Abstract
Backgrounds and Aims
Conceptual and terminological conflicts in inflorescence morphology indicate a lack of understanding of the phenotypic diversity of inflorescences. In this study, an ontogeny-based inflorescence concept is presented considering different meristem types and developmental pathways. By going back to the ontogenetic origin, diversity is reduced to a limited number of types and terms.
Methods
Species from 105 genera in 52 angiosperm families are investigated to identify their specific reproductive meristems and developmental pathways. Based on these studies, long-term experience with inflorescences and literature research, a conceptual framework for the understanding of inflorescences is presented.
Key Results
Ontogeny reveals that reproductive systems traditionally called inflorescences fall into three groups, i.e. ‘flowering shoot systems’ (FSS), ‘inflorescences’ sensu stricto and ‘floral units’ (FUs). Our concept is, first, based on the identification of reproductive meristem position and developmental potential. The FSS, defined as a seasonal growth unit, is used as a reference framework. As the FSS is a leafy shoot system bearing reproductive units, foliage and flowering sequence play an important role. Second, the identification of two different flower-producing meristems is essential. While ‘inflorescence meristems’ (IMs) share acropetal primordia production with vegetative meristems, ‘floral unit meristems’ (FUMs) resemble flower meristems in being indeterminate. IMs produce the basic inflorescence types, i.e. compound and simple racemes, panicles and botryoids. FUMs give rise to dense, often flower-like units (e.g. heads). They occur solitarily at the FSS or occupy flower positions in inflorescences, rendering the latter thyrses in the case of cymose branching.
Conclusions
The ontogenetic concept differs from all existing inflorescence concepts in being based on meristems and developmental processes. It includes clear terms and allows homology statements. Transitional forms are an explicit part of the concept, illustrating the ontogenetic potential for character transformation in evolution.
Keywords: Botryoid, floral unit (FU), flowering sequence, flowering shoot system (FSS), foliage, inflorescence, meristem potential, ontogenetic concept, panicle, position, raceme, thyrse
INTRODUCTION
Increased knowledge of phylogeny, developmental genetics and sexual reproduction has raised interest in the evolutionary and functional significance of inflorescences (e.g. Harder et al., 2004; Benlloch et al., 2007; Prenner et al., 2009; Castel et al., 2010; Endress, 2010; Feng et al., 2011). However, working with inflorescences is difficult. Different concepts and terms have been used, resulting in conflicts and confusion.
The main sources of confusion are: (1) the use of different reference frameworks to define inflorescences (e.g. ‘synflorescences’ sensu Troll, 1964, and Weberling, 1989; ‘uniflorescences/conflorescences’ sensu Briggs and Johnson, 1979; ‘inflorescences/paraclades’ sensu Stauffer, 1963; ‘reproductive units’ sensu van Steenis, 1963, and Schröder, 1987; reviewed in Claßen-Bockhoff, 2000); (2) the mixing of hierarchical levels (e.g. flowering shoot systems vs. inflorescences; discussed in Endress and Doyle, 2009); (3) the use of different terms for the same structure, e.g. dichasium (Seybold, 2011), cymoid (Troll and Weberling, 1989), reduced closed thyrse (Troll, 1964) and cymose inflorescence (e.g. Wagenitz, 2008; see also Castel et al., 2010); and (4) the use of the same term for different things (e.g. cyme sensu Troll, 1964, vs. cyme sensu Judd et al., 1999).
In almost all concepts, adult inflorescences are grouped and named. As a consequence, a huge variety of patterns are compared without regard to origin or development. This approach can be a severe source of misinterpretation. As Bull-Hereñu and Claßen-Bockhoff (2011a, b) recently showed by quantitative developmental studies, open inflorescences (no terminal flower present) may arise from two different meristems. ‘Open I’ meristems continue to elongate while producing lateral meristems in acropetal sequence, whereas ‘open II’ meristems have lost the capacity to elongate and are completely consumed by flower primordia. The two different meristem types are also revealed by molecular and histological data. ‘Open I’ meristems obviously fail to produce a terminal flower, because stem cells are maintained in the central zone of the meristem (e.g. Kwiatkowska, 2004). Stem cell proliferation is genetically regulated by the WUSHEL/CLAVATA3 (WUS/CLA3) system in Arabidopsis thaliana (Bäurle and Laux, 2003) and located in the central zone (CZ) of the meristem, which histologically differs from the peripheral zone by having slightly larger cells. As long as the CZ is present, no terminal flower can be produced. In contrast, ‘open II’ meristems lack a CZ (e.g. Palmer and Palmer, 1982); maintenance of the stem cells ceases due to a collapsing WUS/CLA3 regulatory loop, and all cells of the meristem become homogeneous (mantle core histology).
In the present paper, an ontogeny-based concept for inflorescences is presented taking into account meristem types, developmental pathways and positions within the flowering shoot system. Going back to the meristematic origin of reproductive structures, similar units are revealed as analogous, phenotypic diversity is reduced to a limited number of basic processes, and formerly disregarded characters such as foliage and flowering sequence are given new importance. The finding that similar processes are repeated on different hierarchical levels of the plant's architecture disentangles confusion on inflorescence diversity and terminology and, most importantly, leads to a more natural understanding of inflorescences essential for homology assessments and evolutionary and genetic studies.
MATERIALS AND METHODS
Species from 105 genera in 52 mainly eudicotyledon angiosperm families were investigated (Table 1) to illustrate the diversity of inflorescences. Plant material originates from the Botanical Garden at Mainz University or was collected in the field. Determination was confirmed by the authors. Vouchers (incl. alcohol material) are deposited at the MJG.
Table 1.
Plant material used in the present study (vouchers and/or alcohol material are deposited at MJG; nomenclature following APG III)
| Aceraceae | Acer carpinifolium Siebold & Zucc. |
| Anacardiaceae | Rhus aromatica Aiton, R. copallinum L., R. radicans L. |
| Apiaceae | Astrantia major L., Chaerophyllum bulbosum L., Coriandrum sativum L., Daucus carota L., Pastinaca sativa L., Petagnia saniculifolia Guss., Sanicula marilandica L. |
| Apocynaceae | Apocynun cannabinum L. |
| Asteraceae | Dyssodia decipiens (Bartl.) M. C. Johnst., Echinops bannaticus Rochel ex Schrad., Liatris spicata (L.) Willd., Ligularia stenocephala (Maxim.) Matsum. & Koidz., Matricaria dioscoidea DC., Polycalymma stuartii F. Muell. & Sond. |
| Begoniaceae | Begonia semperflorens Hook. |
| Berberidaceae | Berberis aggregata C. K. Schneid., B. aristata DC., B. bretschneideri Rehder, B. darwinii Hook., B. vulgaris L., B. wilsoniae Hemsl., Mahonia aquifolium (Pursh) Nutt., Mahoberberis × aquiargentii H. Jensen, Nandina domestica Thunb. |
| Boraginaceae | Symphytum asperum Lep. |
| Brassicaceae | Armoracia rusticana G. M. Sch., Cardamine impatiens L., Diplotaxis tenuifolia (L.) DC., Peltaria alliaceae Jacq. |
| Bruniaceae | Berzelia lanuginosa (L.) Brongn., Staavia radiata (L.) Dahl. |
| Buxaceae | Buxus sempervirens L. |
| Campanulaceae | Asyneuma canescens (Waldst. & Kit.) Griseb. & Schenk, Campanula thyrsoides L., Edraianthus pumilio A. DC., E. tenuifolius (Waldst. & Kit.) A. DC., Jasione montana L., Phyteuma canescens Waldst. & Kit. |
| Caprifoliaceae | Weigela florida (Bunge) A. DC. |
| Coriariaceae | Coriaria japonica A. Gray |
| Cornaceae | Cornus mas L., C. florida L., Davidia involucrata Baill. |
| Cucurbitaceae | Bryonia dioica Jacq. |
| Dipsacaceae | Dipsacus sylvestris Huds. |
| Eleagnaceae | Hippophaë rhamnoides L. |
| Euphorbiaceae | Ricinus communis L. |
| Fabaceae | Desmodium canadense (L.) DC., Galega officinalis L., Gleditsia triacanthos L., Melilotus officinalis (L.) Lam., Mimosa pudica L., Trifolium repens L. |
| Fagaceae | Quercus phillyraceoides A. Gray |
| Fumariaceae | Lamprocapnos spectabilis (L.) Fukuhara |
| Geraniaceae | Erodium cicutarium (L.) L'Hér. |
| Hamamelidaceae | Parrotiopsis jacquemontiana Rehder, Sinowilsonia henryi Hemsl. |
| Hydrangeaceae | Deutzia gracilis Siebold & Zucc., Hydrangea arborescens L., H. petiolaris Sieb. et Zucc., H. quercifolia Bartr., Schizophragma hygrangeoides Sieb. et Zucc., Sch. integrifolium Oliv. |
| Hypericaceae | Hypericum perforatum L. |
| Lamiaceae | Salvia apiana Jepson, S. candelabrum Boiss., S. exerta Griseb., S. gravida Epl., S. jurisicii Košanin, S.nutans L., S. verticillata L., S. viridis L. |
| Malvaceae | Alcea rosea L. |
| Marantaceae | Thalia dealbata Fras., T. geniculata L. |
| Meliaceae | Melia azedarach L. |
| Moraceae | Dorstenia contrayerva L., D. indica WALL., D. zanzibarica Oliver |
| Myricaceae | Myrica faya Aiton, M. pensylvanica Mirb. |
| Myrtaceae | Actinodium cunninghamii Schauer, Callistemon citrinus (Curtis) Skeels, C. pallidus (Bonpl.) DC. |
| Oleaceae | Jasminum polyanthum Franch., Phillyrea latifolia L. |
| Papaveraceae | Capnoides sempervirens (L.) Borkh., Chelidonium majus L., Corydalis elata Bureau & Franck, Dicentra eximia (Ker Gawl.) Torr., Macleaya cordata (Willd.) R. Br., Macleaya microcarpa (Maxim.) Fedde, Meconopsis cambrica (L.) Viguier |
| Phytolaccaceae | Phytolacca acinosa Roxb., P. americana L. |
| Plantaginaceae | Digitalis purpurea L., Hebe albicans (Petrie) Cockayne, Veronica caucasica M. Bieb., V. filiformis, V. incana L., V. longifolia L., V. persica Poir., V. teucrium L. |
| Polemoniaceae | Collomia grandiflora Douglas ex Lindl., Phlox drummondii Hook. |
| Primulaceae | Lysimachia atropurpurea L., L. ciliata L., L. ephemerum L., L. nummularia L., L. thyrsiflora L., L. vulgaris L. |
| Ranunculaceae | Anemone nemorosa L., Aquilegia vulgaris L., Delphinium grandiflorum L., Nigella sativa L., Ranunculus lingua L. |
| Resedaceae | Reseda lutea L., R. luteola L. |
| Rhamnaceae | Rhamnus alaternus L. |
| Rosaceae | Agrimonia eupatoria L., Aruncus dioicus (Walter) Fernald, Exochorda racemosa (Lindl.) Rehder, Neviusia alabamensis A. Gray, Sanguisorba minor Scop., Spiraea chamaedryfolia L., S. japonica (L.) Desv., S. nipponica Maxim. |
| Rubiaceae | Cephalanthus occidentalis L. |
| Rutaceae | Diplolaena angustifolia Hook., D. grandiflora Desf., D. microcephala Bartl., Skimmia japonica Thunb. |
| Sapindaceae | Ungnadia speciosa Endl. |
| Saxifragaceae | Ribes sanguineum var. glutinosum (Benth.) Loudon., Tellima grandiflora (Pursh) Douglas ex Lindl. |
| Taccaceae | Tacca chantrieri André |
| Tamaricaceae | Tamarix tetrandra Pall. ex M. Bieb. |
| Trochodendraceae | Trochodendron aralioides Siebold et Zucc. |
| Verbenaceae | Aloysia triphylla Royle, Lantana camara L., Phyla nodiflora (L.) Greene |
| Xanthorrhoeaceae | Hemerocallis middendorffii |
Material was fixed in 70 % ethanol, dissected, critical point dried (CPD 030; BAL-TEC, Pfäffikon, Swizterland), sputtered with gold (SCD 005; BAL-TEC) and documented under a scanning electron microscope (ESEM; Philipps, Eindhoven, the Netherlands). For histological sections, plant material was embedded in paraplast, dissected with a microtome (10 µm) and stained using toluidine blue. All technical work was done according to standard protocols.
