journal of Experimental Botany. 2003;54:1801–1812. doi: 10.1093/jxb/erg213. [DOI] [PubMed] [Google Scholar]
- Dorken ME, Friedman J, Barrett SCH. The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae) Evolution. 2002;56:31–41. doi: 10.1111/j.0014-3820.2002.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Doust AN, Kellogg EA. Inflorescence diversification in the panicoid ‘bristle grass’ clade (Panicaeae, Poaceae): evidence from molecular phylogenies and developmental morphology. American Journal of Botany. 2002;89:1203–1222. doi: 10.3732/ajb.89.8.1203. [DOI] [PubMed] [Google Scholar]
- Dun EA, Ferguson BJ, Beveridge CA. Apical dominance and shoot branching. Divergent opinions or divergent mechanisms? Plant Physiology. 2006;142:812–819. doi: 10.1104/pp.106.086868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiology. 2012;158:487–498. doi: 10.1104/pp.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert CG. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology. 2000;81:532–542. [Google Scholar]
- Elle E. The quantitative genetics of sex allocation in the andromonoecious perennial, Solanum carolinense (L.) Heredity. 1998;80:481–488. [Google Scholar]
- Endress PK. Disentangling confusions in inflorescence morphology: patterns and diversity of reproductive shoot ramification in angiosperms. Journal of Systematics and Evolution. 2010;48:225–239. [Google Scholar]
- Eppley SM, Pannell JR. Density-dependant self-fertilization and male versus hermaphrodite siring success in an androdioecious plant. Evolution. 2007;61:2349–2359. doi: 10.1111/j.1558-5646.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- Evanhoe L, Galloway LF. Floral longevity in Campanula americana (Campanulaceae): a comparison of morphological and functional gender phases. American Journal of Botany. 2002;89:587–591. doi: 10.3732/ajb.89.4.587. [DOI] [PubMed] [Google Scholar]
- Evans TM, Sytsma KJ, Faden RB, Givnish TJ. Phylogenetic relationships in the Commelinaceae: II. A cladistic analysis of rbcL sequences and morphology. Systematic Botany. 2003;28:270–292. [Google Scholar]
- Farzad M, Griesbach R, Weiss MR. Floral color change in Viola cornuta L. (Violaceae): a model system to study regulation of anthocyanin production. Plant Science. 2002;162:225–231. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics. 2004;35:375–403. [Google Scholar]
- Fishbein M, Venable DL. Evolution of inflorescence design: theory and data. Evolution. 1996;50:2165–2177. doi: 10.1111/j.1558-5646.1996.tb03607.x. [DOI] [PubMed] [Google Scholar]
- Forrest J, Thomson JD. Pollinator experience, neophobia and the evolution of flowering time. Proceedings of the Royal Society B: Biological Sciences. 2009;276:935–943. doi: 10.1098/rspb.2008.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Barrett SCH. A phylogenetic analysis of the evolution of wind pollination in the angiosperms. International Journal of Plant Sciences. 2008;169:49–58. [Google Scholar]
- Friedman J, Barrett SCH. The consequences of monoecy and protogyny for mating in wind-pollinated Carex. New Phytologist. 2009a;181:489–497. doi: 10.1111/j.1469-8137.2008.02664.x. [DOI] [PubMed] [Google Scholar]
- Friedman J, Barrett SCH. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Annals of Botany. 2009b;103:1515–1527. doi: 10.1093/aob/mcp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Harder LD. Inflorescence architecture and wind pollination in six grass species. Functional Ecology. 2004;18:851–860. [Google Scholar]
- Gallwey JR. Influences on floral longevity and anthesis rate and their consequences for floral display size. Canada: MSc Thesis, University of Calgary; 2011. [Google Scholar]
- Giblin DE. Variation in floral longevity between populations of Campanula rotundifolia (Campanulaceae) in response to fitness accrual rate manipulation. American Journal of Botany. 2005;92:1714–1722. doi: 10.3732/ajb.92.10.1714. [DOI] [PubMed] [Google Scholar]
- Glaettli M, Barrett SCH. Pollinator responses to variation in floral display and flower size in dioecious Sagittaria latifolia (Alismataceae) New Phytologist. 2008;179:1193–1201. doi: 10.1111/j.1469-8137.2008.02532.x. [DOI] [PubMed] [Google Scholar]