To facilitate ease of reading, the following abbreviations are used: flower meristem (FM), flowering shoot system (FSS), floral unit (FU), floral unit meristem (FUM), inflorescence meristem (IM), reproductive meristem (RM), reproductive unit (RU), vegetative meristem (VM). To distinguish the here defined inflorescences sensu strictu (only reproductive units originating from the inflorescence meristem) from inflorescences sensu latu (all reproductive units traditionally called inflorescences), the latter is given in quotation marks.
RESULTS AND DISCUSSION
A seasonal shoot system, defined as the whole annual plant or the seasonal growth unit of perennial herbs and woody plants, has VMs at terminal and axillary positions. When entering the reproductive stage it becomes a flowering shoot system (FSS; ‘seasonal growth unit’ sensu Briggs and Johnson, 1979). All (many annuals) or part of the VMs become reproductive, while the other meristems remain vegetative, continue to grow or persist as inhibited buds.
Meristem types
RMs differ from VMs in size, shape and/or phyllotactic pattern (e.g. Stebbins, 1973; Kwiatkowska, 2008). Based on ontogenetic investigations three different RMs can be distinguished: the IM, FM and FUM (Fig. 1, Table 2).
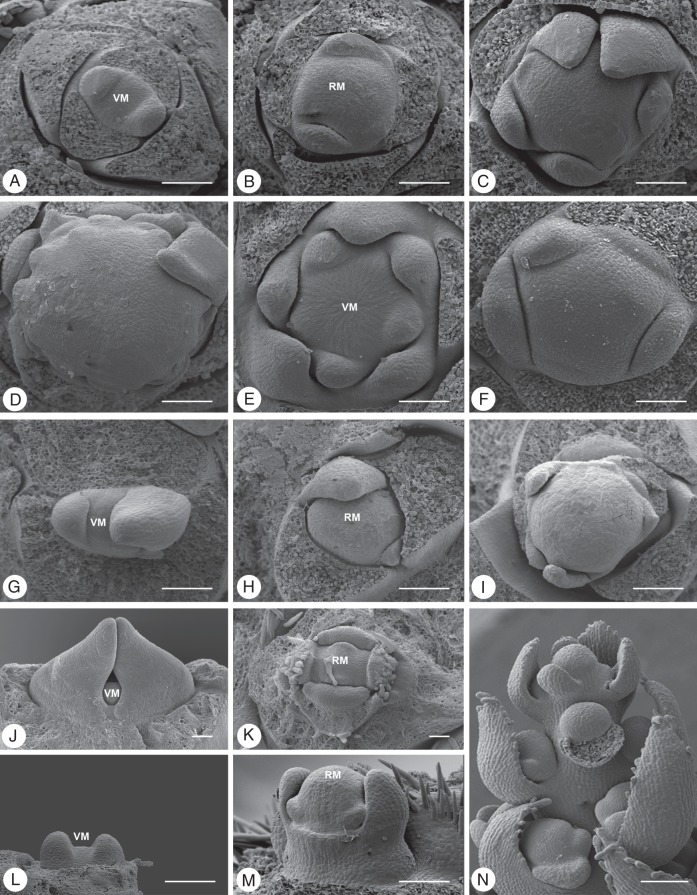
Fig. 1.
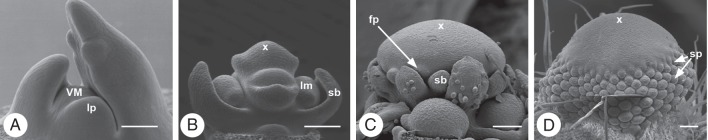
Meristem types. (A) Bocconia frutescens, Papaveraceae: vegetative meristem (VM) with leaf primordia (lp), no axillary buds present (original: S. Gleissberg, Ohio University). (B) Hypericum perforatum Hypericaceae: inflorescence meristem (x) with early developing lateral meristems (lm) and subtending bract primordia (sb). (C) Matricaria dioscoidea, Asteraceae: floral unit meristem (x) with flower primodria (fp) and subtending bract primordia (sb). (D) Anemone nemorosa, Ranunculaceae: flower meristem (x) producing stamen primordia (sp). ‘x’ in B–D indicates the tip of the respective meristem. Scale bars = 100 µm.
Table 2.
Basic meristem types and their characteristics
| Meristem type | Acropetal activity | Apical meristem | Meristem shape | Lateral primodia | Axillary meristem development | Sequence of development |
|---|---|---|---|---|---|---|
| Vegetative meristem (VM) | infinite | indeterminate | small, usually dome-shaped | leaves | delayed | acropetal |
| Inflorescence meristem (IM) | limited | indeterminate (→ determinate) | enlarged, dome-shaped or flat | bracts | immediate | acropetal |
| Floral unit meristem (FUM) | lacking | determinate | enlarged, dome-shaped or flat, ‘naked’ | minute bracts, if at all | immediate | centripetal or centrifugal (cymose) |
| Flower meristem (FM) | lacking | determinate | enlarged, dome-shaped or flat, ‘naked’ | floral organs | lacking | centripetal (centrifugal) |
Vegetative meristems (Fig. 1A)
VMs are characterized by indeterminate growth and acropetal production of leaf primordia and axillary meristems (Kwiatkowska, 2008). This process is called ‘segregation’ in the present study and is defined as the continuous production of new meristematic tissue based on stem cell activity. Node and internode production accompanies this process. The meristem is indeterminate as the ongoing activity of segregation does not allow formation of a terminal RU. Leaves tend to overgrow and protect the active apical meristem (Figs 1A, 2J and 3A). Their axillary meristems either remain inactive or develop with a delay, producing vegetative shoots or RUs in the same or one of the following seasons. Histological sections show the characteristic zonation of the meristem with a central zone, peripheral zone and rib zone (Clowes, 1961; reviewed by Kwiatkowska, 2008; Fig. 3B).
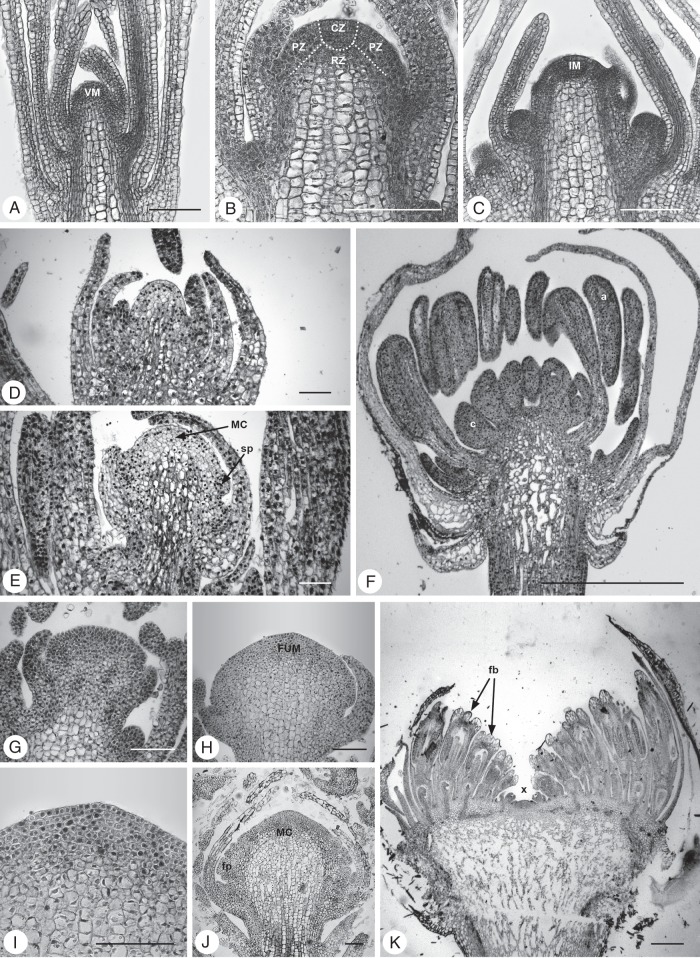
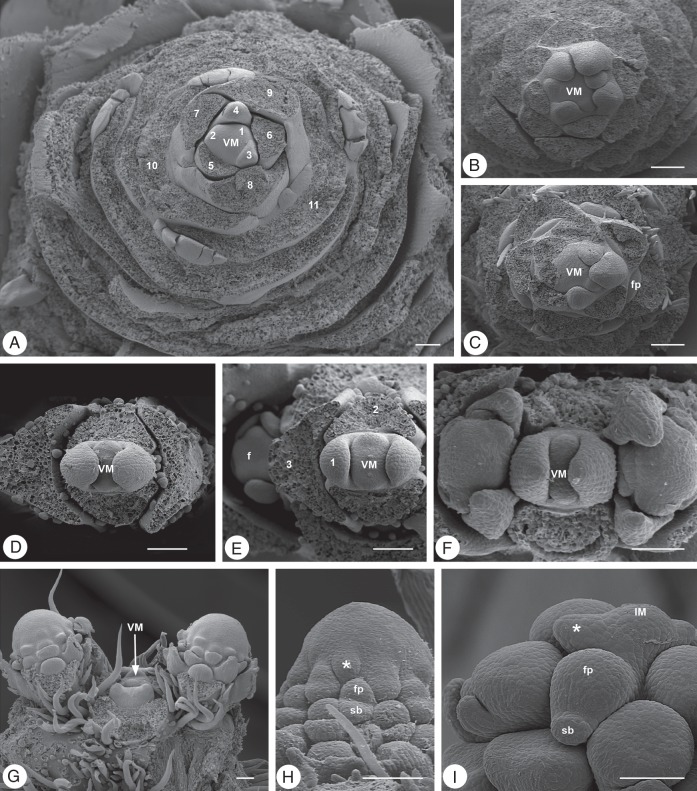
Fig. 2.
Vegetative and reproductive meristems. (A–D) Veronica longifolia, Plantaginaceae: (A) vegetative meristem (VM), (B) early reproductive meristem (RM), (C, D) young and older raceme meristems. (E, F) Trochodendron aralioides, Trochodendraceae: (E) vegetative meristem (VM), (F) botryoid meristem. (G–I) Phytolacca acinosa, Phytolaccaceae: (G) vegetative meristem (VM), (H) early reproductive meristem (RM), (I) raceme meristem. (J, K) Schizophragma integrifolium, Hydrangeaceae: (J) vegetative meristem (VM), (K) early panicle meristem (RM). (L–N) Hebe albicans, Plantaginaceae: (L) vegetative meristem (VM), (M) early raceme meristem (RM), (N) developing raceme with decreasing apical meristem (x) size. Scale bars = 100 µm (pictures of same species at same scale).
Fig. 3.
Histological zonation of meristems. (A–C) Actinodium cunninghamii, Myrtaceae: (A) vegetative meristem (VM), (B) zonation of VM with central zone (CZ), peripheral zone (PZ) and rib zone (RZ), (C) indeterminate inflorescence meristem (IM) with bracts and lateral buds. (D–F) Ranunculus ficaria, Ranunculaceae: (D) early flower meristem, (E) enlarged ‘naked’ flower meristem with first floral stamen primodia (sp) and mantle core (MC) histology, (F) young flower; a, anther; c, carpel. (G–K) Erigeron canadensis, Asteraceae: (G) early reproductive meristem, (H) enlarged ‘naked’ head meristem (FUM), (I) detail from (H) showing homogeneous meristem, (J) developing head with mantle core (MC) histology and first flower primordia (fp), (K) young head with flower buds (fb); terminal flower absent (x). Scale bars: (A–E, G–K) = 100 µm; (F) = 1 mm.
Inflorescence meristems (Fig. 1B)
IMs share with VMs the acropetal segregation of lateral primordia, but differ from them in their increased size and/or volume often combined with different shape and phyllotaxis (Fig. 2). They usually have limited activity (Stebbins, 1973; Kwiatkowska, 2008; Bull-Hereñu and Claßen-Bockhoff, 2011b). Correspondingly, inflorescences are ephemeral units which drop off after seed-set (see van Steenis, 1963; Schröder, 1987; Claßen-Bockhoff, 2000). Axillary meristems from IMs develop immediately (Figs 1B: lm and 3C) giving rise to flowers or lateral partial inflorescences. These are subtended by bracts which are smaller than vegetative leaves and normally do not protect the IM (Figs 1B: sb and 2K, M). The fast developing axillary meristems obviously suppress the developmental programme of their subtending bracts, which is taken as a general indicator for RMs.
Flower meristems (Fig. 1D)
FMs clearly differ from VMs and IMs (Table 2). They have lost the capacity to continuously produce new organs and are characterized by mantle core histology (Bull-Hereñu and Claßen-Bockhoff, 2011a; Fig. 3E: MC). Correspondingly, FMs are determinate and normally used up completely. In the present study floral organ production is called ‘fractionation’ and is defined as the subdivision of an already existing ‘naked’ meristem lacking stem cells.
Floral organs are usually produced in centripetal direction (Fig. 1D), but centrifugal (Rudall, 2010) or irregular developmental sequences (Kirchoff, 1983) also occur. Internodes are usually inhibited. According to the euanthium theory (Arber and Parkin, 1907) floral organs are leaf homologues without axillary meristems.
FMs are unique in differentially expressing meristematic activity, giving rise to inferior ovaries, hypanthia, androgynophores or stamen–petalum complexes (e.g. Endress, 1994; Leins and Erbar, 2008; Ronse de Craene, 2010). Floral organs originate from single primordia, super primordia (forming fascicles) or ring meristems (resulting in ‘congenitally fused’ tubes). They obviously respond to specific spatial constraints by undergoing metatopic dislocations (e.g. obdiplostemony, see Leins and Erbar, 2008) and dédoublements (e.g. stamen pairs, see Ronse de Craene, 2010). They are able to expand during floral organ production generating space for new organs (e.g. cyclical polyandry, see Ronse de Craene and Smets, 1987). Flowers differ in sex and symmetry even on the same plant (e.g. Asteraceae, see Uexküll-Gyllenband, 1901), present exciting examples of synorganization (e.g. Marantaceae, see Claßen-Bockhoff and Heller, 2008) and are able to generate novel structures (e.g. paracorolla, see Bernhard, 1999).
Floral unit meristems (Fig. 1C)
FUMs, although giving rise to structures that bear many flowers, are nearer to FMs than to IMs. They share the initial ‘naked’ stage (Fig. 1C, D) and the mantle core histology with FMs (Fig. 3J: MC). They also present a simple or complex fractionation process of lateral meristems which in the end convert into flower primordia. This contrasts with the fractionation of FMs that directly produce floral organs. Usually the FUM expands during fractionation promoting further subdivisions of the tissue (comp. scales in Fig. 3G, J, K). Bract production is highly suppressed in FUMs. Often only the outer flower primordia have bracts or bracts are completely lacking. FUMs can appear solitarily in the plant body or form part of inflorescences.
Tucker and Grimes (1999) already mentioned that IMs can have different histologies. But here, FUMs are identified for the first time as a second type of flower-producing meristems. They are based on the ‘open II’ meristems described by Bull-Hereñu and Claßen-Bockhoff (2011b), but are more generally defined, also including cymose structures.
Meristem position – types of FSSs
FSSs are leafy shoots (Figs 4A and 5A) producing RUs at different positions (Figs 4B and 5B: dark red). In ‘open’ FSSs, only meristems in vegetative leaf axils become reproductive, while the shoot apical meristem remains vegetative (Fig. 6A–E, R; Table 3). In ‘closed’ FSSs, only the terminal meristem becomes reproductive (Fig. 6F, L, S; Table 3) or terminal and axillary meristems come to flower (Fig. 6G–J; Table 3). FSSs are often termed ‘frondo-bracteose inflorescences’ (Troll, 1964; Weberling, 1989). However, it is important to state that FSSs are composed of RUs arising from different RMs (Fig. 4B), while an inflorescence in the here used definition originates from a single one (Fig. 4C).