- Goldblatt P, Rodriguez A, Powell MP, et al. Iridaceae ‘out of Australasia’? Phylogeny, biogeography, and divergence time based on plastid DNA sequences. Systematic Botany. 2008;33:495–508. [Google Scholar]
- Goosey L, Sharrock R. The Arabidopsis compact inflorescence genes: phase-specific growth regulation and the determination of inflorescence architecture. The Plant Journal. 2001;26:549–559. doi: 10.1046/j.1365-313x.2001.01053.x. [DOI] [PubMed] [Google Scholar]
- Grimes J. Inflorescence morphology, heterochrony, and phylogeny in the Mimosoid tribes Ingeae and Acacieae (Leguminosae: Mimosoideae) Botanical Review. 1999;65:317–347. [Google Scholar]
- Gross WE. Dependence of hummingbird movement within inflorescences on the spatial arrangement of flowers. Canada: MSc Thesis, University of Calgary; 2003. [Google Scholar]
- Guenot B, Bayer E, Kierzkowski D, Smith RS, Mandel T, Zádníková P, Benková E, Kuhlemeier C. PIN1-independent leaf initiation in. Arabidopsis. Plant Physiology. 2012;159:1501–1510. doi: 10.1104/pp.112.200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. Mating cost of large floral displays in hermaphrodite plants. Nature. 1995;373:512–515. [Google Scholar]
- Harder LD, Barrett SCH. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman and Hall; 1996. pp. 140–190. [Google Scholar]
- Harder LD, Johnson SD. Adaptive plasticity of floral display size in animal-pollinated plants. Proceedings of the Royal Society B: Biological Sciences. 2005;272:2651–2657. doi: 10.1098/rspb.2005.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Johnson SD. Function and evolution of aggregated pollen in angiosperms. International Journal of Plant Sciences. 2008;169:59–78. [Google Scholar]
- Harder LD, Johnson SD. Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist. 2009;183:530–545. doi: 10.1111/j.1469-8137.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- Harder LD, Thomson JD. Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. American Naturalist. 1989;133:323–344. [Google Scholar]
- Harder LD, Wilson WG. Floral evolution and male reproductive success: optimal dispensing schedules for pollen dispersal by animal-pollinated plants. Evolutionary Ecology. 1994;8:542–559. [Google Scholar]
- Harder LD, Barrett SCH, Cole WW. The mating consequences of sexual segregation within inflorescences of flowering plants. Proceedings of the Royal Society B: Biological Sciences. 2000;267:315–320. doi: 10.1098/rspb.2000.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Williams NM, Jordan CY, Nelson WA. The effects of floral design and display on pollinator economics and pollen dispersal. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination. Cambridge, UK: Cambridge University Press; 2001. pp. 297–317. [Google Scholar]
- Harder LD, Jordan CY, Gross WE, Routley MB. Beyond floricentrism: the pollination function of inflorescences. Plant Species Biology. 2004;19:137–148. [Google Scholar]
- Harlan JR, de Wet JMJ, Price EG. Comparative evolution of cereals. Evolution. 1973;27:311–325. doi: 10.1111/j.1558-5646.1973.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Harris EM. Capitula in the Asteridae: a widespread and varied phenomenon. Botanical Review. 1999;65:348–369. [Google Scholar]
- Heinrich B. Energetics of pollination. Annual Review of Ecology and Systematics. 1975;6:139–170. [Google Scholar]
- Hempel FD, Feldman LJ. Bi-directional inflorescence development in Arabidopsis thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta. 1994;192:276–286. [Google Scholar]
- Herrera CM. Multiplicity in unity: plant subindividual variation and interactions with animals. Chicago, IL: University of Chicago Press; 2009. [Google Scholar]
- Herrera J. The role of colored accessory bracts in the reproductive biology of Lavandula stoechas. Ecology. 1997;78:494–504. [Google Scholar]
- Husband BC, Schemske DW. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution. 1996;50:54–70. doi: 10.1111/j.1558-5646.1996.tb04472.x. [DOI] [PubMed] [Google Scholar]
- Ishii HS, Harder LD. Phenological associations of gender with floral morphology and integration in protandrous Delphinium glaucum. Journal of Ecology. 2012;100:1029–1038. [Google Scholar]
- Ishii HS, Sakai S. Effects of display size and position on individual floral longevity in racemes of Narthecium asiaticum (Liliaceae) Functional Ecology. 2001;15:396–405. [Google Scholar]