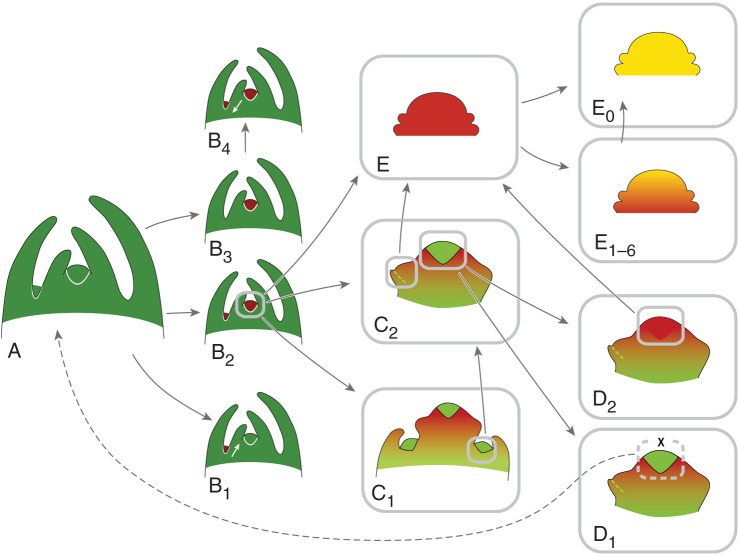
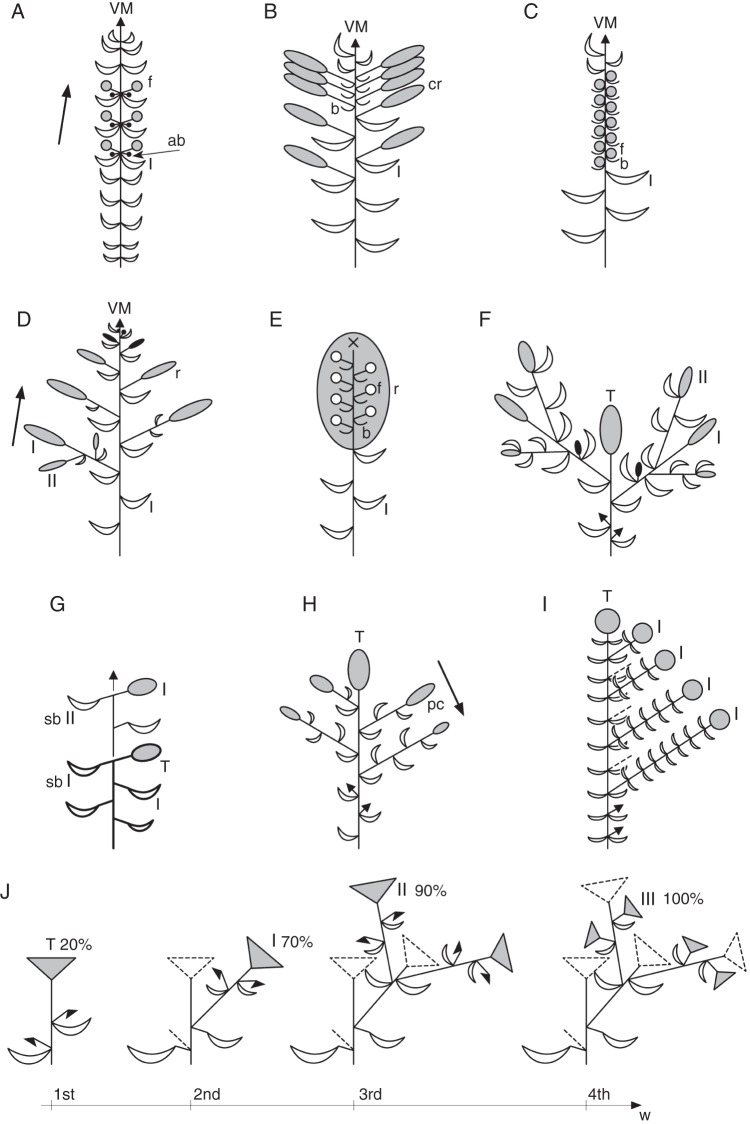
Fig. 4.
Meristems and developmental pathways. The combination of different meristem types and developmental pathways allows the deduction of all forms of flowering shoot systems (see Figs 5 and 6). (A) Tip of a shoot system with vegetative meristems (green) in terminal and axillary position. (B) Transition to reproductive meristems (dark red), (B1) at axillary positions (white arrow: acropetal development), (B2) terminal and axillary position, (B3) terminal position, (B4) terminal position followed by induced axillary meristems (white arrow: basipetal sequence); (C) early reproductive meristem with indeterminate tip (light green zone), (C1) producing lateral partial inflorescences (compound inflorescences), (C2) producing lateral flowers or floral units (simple inflorescences). (D) Tip of late reproductive meristem, (D1) remaining indeterminate (cross) and rarely returning to vegetative growth (grey dashed line), (D2) merging into the determinate stage (light red; terminal flower/FU). (E) Determinate reproductive meristem (red) originating a floral unit (E1–6: red–yellow grade) or flower (E0: yellow). Grey frames: meristems and meristem parts exemplarily illustrating possible developmental pathways; arrows: developmental alternatives; green–red colour gradient: increasing reproductive signals in inflorescence meristems; red–yellow colour gradient: floral unit meristem fractionating into sub-meristems before starting flower production.
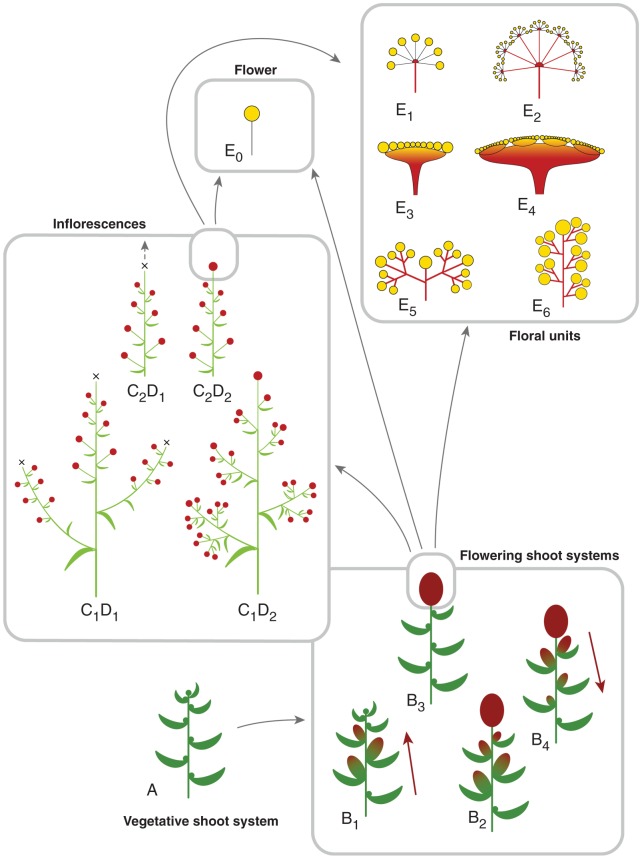
Fig. 5.
Flowering shoot systems, inflorescences and floral units. (A) Annual shoot system with vegetative meristems at terminal and lateral positions (green dots). (B) Flowering shoot system (FSS) with reproductive meristems (RM), (B1) open (also proliferating) monopode with RMs at lateral positions (arrow: acropetal sequence), (B2) RMs at terminal and lateral positions, (B3) only terminal position and (B4) terminal and lateral positions (arrow: basipetal sequence); dark green: vegetative part of the FSS; red ovals: RUs; green–red colour gradient: lateral shoots with varying degree of vegetative vs. reproductive signals. (C1D1–C2D2) Basic types of inflorescences: (C1D1) compound raceme, (C1D2) panicle, (C2D1) raceme (arrow: rarely proliferating), (C2D2) botryoid; light green: branching within inflorescence; light red dots: reproductive units (FUs, flowers). (E0) Flower (yellow). (E1–6) Selection of floral units (FU) (red–yellow colour grade): (E1) umbel, (E2) secondary umbel, (E3) head, (E4) secondary head, (E5) cymose FU or cyme depending on terminal or lateral position, (E6) cyathium. Arrow A to B: vegetative shoot systems merge into flowering shoot systems by forming reproductive units; remaining arrows: reproductive units in a flowering shoot system (dark red ovals) correspond to an inflorescence (C1D1–C2D2), flower (E0) or floral unit (FU) (E1–6); RUs in an inflorescence (light red dots) correspond to flowers (E0) or FUs (E1–6) (compare with Figs 4 and 6).
Fig. 6.
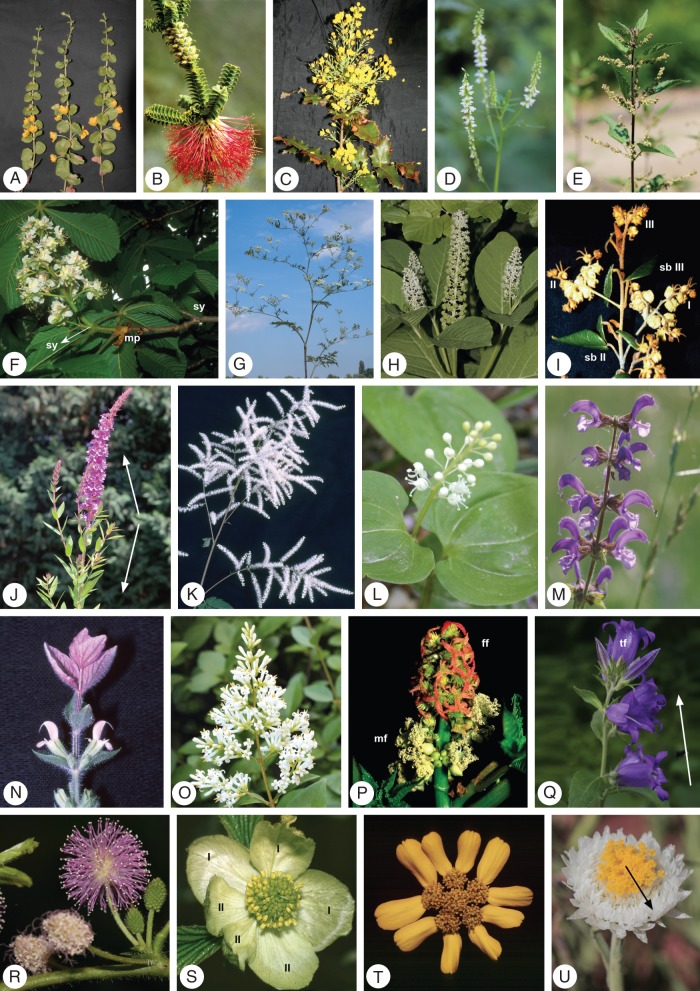
Examples of flowering shoot systems, inflorescences and floral units (see Table 3 for formulas and characteristics). (A–E) Open flowering shoot system (FSS): (A) Lysimachia nummularia, Primulaceae: lateral flowers in leaf axils; (B) Beaufortia squarrosa, Myrtaceae: lateral flowers in leaf axils; (C) Mahonia aquifolium, Berberidaceae: racemes in leaf and bract axils; (D) Melilotus alba, Fabaceae: lateral racemes in leaf axils; (E) Urtica dioica, Urticaceae: cymose floral units (FU) at axillary position. (F–J) Closed FFS: (F) Aesculus hippocastanum, Hippocastaneaceae: terminal inflorescence – shoot connection is monopodial (mp) or sympodial (sy) depending on flowering; (G) Chaerophyllum bulbosum, Apiaceae: modular shoot construction with FUs (secondary umbels) up to the 3rd branch order; (H) Phytolacca acinosa, Phytolaccaceae: leafy FSS with dominant terminal raceme and smaller racemes of 1st order; (I) Lasiopetalum cordifolium, Malvaceae: extreme sympodial–monochasial branching pattern with cymose FUs up to the 3rd branch order (I–III), FUs stand opposite the subtending bracts of next order units; (J) Lythrum salicaria, Lythraceae: terminal thyrse (raceme with cymes) with basipetal paraclades (arrow). (K–Q) Inflorescences: (K) Aruncus dioicus, Rosaceae: compound raceme; (L) Maianthemum bifolium, Liliaceae: raceme; (M) Salvia pratensis, Lamiaceae: thyrse (spike with cymes); (N) Salvia viridis, Lamiaceae: tuft of petaloid leaves above the uppermost flowers; (O) Ligustrum vulgare, Oleaceae: panicle; (P) Ricinus communis, Euphorbiaceae: thyrse (botryoid with cymes); (Q) Campanula medium, Campanulaceae: botryoid with acropetal flowering sequence (arrow) and premature terminal flower (tf). (R–U) Floral units: (R) Mimosa pudica, Fabaceae–Mimosoideae: heads in accessory positions; (S) Parrotiopsis jacquemontiana, Hamamelidaceae: head with two stipulated extrafloral bracts (I, II); (T) Dyssodia decipiens, Asteraceae: secondary head with ray flowers at the outer heads; (U) Polycalymma stuartii, Asteraceaea: thyrse (botryoid with cymes bearing heads) and centrifugal (ordinal) flowering sequence (arrow), surrounded by strawy bracts.