- Ishii HS, Hirabayashi Y, Kudo G. Combined effects of inflorescence architecture, display size, plant density and empty flowers on bumble bee behaviour: experimental study with artificial inflorescences. Oecologia. 2008;156:341–350. doi: 10.1007/s00442-008-0991-4. [DOI] [PubMed] [Google Scholar]
- Itagaki T, Sakai S. Relationship between floral longevity and sex allocation among flowers within inflorescences in Aquilegia buergeriana var. oxysepala (Ranunculaceae) American Journal of Botany. 2006;93:1320–1327. doi: 10.3732/ajb.93.9.1320. [DOI] [PubMed] [Google Scholar]
- Iwata T, Nagasaki O, Ishii HS, Ushimaru A. Inflorescence architecture affects pollinator behaviour and mating success in Spiranthes sinensis (Orchidaceae) New Phytologist. 2012;193:196–203. doi: 10.1111/j.1469-8137.2011.03892.x. [DOI] [PubMed] [Google Scholar]
- Janssen JM, Lindenmayer A. Models for the control of branch positions and flowering sequences of capitula in Mycelis muralis (L.) Dumont (Compositae) New Phytologist. 1987;105:191–220. [Google Scholar]
- Jersáková J, Johnson SD. Protandry promotes male pollination success in a moth-pollinated orchid. Functional Ecology. 2007;21:496–504. [Google Scholar]
- Jesson LK, Barrett SCH. Experimental tests of the function of mirror-image flowers. Biological Journal of the Linnean Society. 2005;85:167–179. [Google Scholar]
- Johnson SD, Neal PR, Harder LD. Pollen fates and the limits on male reproductive success in an orchid population. Biological Journal of the Linnean Society. 2005;86:175–190. [Google Scholar]
- Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E. An auxin-driven polarized transport model for phyllotaxis. Proceedings of the National Academy of Sciences, USA. 2006;103:1633–1638. doi: 10.1073/pnas.0509839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CY, Harder LD. Manipulation of bee behavior by inflorescence architecture and its consequences for plant mating. American Naturalist. 2006;167:496–509. doi: 10.1086/501142. [DOI] [PubMed] [Google Scholar]
- Kapustjansky A, Chittka L, Spaethe J. Bees use three-dimensional information to improve target detection. Naturwissenschaften. 2010;97:229–233. doi: 10.1007/s00114-009-0627-5. [DOI] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ. Effects of floral display size on male and female reproductive success in Mimulus ringens. Annals of Botany. 2012;109:563–570. doi: 10.1093/aob/mcr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. The influence of floral display size on selfing rates in Mimulus ringens. Heredity. 2004;92:242–248. doi: 10.1038/sj.hdy.6800402. [DOI] [PubMed] [Google Scholar]
- Kellogg EA. A model of inflorescence development. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Melbourne, Australia: CSIRO; 2000. pp. 84–88. [Google Scholar]
- Klinkhamer PGL, de Jong TJ. Attractiveness to pollinators: a plant's dilemma. Oikos. 1993;66:180–184. [Google Scholar]
- Kohn JR, Barrett SCH. Floral manipulations reveal the cause of male fitness variation in experimental populations of Eichhornia paniculata (Pontederiaceae) Functional Ecology. 1992;6:590–595. [Google Scholar]
- Kudo G, Kasagi T. Floral sex allocation in Corydalis ambigua populations visited by different pollinators. Écoscience. 2004;11:218–227. [Google Scholar]
- Kudo G, Ishii HS, Hirabayashi Y, Ida TY. A test of the effect of floral color change on pollination effectiveness using artificial inflorescences visited by bumblebees. Oecologia. 2007;154:119–128. doi: 10.1007/s00442-007-0820-1. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska D. Flowering and apical meristem growth dynamics. Journal of Experimental Botany. 2008;59:187–201. doi: 10.1093/jxb/erm290. [DOI] [PubMed] [Google Scholar]
- Lau JA, Miller RE, Rausher MD. Selection through male function favors smaller floral display size in the common morning glory Ipomoea purpurea (Convolvulaceae) American Naturalist. 2008;172:63–74. doi: 10.1086/588080. [DOI] [PubMed] [Google Scholar]
- Leclerc-Potvin C, Ritland K. Modes of self-fertilization in Mimulus guttatus (Scrophulariaceae): a field experiment. American Journal of Botany. 1994;81:199–205. [Google Scholar]
- Li P, Johnston MO. Heterochrony in plant evolutionary studies through the twentieth century. Botanical Review. 2000;66:57–88. [Google Scholar]