Table 3.
Formulas for flowering shoot systems (FSSs) and reproductive units based on meristem position (Figs 4B and 5B), meristem type and developmental programme (Figs 4C–E and 5C–E); species are arranged after Fig. 6.
| Species | FSS formula | Term |
|---|---|---|
| Lysimachia nummularia | h B1 pr E0 | Proliferating monopode (FSS) with axillary flowers |
| Beaufortia squarrosa | w B1 pr E0 | Proliferating monopode (FSS) with axillary flowers |
| Mahonia aquifolium | w B1 pr C1D1 | Proliferating monopode (FSS) with axillary (compound) racemes (I) |
| Melilotus alba | h B1 C2D1 | Monopode (FSS) with axillary racemes up to ∼ 2nd order (I) |
| Urtica dioica | h B1 E5 | Monopode (FSS) with axillary cy-FUs of 2nd order (see Bernbeck 1932) |
| Aesculus hippocastanum | w B3 C2D2 E5 | Terminal (FSS) thyrse (I: botryoid with cymes), terminal flower sometimes suppressed |
| Chaerophyllum bulbosum | h B2 E2 | Terminal and lateral (FSS) secondary umbels up to 4th order (FU) |
| Phytolacca acinosa | h B4 C2D1 | Terminal and basipetal (FSS) racemes (I) up to ∼ 2nd order |
| Lasiopetalum cordifolium | w B2 E5 | Terminal and lateral (FSS) cy-FUs up to high orders (monochasia) |
| Lythrum salicaria | h B4 C2D1 E5 | Terminal and basipetal (FSS) thyrses (I: spike with cymes) |
| Aruncus dioicus | w B4 C1D1 | Terminal and basipetal (FSS) compound racemes (I) |
| Maianthemum bifoilium | h B3 C2D1 | Terminal (FSS) raceme (I) |
| Salvia pratensis | h B4 C2D1 E5 | Terminal and basipetal (FSS) thyrses (I: spike with cymes) |
| Salvia viridis | h B4pr C2D1 E5 | Terminal and basipetal (FSS) proliferating thyrse (I: spike with cymes) |
| Ligustrum vulgaris | w B3 C1D2 | Terminal (FSS) panicle (I) |
| Ricinus communis | h B4 C2D2 E5 | Terminal and basipetal (FSS) thyrses up to ∼ 4th order (I: botyroid with cymes) |
| Campanula medium | h B4 C2D2 | Terminal and basipetal (FSS) botryoids (I) |
| Mimosa pudica | h B1 acE3 p | Proliferating monopode (FSS) with heads (FU) at accessory shoot positions |
| Parrotiopsis jacquemontiana | w B3 E3 p | Terminal (FSS) head (FU; with extrafloral bracts |
| Dyssodia decipiens | h B4 E4 p | Terminal and basipetal (FSS) secondary heads (FU; with ray flowers) |
| Polycalymma stuartii | h B3 C2D2 E5 E3 p | Terminal (FSS) thyrse (I: botryoid with cymes bearing heads up to 5th order) (with extrafloral bracts) |
Abbreviations: ac, accessory shoot; pr, proliferating; cy, cymose; FSS, flowering shoot system; FU, floral unit; h, herbaceous; I, inflorescence; p, pseudanthium; w, woody.
Open flowering shoot systems
FSSs with RMs only at axillary positions are characterized by an indeterminate shoot apical meristem (Figs 4B1 and 5B1). Dependent on its vegetative vigour the shoot tip continues to grow (proliferation; Fig. 6A–C) or ceases growth after the production of axillary RUs.
RUs originate in acropetal sequence, thus following the normal growth of VMs. They often appear a few nodes below the active shoot apical meristem (Fig. 7A, E). They either arise directly from the leaf axils resulting in axillary RUs as seen in Lysimachia nummularia (Figs 6A, 7E and 8A), Beaufortia squarrosa (Fig. 6B) or Mahonia aquifolium (Figs 6C and 8B) or they are formed at lateral shoots of first or higher branch order as in many Fabaceae (e.g. Melilotus albus, Figs 6D and 8D). Sometimes, accessory buds produce additional RUs (e.g. Lysimachia nummularia, Fig. 8A). Sometimes the central bud of the leaf axil remains vegetative while surrounding accessory buds come to flower (Mimosa pudica, Fig. 6R). This clearly indicates that the reproductive stimulus affects only part of the axillary meristem, while the other part remains vegetative (see Sell, 1995).
Fig. 7.
Proliferating flowering shoot system and leaf/bract development. (A–G) Open flowering shoot systems (FSS): (A) Mahoberberis aquiargentii, Berberidaceae: axillary botryoids arising far below the vegetative apex (VM), 1–11 sequence of leaf formation, youngest lateral primordium in the axil of leaf 10; (B, C) Callistemon citrinus, Myrtaceae: vegetative meristem (VM) before (A) and after (B) axillary flower production (fp); (D, E) Lysimachia nummularia, Primulaceae: vegetative meristem (VM) before (D) and after (E) axillary flower (f) production; flower in axil of leaf below the vegetative apex (one leaf of each of the three distal pairs numbered); (F) Veronica teucrium, Plantaginaceae: axillary raceme primordia below vegetative meristem (VM); (G) Lantana camara, Verbenaceae: axillary head primordia below vegetative meristem (VM). (H, I) Bract-flower primordia in developing inflorescences: (H) Trifolium repens, Fabaceae: developing raceme with flowers (fp) and subtending bracts (sb) originating from the same primordium (asterisk); (I) Spiraea japonica, Rosaceae: detail from developing raceme showing subtending bracts (sb) dislocated by their large flower primordia (fp) in a concaulescent manner; asterisk: common primordium for bract and flower. Scale bars = 100 µm.
Fig. 8.
Schematic side views of flowering shoot systems (compare with Fig. 6). (A) Lysimachia nummularia, Primulaceae: proliferating flowering shoot system (FSS) with leaves (l), axillary flowers (f) and accessory flower buds (ab); arrow: acropetal development. (B) Mahonia aquifolium, Berberidaceae: proliferating FSS with simple and compound racemes (cr) in the axils of leaves (l: proximal) and bracts (b: distal). (C) Callistemon citrinus, Myrtaceae: proliferating FSS with flowers (f) in the axils of bracts (b). (D) Securigera varia, Fabaceae: open FSS with lateral racemes (r) of 1st (I) and 2nd (II) order developing in acropetal order (arrow) in leaf axils (l). (E) Linaria vulgars, Plantaginaceae: terminal raceme (grey: r) with flowers (f) subtended by bracts (b); the ‘frondo-bracteose’ aspect of the FSS results from the leaves below and the bracts within the inflorescence. (F) Phytolacca acinosa, Phytolaccaceae: terminal raceme (T) with paraclades of 1st (I) and 2nd (II) order; black ovals: reproductive buds; arrows: vegetative buds. (G) Lasiopetalum cordifolium, Malvaceae: extremely monochasially branched FSS with terminal cymose FU (T) overtopped by the axillary branch of 1st (I) order arsing from the uppermost leaf axil (sb I). (H) Lythrum salicaria, Lythraceae: terminal thyrse (T: spike with cymes) with paraclades (pc); arrow: basipetal development. (I) Hypericum perforatum, Hypericaceae: closed FSS with terminal (T) and lateral (I) thyrses (botryoids with cymes). (J) Chaerophyllum bulbosum, Apiaceae: developmental changes of the FSS during its 4-week development (w): terminal FU (T: secondary umbel) starts flowering while lateral FUs (I) are still in bud stage; after anthesis of T, 1st-order branches elongate and flower (2nd week), after their anthesis 2nd-order branches continue (3rd week) finally producing FUs of 3rd order (III; 4th week); as all FUs of the same order are simultaneously protandrous and among orders flower successively, multicyclical dichogamy results; number of male flowers (%) increase with age, i.e. secondary umbel order (andromonoecy).
FSSs may show bracteose subtending leaves as in Mahonia aquifolium (Fig. 6C and 8B), where only the proximal RUs originate from leaf axils (l), while the distal ones are subtended by bracts (b). A clear alteration between leaves and flower subtending bracts is the rule in the likewise proliferating Callistemon citrinus (Myrtaceae; Fig. 8C). Although the shoot apical meristem remains vegetative all the time (Fig. 7B, C), the axillary RUs obviously inhibit the developmental programme of the leaves, indicating that the floral stimulus on the axillary meristems has been expressed early enough to affect leaf development at the main axis.
Closed flowering shoot systems
Closed FSSs are defined by always being crowned by a terminal RU. This is ontogenetically characterized by the enlargement of the vegetative shoot apical meristem that merges into an RM (inflorescence, floral unit or flower meristem; Fig. 2). Further apical growth is thereby ceased. In woody plants such as Aesculus hippocastanum (Fig. 6F), terminal RU production influences the branching pattern of the canopy. In seasons without flower production, shoots are monopodial, whereas they are sympodial in flowering seasons.