- Lifschitz E, Eviatar T, Rozman A, et al. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proceedings of the National Academy of Sciences, USA. 2006;103:6398–6403. doi: 10.1073/pnas.0601620103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DG, Webb CJ. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. I. Dichogamy. New Zealand Journal of Botany. 1986;24:135–162. [Google Scholar]
- Malcomber ST, Preston JC, Reinheimer R, Kossuth J, Kellogg EA. Developmental gene evolution and the origin of grass inflorescence diversity. Advances in Botanical Research. 2006;44:425–481. [Google Scholar]
- Mann LK. The Allium inflorescence: some species of the section Molium. American Journal of Botany. 1959;46:730–739. [Google Scholar]
- Meagher TR, Delph LF. Individual flower demography, floral phenology and floral display size in Silene latifolia. Evolutionary Ecology Research. 2001;3:845–860. [Google Scholar]
- Morales CM, Travaset A, Harder LD. Sterile flowers increase pollinator attraction and promote female success in the Mediterranean herb, Leopoldia comosa. Annals of Botany. 2013;111:103–111. doi: 10.1093/aob/mcs243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ. The aerodynamics of wind pollination. Botanical Review. 1985;51:328–386. [Google Scholar]
- Ohashi K, Yahara T. Behavioral responses of pollinators to variation in floral display size and their influences on the evolution of floral traits. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination. Cambridge, UK: Cambridge University Press; 2001. pp. 274–296. [Google Scholar]
- Park SJ, Jiang K, Schatz MC, Lippman ZB. Rate of meristem maturation determines inflorescence architecture in tomato. Proceedings of the National Academy of Sciences, USA. 2012;109:639–644. doi: 10.1073/pnas.1114963109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Tabla V, Abdala-Roberts L, Rojas JC, Navarro J, Salinas-Peba L. Floral longevity and scent respond to pollen manipulation and resource status in the tropical orchid Myrmecophila christinae. Plant Systematics and Evolution. 2012;282:1–11. [Google Scholar]
- Pozner R, Zanotti C, Johnson LA. Evolutionary origin of the Asteraceae capitulum: insights from Calyceraceae. American Journal of Botany. 2012;99:1–13. doi: 10.3732/ajb.1100256. [DOI] [PubMed] [Google Scholar]
- Preston KA. Can plasticity compensate for architectural constraints on reproduction? Patterns of seed production and carbohydrate translocation in Perilla frutescens. Journal of Ecology. 1999;87:697–712. [Google Scholar]
- Prusinkiewicz P. Art and science for life: designing and growing virtual plants with L-systems. Acta Horticulturae. 2004;630:15–28. [Google Scholar]
- Prusinkiewicz P, Lindenmayer A. The algorithmic beauty of plants. New York: Springer-Verlag; 1990. [Google Scholar]
- Prusinkiewicz P, Runions A. Computational models of plant development and form. New Phytologist. 2012;193:549–569. doi: 10.1111/j.1469-8137.2011.04009.x. [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E. Evolution and development of inflorescence architectures. Science. 2007;316:1452–1456. doi: 10.1126/science.1140429. [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, et al. Control of bud activation by an auxin transport switch. Proceedings of the National Academy of Sciences, USA. 2009;106:17431–17436. doi: 10.1073/pnas.0906696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebocho AB, Bliek M, Kusters E, et al. Role of EVERGREEN in the development of the cymose Petunia inflorescence. Developmental Cell. 2008;15:437–447. doi: 10.1016/j.devcel.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Reeves PH, Ellis CM, Ploense SE, et al. A regulatory network for coordinated flower maturation. PLoS Genetics. 2012;8:e1002506. doi: 10.1371/journal.pgen.1002506. http://dx.doi.org/10.1371/journal.pgen.1002506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- Reinheimer R, Zuloaga FO, Vegetti AC, Pozner R. Diversification of inflorescence development in the PCK clade (Poaceae: Panicoideae: Paniceae) American Journal of Botany. 2009;96:549–564. doi: 10.3732/ajb.0800245. [DOI] [PubMed] [Google Scholar]