Closed FSSs are either characterized by a single terminal unit, i.e. an inflorescence (e.g. Aesculus hippocastanum, Fig. 6F), a flower (e.g. Magnolia grandiflora, not shown) or a FU (e.g. Parrotiopsis jacquemontiana, Fig. 6S), or they bear several to many RUs which are arranged according to the monopodial and/or sympodial architecture of the respective FSS (Figs 6G–I and 8F, G). In extreme monochasial sympodia (Figs 6I and 8G) the terminal RU of each branch order is overtopped by its uppermost axillary shoot, resulting in an apparent main axis but which is composed of sympodial units. This can be easily recognized by the position of the leaves which is opposite to the RU. Closed FSSs are often characterized by a mixture of frondose and bracteose leaves as leaves are associated with the vegetative and bracts with the reproductive parts of the FSS (Fig. 8E).
While open FSSs usually flower in an acropetal sequence, closed FSSs also flower successively, basipetally or simultaneously.
Successive flowering is usually found in closed FSS with modular construction in which RUs are repeated on different branch orders. The terminal unit starts flowering followed by the RUs of 1st order, 2nd order and so on. The underlying branching pattern can be monopodial (e.g. Chaerophyllum bulbosum, Fig. 6G) or sympodial (Fig. 6H, I). Each time, total flowering time of the FSS is extended.
Architecture not only influences sequence and duration of flowering time, but provides a specific spatio-temporal arrangement directly affecting the plant's sexual reproductive success (e.g. see Diggle, 2003; Harder et al., 2004). If successive flowering among branch orders is combined with dichogamy and simultaneous flowering within each branch order, multicyclic dichogamy may be the result and increase the plant's chance for outcrossing (Lloyd and Webb, 1986; Schlessman and Graceffa, 2002; Narbona et al., 2005). If the RUs differ in size and sex with age as is often found in andromonoecious Apiaceae (Reuther and Claßen-Bockhoff, 2010), the FSS acts as pollen receptor first and as pollen donator in the late season (Fig. 8J).
FSSs with RUs at different branch orders were termed anthoclades by Goebel (1931). He introduced this term to point to the regular alteration between vegetative leaves and RUs. However, according to the ontogeny-based inflorescence concept, there is no alteration at all. Instead, the FSS is primarily a vegetative shoot that only produces RMs at the tip of each branch order.
Basipetal flowering can be observed in FSSs in which the terminal RU is enriched by delayed downwards developing RUs (paraclades sensu Stauffer, 1963; Figs 6J and 8H). The terminal RU develops first and then the axillary buds originating from the vegetative leaves immediately below the terminal RU subsequently start to develop and flower in basipetal direction (Fig. 9A–E). If the terminal RU flowers in acropetal sequence, the entire system is characterized by bidirectional flowering (see Lythrum salicaria, Fig. 6J).
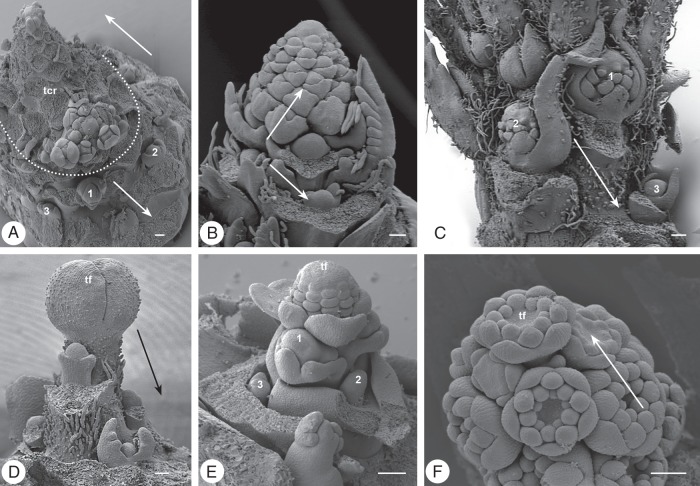
Fig. 9.
Paraclades and inflorescences. (A–D) Paraclade formation: (A) Armoracia rusticana, Brassicaceae: terminal compound raceme (tcr, dotted line) and basipetally following raceme primordia (arrow: 1–3); (B) Sanguisorba minor, Rosaceae: terminal raceme (upwards arrow) and basipetally developing buds (downwards arrow) (C) Veronica longifolia, Plantaginaceae: axillary racemes (1–3) below the terminal one with decreasing size (arrow); (D) Meconopsis cambrica, Papaveraceae: terminal flower (tf) with basipetal paraclades (arrow). (E) Aquilegia vulgaris, Ranunculaceae: terminal flower (tf) and axillary flowers in basipetal developmental sequence (1–3). (F) Inflorescence development: Deutzia gracilis, Hydrangeaceae: botryoid with premature terminal flower (tf); arrows indicate acropetal development. Scale bars = 100 µm.
Examples appear in many families, e.g. Brassicaceae (Fig. 9A), Rosaceae (Fig. 9B) and Asteraceae (Fig. 10B). Usually, on the lateral branches, the number of vegetative leaves increases and the size of the RU decreases with increasing distance from the terminal unit (Fig. 8H). Hempel and Feldmann (1994) illustrated that basipetal shoots can be induced by light in Arabidopsis thaliana, indicating that formerly vegetative buds become secondarily stimulated to flower by signals transferred from the flowering apex downwards. As the proximal branches are older than the distal ones, they have already developed leaf primordia when the reproductive stimulus changes their developmental programme. Their extended vegetative part can thus be explained by the longer lasting influence of vegetative signals compared with distal branches.
Fig. 10.
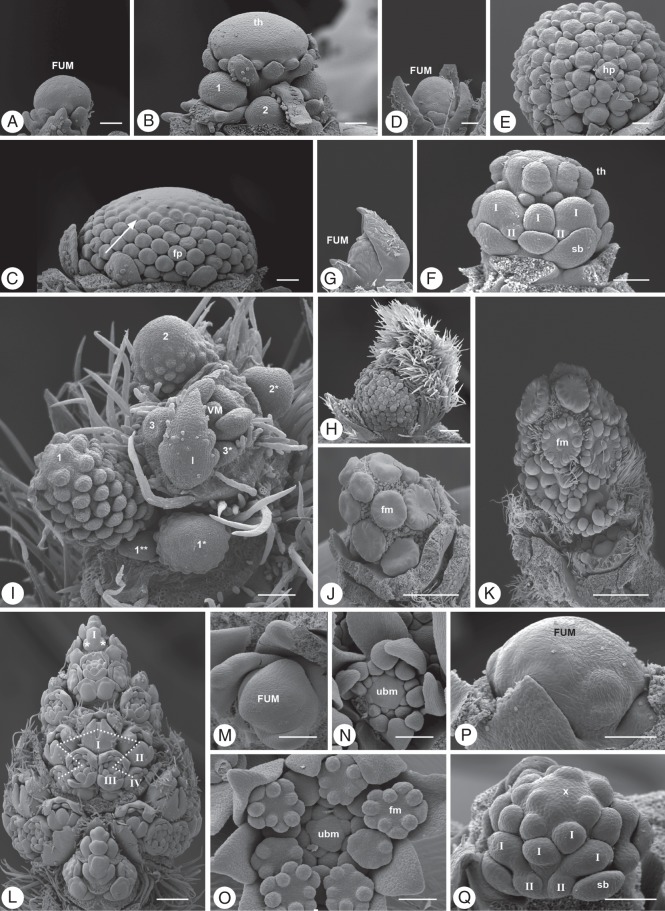
Floral units. (A–C) Matricaria dioscoidea, Asteraceae: three developmental stages showing the increasing size of the head primordium. (A) FUM, floral unit meristem; (B) th, terminal head; 1-3, basipetal formation of axillary heads; (C) fp, flower primordium; arrow, acropetal flower formation. (D, E) Echinops bannaticus, Asteraceae: (D) young floral unit meristem already fractionating head meristems; (E) young secondary head composed of head primordia (hp). (F) Polycalymma stuartii, Asteraceae: terminal head (th) preceding the lateral ones which continue to fractionate heads of higher order (I, II; sb. subtending bract). (G, H) Davidia involucrata, Cornaceae: two developmental stages showing the increase in primordium size during fractionation. (I) Mimosa pudica, Fabaceae–Mimosoideae: open flowering shoot system (VM) with heads in different developmental stages [1–3; same number and asterisks indicate accessory floral units in same leaf (l) axil]. (J, K) Parrotiopsis jacquemontiana, Hamamelidaceae: almost synchronous development of flowers (fm) in young and older developmental stages. (L) Salvia verticillata, Lamiaceae: thyrse (spike with cymes) developing along the main axis in acropetal order (a lateral thyrse arises from a leaf axil below the marked cyme); I–IV flower order, asterisk: lateral meristems of a young dichasial cyme. (M–O) Chaerophyllum bulbosum, Apiaceae: (M) young FUM; (N) umbellet meristem (ubm) with flowers in bud; (O) umbellet (ubm) with developing flowers (fm). (P, Q) Astrantia major, Apiaceae: two different developmental stages showing fractionation (I, II: umbel orders). Scale bars: (A–F, M–Q) = 100 µm, (G, H) = 200 µm, (J, K) = 500 µm. All pictures of the same species at the same scale.
Simultaneous flowering is often found in RUs of the same branch order. It indicates that the stimulus to produce RUs affects all shoot tips of the FSSs at the same time. Consequently, in much branched FSS, for example in Hypericum perforatum (Fig. 8I), the first produced, proximal branches bear more vegetative leaves below their terminal RU than the later, distal ones. Dependent on the vegetative vigour of the single branches a slight median promotion and/or a delayed development of the most proximal branches occasionally occur.
Meristem development – types of RUs
Depending on their meristem of origin (Figs 1 and 4) RUs are inflorescences, flowers or FUs (Fig. 5). Flowers and FUs appear solitarily or are part of an inflorescence.
Inflorescence meristems
Inflorescences are RUs originating from RMs with limited activity. All IMs begin with an indeterminate central zone organization (Fig. 4C: light green). Four basic types of inflorescences can be distinguished: compound and simple racemes (Fig. 5C1D1, C2D1), panicles and botryoids (Fig. 5C1D2, C2D2). They differ in the final meristematic condition, either persisting indeterminate (with central zone histology; Fig. 4D: light green) or becoming determinate (with mantle core histology; Fig. 4D: red), giving rise to open (terminal flower absent) or closed (terminal flower present) inflorescences (Fig. 5). They also differ in their meristem activity producing compound (Fig. 4C1) or simple (Fig. 4C2) inflorescences.
Flowers and partial inflorescences are often subtended by bracts which appear before their axillary meristems (Fig. 2N). However, both structures can also arise simultaneously from a single roundish primordium (Fig. 7H). In the extreme case, the subtending bract is dislocated by the rapid growth of its axillary meristem (Fig. 7I) resulting in a concaulescent position in the adult stage. It is evident that the earlier the axillary meristem develops, i.e. the higher the reproductive stimulus at the respective site, the more suppressed is the develomental programme of the associated leaf.
Open inflorescences: compound and simple racemes (Fig. 5C1D1, C2D1) arise from indeterminate IMs that continuously produce lateral partial inflorescences and flowers (or FUs) in acropetal direction (Fig. 4C). Stem cells are a substantial part of the IM and prevent terminal flower production. The meristem finally ceases growth and the inflorescence often ends in a sterile tip (‘open I’-meristem sensu Bull-Hereñu and Claßen-Bockhoff, 2011a, b).
If the axillary meristems subtended by bracts directly merge into flower production, a simple raceme is formed (Figs 4C2D1, 5C2D1 and 6L). ‘Raceme’ is used here as a collective name for all its derivatives, ranging from typical racemes (pedicillate flowers on elongated axis) to spikes (sessile flowers on elongated axis) and spadices (sessile flowers on thickened elongated axis) and from umbel-like (pedicillate flowers on short axis) to head-like racemes (sessile flowers on short, often thickened axis). In the extreme case, the latter resemble true umbels and heads arising from FUMs (Fig. 5E1, E3).
If the axillary meristems produce flowers on higher branch orders, compound racemes are formed (Fig. 6K). They are characterized by a disjunct appearance based on the abrupt alteration of indeterminate raceme and determinate FMs (Fig. 5C1D1). According to Bull-Hereñu and Claßen-Bockhoff (2013), meristems producing compound racemes enlarge twice, first when the VM becomes reproductive, and second when the IM merges into terminal raceme production. This indicates that in compound inflorescences, racemes develop as entities. The disjunct appearance results from the ‘transient’ status of the main axis (and not necessarily from transient lateral meristems as supposed by Prusinkiewicz et al., 2007) producing lateral racemes before merging into the terminal one.
In rare cases, the tip of the indeterminate IM returns to vegetative growth, resulting in proliferating inflorescences (Fig. 4D: dotted arrow). Examples are Actinodium cunninghammii Schau. (Claßen-Bockhoff et al., 2013), a woody plant continuing growth after flowering, and all inflorescences producing a tuft of sterile leaves above their flowers before ceasing growth (e.g. Ananas comosus, Bartholomew, 1977; Salvia viridis, Fig. 6N). Proliferating inflorescences (Fig. 4D1) differ from proliferating FSSs (Fig. 4B1) by secondarily resuming vegetative growth at a previous IM.
Rarely, terminal flowers appear in open inflorescences, either as naturally occurring peloria (e.g. Digitalis purpurea, Rudall and Bateman, 2003) or induced in mutants of Arabidopsis (e.g. Shannon and Meeks-Wagner, 1991), Antirrhinum (Bradley et al., 1996) or Pisum (Singer et al., 1999; reviewed by Bull-Hereñu and Claßen-Bockhoff, 2011a). It is assumed that in these cases the tip of the IM is changed from an indeterminate (central zone histology) to a determinate (mantle core histology) condition, most likely by a collapse of the WUS/CLA3 regulatory loop and the associated loss of stem cell activity (Carles and Fletcher, 2003).
Closed inflorescences: panicles and botryoids (Fig. 5C1D2, C2D2) arise from an IM that becomes determinate during development (Fig. 4C1D2, C2D2; Kwiatkowska, 2008). Correspondingly, the terminal and all lateral meristems produce flowers. Contrary to open inflorescences, the number of flowers in closed inflorescences is limited as no meristem is left after flower production. Correspondingly, the initial size of the meristem is correlated with the number of flowers being produced (Bull-Hereñu and Claßen-Bockhoff, 2011b, 2013).
If only flowers of 1st order appear, a botryoid is formed (Fig. 6P, Q). If also flowers of higher orders appear, a panicle results, i.e. a branched and all-around closed inflorescence (Fig. 6O). Panicles have often a conjunct, pyramidal shape with an upward-decreasing branching pattern. This is based on the meristem condition being used completely (Bull-Hereñu and Claßen-Bockhoff, 2011b). If all flowers are arranged in a plane, the panicle is called a corymb (e.g. Sambucus nigra), whereas if the proximal branches overtop the distal ones, it is called an anthela (e.g. Filipendula ulmaria).
According to the general acropetal activity of IMs, flowering sequence is usually acropetal in open and closed inflorescences. However, terminal units, i.e. terminal racemes and terminal flowers (Fig. 9F), tend to develop faster than their immediate neighbours. In long inflorescences, the meristem might be too weak to fully develop all flowers, resulting in floral buds at the tip of open inflorescences and only facultatively developed terminal flowers in closed inflorescences (e.g. Agrimonia eupatoria).
Floral unit meristems
FUMs are here considered for the first time. They lack the capacity of IMs to produce organs in an acropetal order. Instead, they resemble FMs in their ‘naked’ shape, in the process of fractionating subunits and in mantle core histology (Fig. 1C; Table 2). Fractionation is fast (sometimes almost simultaneous) and often occurs along with meristem enlargement due to polydirectional mitotic activity in the meristem (Figs 3G–K and 10). As a consequence, the meristem does not elongate but expands to all sides, usually maintaining its original flat or globular shape.
Centripetal fractionation is found in the FUs of Asteraceae (Figs 1C and 10A–F) and Apiaceae (Fig. 10M–Q). Diversity in the internal structure of a centripetal FU results from the number of fractionation steps (simple vs. compound FUs), the absence vs. presence of a terminal unit (open vs. closed FUs), the degree of density vs. intercalary meristem activity and bract development.
If flowers are produced after the first step of fractionation, simple FUs (heads, umbels) arise. If the first fractionated sub-meristems continue to subdivide, secondary FUs develop (Fig. 6T). Secondary heads originate from a single FU meristem which enlarges during fractionation providing space for more heads (Fig. 10D–F; Claßen-Bockhoff, 1992). They should not be confused with compound heads being inflorescences with heads at flower position.
In Asteraceae, simple heads are open, while secondary heads usually end in a terminal unit (Kunze, 1969; Claßen-Bockhoff, 1996). In secondary units, heads are densely aggregated (e.g. Dyssodia decipiens, Fig. 6T) or even arranged on a secondary receptacle (e.g. Gilberta tenuifolia, Myriocephalum gracilis, Craspedia globosa, Claßen-Bockhoff, 1996). This increases the analogous similarity between secondary heads, simple heads and flowers. Repeated fractionation tends to reduce the meristem size of the individual heads. As a consequence, the number of flowers per head decreases, finally resulting in ‘one-flowered units’ (Kunze, 1969; Claßen-Bockhoff, 1996). From the developmental point of view, these one-flowered units develop like terminal flowers using the whole existing meristem tissue (e.g. Echinops exaltatus, Leins and Gemmeke, 1979; Gundelia tournefortii, Claßen-Bockhoff et al., 1989).
In Apiaceae, the umbel (usually termed ‘umbellet’) can be open (e.g. Anthriscus sylvestris), closed (e.g. Chaerophyllum bulbosum) or flexible (e.g. Daucus carota) (Reuther and Claßen-Bockhoff, 2010). Investigations in Daucus carota (Bull-Hereñu and Claßen-Bockhoff, 2010) indicate that terminal flower production depends on spatial conditions at the determinate FUM (Fig. 4E1–6) and should not be confused with the transition of indeterminate to determinate meristems in IMs (Fig. 4C2D2). The secondary umbels (called ‘umbel’ in Apiaceae) are usually open, i.e. they do not have a terminal umbellet. Most likely, this is due to spatial constraints but relevant studies are presently lacking.
In contrast to the dense aggregation in Asteraceae (Fig. 5E3, E4), flowers and umbellets in Apiaceae are petiolated due to intercalary meristem activity (Fig. 5E1, E2). They thus simulate short racemes, i.e. inflorescences (Fig. 5C2D1). In the extreme case, as seen in Dorema aucheri from Iran (Ajani and Claßen-Bockhoff, 2012), umbellets can be arranged at up to 30-cm-long shoots representing a single umbel with considerable internode elongation.
Bracts (paleae, involucellar bracts) are usually minute or lacking completely. However, at the outer margin of the FUMs involucral bracts appear. In Asteraceae, they are usually present, whereas they may be absent in Apiaceae. As bud protectors (green bracts), attractors (petaloid bracts) or propagators (spiny bracts), they act like sepals and petals transforming the FU into a flower-like unit or pseudanthium (Troll, 1928; Claßen-Bockhoff, 1990, 1991a). Pseudanthia also result from enlarged ray flowers appearing at the periphery of simple (e.g. Bellis perennis) and secondary heads (e.g. Dyssodia decipiens, Fig. 6T) and of simple (e.g. Tordylium apulum) and secondary umbels (e.g. Artedia squamata).
Further examples of centripetal FUs are Saururus cernuus and Houttuynia cordata (both Saururaceae; Tucker, 1979, 1981), Parrotiopsis jacquemontiana (Hamamelidaceae, Figs 6S and 10J, K), Mimosa pudica (Fabaceae-Mimosoideae, Figs 6R and 10I) and Davidia involucrata (Cornaceae; Fig. 10G, H). They all represent pseudanthia and illustrate that FUs not only resemble flowers at the meristem level but also in their outer appearance.
Centrifugal fractionation is usually the result of cymose branching. Diversity results from the degree of branching (number of branch orders), from number and position of the consecutive meristems (mono-/dichasial cincinnus, bostryx, drepanium, rhipidum) and from position of the centrifugally developing FUM (solitary or part of an inflorescence).