- Reuther K, Claßen-Bockhoff R. Diversity behind uniformity – inflorescence architecture and flowering sequence in Apiaceae-Apioideae. Plant Diversity and Evolution. 2010;128:181–220. [Google Scholar]
- Routley MB, Husband BC. Responses to selection on male-phase duration in Chamerion angustifolium. Journal of Evolutionary Biology. 2005;18:1050–1059. doi: 10.1111/j.1420-9101.2005.00896.x. [DOI] [PubMed] [Google Scholar]
- Sage TL, Strumas F, Cole WM, Barrett SCH. Differential ovule development following self- and cross-pollination: the basis of self-sterility in Narcissus triandrus (Amaryllidaceae) American Journal of Botany. 1999;86:855–870. [PubMed] [Google Scholar]
- Sage TL, Price M, Waser NM. Self-sterility in Ipomopsis aggregata (Polemoniaceae) is due to prezygotic ovule degeneration. American Journal of Botany. 2006;93:254–262. doi: 10.3732/ajb.93.2.254. [DOI] [PubMed] [Google Scholar]
- Sargent RD, Goodwillie C, Kalisz S, Rees RH. Phylogenetic evidence for a flower size and number trade-off. American Journal of Botany. 2007;94:2059–2062. doi: 10.3732/ajb.94.12.2059. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Ashman T-L. The evolution of floral longevity: resource allocation to maintenance versus construction of repeated parts in modular organisms. Evolution. 1995;49:131–139. doi: 10.1111/j.1558-5646.1995.tb05965.x. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Dubuc M. The evolution of inflorescence size and number: a gamete-packaging strategy in plants. American Naturalist. 1990;135:841–857. [Google Scholar]
- Schoen DJ, Lloyd DG. Self- and cross-fertilization in plants. III. Methods for studying modes and functional aspects of self-fertilization. International Journal of Plant Sciences. 1992;153:381–393. [Google Scholar]
- Schulte PJ, Brooks JR. Branch junctions and the flow of water through xylem in Douglas-fir and ponderosa pine stems. Journal of Experimental Botany. 2003;54:1597–1605. doi: 10.1093/jxb/erg169. [DOI] [PubMed] [Google Scholar]
- Schultz EA, Haughn GW. Genetic analysis of the floral initiation process (FLIP) in Arabidopsis. Development. 1993;119:745–765. [Google Scholar]
- Sell Y. Physiological and phylogenetic significance of the direction of flowering in inflorescence complexes. Flora. 1980;169:282–294. [Google Scholar]
- Shahri W, Tahir I. Flower senescence – strategies and some associated events. Botanical Review. 2011;77:152–184. [Google Scholar]
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. A plausible model of phyllotaxis. Proceedings of the National Academy of Sciences, USA. 2006;103:1301–1306. doi: 10.1073/pnas.0510457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. The Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souer E, Rebocho AB, Bliek M, Kusters E, de Bruin RAM, Koes R. Patterning of inflorescences and flowers by the F-box protein DOUBLE TOP and the LEAFY homolog ABERRANT LEAF AND FLOWER of Petunia. The Plant Cell. 2008;20:2033–2048. doi: 10.1105/tpc.108.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. Flowering plants: evolution above the species level. Cambridge, MA: Belknap; 1974. [Google Scholar]
- Sun JF, Gong YB, Renner SS, Huang S-Q. Multifunctional bracts in the dove tree Davidia involucrata (Nyssaceae: Cornales): rain protection and pollinator attraction. American Naturalist. 2008;171:119–124. doi: 10.1086/523953. [DOI] [PubMed] [Google Scholar]
- Sun S, Zhang DY, Ives AR, Li QJ. Why do stigmas move in a flexistylous plant? Journal of Evolutionary Biology. 2011;24:497–504. doi: 10.1111/j.1420-9101.2010.02181.x. [DOI] [PubMed] [Google Scholar]
- Thomas MM, Rudall PJ, Ellis AG, Savolainen V, Glover BJ. Development of a complex floral trait: the pollinator-attracting petal spots of the beetle daisy, Gorteria diffusa (Asteraceae) American Journal of Botany. 2009;96:2184–2196. doi: 10.3732/ajb.0900079. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Barrett SCH. Temporal variation of gender in Aralia hispida Vent. (Araliaceae) Evolution. 1981;35:1094–1107. doi: 10.1111/j.1558-5646.1981.tb04979.x. [DOI] [PubMed] [Google Scholar]
- Toräng P, Ehrlén J, Ågren J. Facilitation in an insect-pollinated herb with a floral display dimorphism. Ecology. 2006;87:2113–2117. doi: 10.1890/0012-9658(2006)87[2113:fiaihw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tripp EA. Evolutionary relationships within the species-rich genus Ruellia (Acanthaceae) Systematic Botany. 2007;32:628–649. [Google Scholar]
- Troll W. Die Infloreszenzen: Typologie und Stellung im Aufbau des Vegetationskörpers, Band 1. Stuttgart: Fischer; 1964. [Google Scholar]
- Troll W. Die Infloreszenzen: Typologie und Stellung im Aufbau des Vegetationskörpers, Band 2. Stuttgart: Fischer; 1969. [Google Scholar]
- Tucker SC. Floral ontogeny in legume genera Petalostylis, Labichea, and Dialium (Caesaplinoideae: Cassieae), a series in floral reduction. American Journal of Botany. 1998;85:184–208. [PubMed] [Google Scholar]
- Tucker SC, Grimes J. The inflorescence: introduction. Botanical Review. 1999;65:303–316. [Google Scholar]
- Urzay J, Smith SGL, Thompson E, Glover BJ. Wind gusts and plant aeroelasticity effects on the aerodynamics of pollen shedding: a hypothetical turbulence-initiated wind-pollination mechanism. Journal of Theoretical Biology. 2009;259:785–792. doi: 10.1016/j.jtbi.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Vaughton G, Ramsey M. Pollinator-mediated selfing erodes the flexibility of the best-of-both-worlds mating strategy in Bulbine vagans. Functional Ecology. 2010;24:374–382. [Google Scholar]
- Vekemans D, Viaene T, Caris P, Geuten K. Transference of function shapes organ identity in the dove tree inflorescence. New Phytologist. 2012;193:216–228. doi: 10.1111/j.1469-8137.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- Verbeek NAM, Boasson R. Flowering height and postfloral elongation of flower stalks in 13 species of angiosperms. Canadian Journal of Botany. 1995;73:723–727. [Google Scholar]
- Vogler DW, Peretz S, Stephenson AG. Floral plasticity in an iteroparous plant: the interactive effects of genotype, environment, and ontogeny in Campanula rapunculoides (Campanulaceae) American Journal of Botany. 1999;86:482–494. [PubMed] [Google Scholar]
- de Waal C, Anderson B, Barrett SCH. The natural history of pollination and mating in bird-pollinated Babiana (Iridaceae) Annals of Botany. 2012a;109:667–679. doi: 10.1093/aob/mcr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal C, Barrett SCH, Anderson B. The effect of mammalian herbivory on inflorescence architecture in ornithophilous Babiana (Iridaceae): implications for the evolution of a bird perch. American Journal of Botany. 2012b;99:1096–1103. doi: 10.3732/ajb.1100295. [DOI] [PubMed] [Google Scholar]
- Waddington KD, Heinrich B. The foraging movements of bumblebees on vertical ‘inflorescences’: an experimental analysis. Journal of Comparative Physiology. 1979;134:113–117. [Google Scholar]
- Weberling F. Morphology of flowers and inflorescences. Cambridge, UK: Cambridge University; 1992. [Google Scholar]
- Weller SG, Sakai AK, Culley TM, Campbell DR, Dunbar-Wallis AK. Predicting the pathway to wind pollination: heritabilities and genetic correlations of inflorescence traits associated with wind pollination in Schiedea salicaria (Caryophyllaceae) Journal of Evolutionary Biology. 2006;19:331–342. doi: 10.1111/j.1420-9101.2005.01038.x. [DOI] [PubMed] [Google Scholar]
- Wesselingh RA, Arnold ML. A top-down hierarchy in fruit set on inflorescences in Iris fulva (Iridaceae) Plant Biology. 2003;5:651–660. [Google Scholar]
- Worley AC, Barrett SCH. Evolution of floral display in Eichhornia paniculata (Pontederiaceae): direct and correlated responses to selection on flower size and number. Evolution. 2000;54:1533–1545. doi: 10.1111/j.0014-3820.2000.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Worley AC, Houle D, Barrett SCH. Consequences of hierarchical allocation for the evolution of life-history traits. American Naturalist. 2003;161:153–167. doi: 10.1086/345461. [DOI] [PubMed] [Google Scholar]
- Wyatt R. Inflorescence architecture: how flower number, arrangement, and phenology affect pollination and fruit-set. American Journal of Botany. 1982;69:585–594. [Google Scholar]
- Yakimowski SB, Glaettli M, Barrett SCH. Floral dimorphism in plant populations with combined versus separate sexes. Annals of Botany. 2011;108:765–776. doi: 10.1093/aob/mcr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD. Leaf-produced floral signals. Current Opinion in Plant Biology. 2008;11:541–547. doi: 10.1016/j.pbi.2008.06.009. [DOI] [PubMed] [Google Scholar]