Solitary FUs are represented by the cyathia in Euphorbia (Prenner and Rudall, 2007); further likely examples include Diplolaena spp. (Rutaceae; Claßen-Bockhoff et al., 1991), Dorstenia spp. (Moraceae), Hemerocallis spp. (Xanthorrhoeaceae) and Tacca chantrieri (Taccaceae) (Fig. 5E5, E6).
FUs as parts of an inflorescence are cymes. Cymes are traditionally defined as lateral branches of an inflorescence producing flowers exclusively from the axils of their one (monochasial cyme) or two prophylls (dichasial cyme) (Endress, 2010). From the developmental point of view, cymes originate from FUMs and are thus ontogenetically more similar to flowers than to inflorescences. Both FMs and FUMs are determinate. But whereas FMs are used completely for floral organ production, cymose FUMs divide into the terminal flower and one or two lateral parts maintaining meristematic activity (Fig. 10L: I, asterics). Each of the lateral parts gives rise to a subtending bract and an axillary meristem from which repetitive di- and/or monochasial branching may continue. By retaining part of the meristematic activity for further branching, cymes are theoretically able to produce an infinite number of flowers.
Cymes are not involved in the basic branching pattern of the inflorescence. Consequently, cymes appear in all inflorescence types, i.e. in compound and simple racemes or spikes (e.g. Lythrum salicaria, Fig. 6J; Salvia pratensis, Fig. 6M; S. verticillata, Fig. 10L), panicles and botryoids (e.g. Ricinus communis, Fig. 6P). They usually produce flowers, but in the thyrse of Polycalymma stuartii (Fig. 6U), an Australian member of the Angianthinae (Asteraceae), they produce heads. The IM segregates a few lateral meristems and a large terminal head primordium (Fig. 10F: th). The terminal head develops rapidly, whereas the lateral meristems divide into head meristems of 1st order (Fig. 10F: I) and lateral parts giving rise to head primordia of 2nd order and their associated prophylls (Fig. 10F: II). Cymose branching starts dichasial and ends in monochasial branches producing heads up to the 5th (–9th) order (Claßen-Bockhoff, 1996).
Traditionally, inflorescences with cymes were termed thyrses (Endress, 2010) and contrasted with panicles (Troll, 1964; Weberling, 1989). However, according to the ontogeny-based concept neither panicles and thyrses nor thyrses among themselves are equivalent: panicles represent one of the four basic types of inflorescence architecture (irrespective of ending in flowers or cymes) (Fig. 4C1D2) and thyrses represent any type of inflorescence with cymes. Moreover, the term thyrse has been used to designate FSSs, inflorescences or FUs. To avoid confusion, the term thyrse should be restricted to the inflorescence level and not be mixed with sympodially branched FSSs or cymose FUs (e.g. cyathium in Euphorbia, Fig. 5 E6).
Simultaneous fractionation. A third type of fractionation has been observed in Thalia geniculata and T. dealbata, (Marantaceae) inflorescences of which bear flower pairs characteristic for the family (Kunze, 1985; Kirchoff, 1986; Claßen-Bockhoff, 1991b). The two flowers are mirror images of each other, lack subtending bracts and develop simultaneously. Ontogenetic studies indicate that both flowers originate from a single FUM splitting completely into two halves, i.e. without leaving any rest (Wasner and Claßen-Bockhoff, 2010). A similar mode of fractionation appears in the male units of Ricinus communis (Euphorbiaceae). Their meristems divide into several sub-meristems which each split completely into two halves. With increasing meristem size, all fractions and derivates repeat splitting before anthers are formed. While Prenner et al. (2008) interpret the male unit as a flower with stamen fascicles, Claßen-Bockhoff and Frankenhäuser (2008) identify it at as a FU with simultaneous steps of fractionation up to the 4th order. This interpretation is supported by ontogenetic studies in Urticaceae and Moraceae (Bernbeck, 1932), whose FUMs show a similar type of repeated simultaneous fractionation. The given examples (and flower pairs in general) may be explained by being extreme forms of cymes or racemes (see Douglas and Tucker, 1996), but relevant investigations are largely lacking.
Conceptual framework
By setting the focus on ontogeny, inflorescence analysis proceeds in four steps. First, the flowering shoot system (FSS) has to be identified as the general reference system followed by, second, the position of reproductive units (RUs) within the FSS, third, the type of RU and, fourth, the developmental processes shaping the RU phenotype. We here restrict the term ‘inflorescence’ to a RU originating from an IM and contrast it with FSSs and FUs.
Inflorescences originate from IMs which ontogenetically differ from VMs by enlargement and limited activity. IMs are indeterminate at least in early stages, maintaining the capacity to elongate by acropetal segregation. Inflorescences are characterized by bracts and only rarely resume vegetative growth.
Basic types of inflorescences are defined by their degree of branching (meristem activity) and their meristem tips (indeterminate vs. determinate). They include simple and compound racemes, botryoids and panicles and their derivates. Thyrses arise from all basic inflorescences if cymes are produced instead of flowers.
Inflorescences appear in terminal and/or lateral positions within an FSS. In perennial and woody plants, IMs may be produced in the preceding season, hibernate and come to flower without producing a vegetative shoot system.
FSSs originate from VMs and include all flower arrangements produced in one season. They are leafy and often continue growth after the production of lateral RUs (proliferating FSS). FSSs show all kinds of monopodial and sympodial branching patterns and produce RUs at terminal and lateral positions of diverse orders. Consequently, a single FSS usually produces more than one RU (either being an inflorescence, FU or single flower). RUs of different orders develop successively, thereby substantially extending total flowering time of the FSS.
FUs originate from FUMs, which differ from FMs by undergoing fractionation before floral organ production. As the meristem tip is determinate, i.e. not able to elongate by segregation, space for ongoing flower production is provided by meristem expansion. According to present knowledge, all cymes and a high number of flower-like units (pseudanthia) originate from FUMs.
General reference system
Although Goebel (1931) had already distinguished ‘synflorescences’ (a leafy FSS with lateral inflorescences) from ‘inflorescences’ (a bracteose flower-bearing unit different from the vegetative part of the plant), little attention has been given to the different hierarchical levels of flowering in the concept of synflorescences introduced by Troll (1964). This concept, dominating decades of inflorescence morphology, is most important in pointing to careful morphological analyses (see, for example, Weberling, 1989), but failed in clearly separating inflorescences from FSSs. Instead, both units were united under the term ‘synflorescence’. Consequently, foliage and flowering sequence were disregarded as useful criterions for inflorescence identification and proliferation was misinterpreted (see Claßen-Bockhoff, 2000).
Foliage. At the beginning of the 20th century, foliage played an important role in characterizing reproductive systems (Parkin, 1914; Pilger, 1921, 1922; Goebel, 1931). However, with the definition of synflorescences as FSSs, foliage became obsolete and ‘frondo-bracteose inflorescences’ were widely accepted. According to the ontogenetic view, leaves arise from VMs and bracts from RMs. Frondo-bracteose foliage thus most likely characterizes only FSSs, although additional studies are needed to confirm this view. Endress and Doyle (2009) recently discussed the problem using the example of solitary flowers originating from vegetative leaf axils. They found the degree of branching to be more fundamental than foliage and subsumed these flowers under the term ‘raceme’. However, considering ontogeny, each of these flowers arises from its own RM, rendering the example a monopodial FSS with single flowers instead of an inflorescence.
Flowering sequence. Parkin (1914) and Maresquelle (1970) assumed that inflorescences evolved from terminal flowers by basipetal enrichment. To explain the genesis of acropetal flowering sequence, Maresquelle (1970) and Sell (1976) introduced the process of racemization. However, there is no ontogenetic evidence that supports that such a process actually exists. Instead, acropetal flowering sequence reflects the normal acropetal growth in FSSs and acropetal organ segregation in inflorescences and FUs. Basipetal flowering sequence, by contrast, is rarely found in inflorescences and FUs (e.g. racemes in Sanguisorba minor, secondary heads in Echinops spp.) and is not fully understood. It is, however, common in FSSs and interpreted as the expression of a downward extending reproductive impulse stimulating axillary VMs to become reproductive (Hempel and Feldman, 1994). Stauffer (1963) concluded that basipetal RUs should be handled separate from the main inflorescence and called ‘paraclades’.
Proliferation. Proliferating shoot systems (‘intercalary inflorescences’ sensu Parkin, 1914; ‘proliferating synflorescences’ sensu Troll, 1964; Weberling, 1989; ‘auxotelic conflorescences’ sensu Briggs and Johnson, 1979) have been controversially interpreted in the past. While Briggs and Johnson (1979) postulated a ‘flexible condition’ at the shoot apical meristem to either proliferate or cease growth, Troll (1964) and Weberling (1989) understood proliferation as a derived process following the loss of the terminal flower (truncation) and the return of the meristem tip to vegetative growth. When studying the reproductive systems of basal angiosperms, which often have ‘proliferating synflorescences’, Weberling (1988) correspondingly found many highly derived branching patterns. However, following the ontogenetic view, proliferating FSSs are flowering monopods with a high vegetative vigour only coming to flower at lateral meristems (see also Hallé et al., 1978; Sell, 1982; Claßen-Bockhoff, 2000).
Inflorescences and FUs
Confusing terminology is one of the major problems in inflorescence morphology (Endress, 2010). It is mainly based on inadequate definitions, mixing of reference levels and different terminological schools. One of the main aims of the present approach is its contribution to solve these problems.
Terminology. Inflorescence diversity is reduced to four basic types. These types differ from the recently used main classes of reproductive systems as ‘cymose and racemose inflorescences, thyrses and panicles’ (Prenner et al., 2009) or ‘racemes, cymes and panicles’ (Prusinkiewicz et al., 2007; Castel et al., 2010; Rijpkemaa et al., 2010) which all refer to the branching pattern and the presence vs. absence of a terminal flower as the most important parameters. Our ontogenetic studies confirm the significance of these two parameters, but confine their use to certain reference levels. Although branching patterns look similar, sympodial branching within an FSS is not the same as cymose branching within an FU and monopodial FSSs are not the same as racemes or heads. Instead, the basic morphological principles (e.g. axillary branching) and the same physiological processes (e.g. hormone regulation) are found repetitively on different meristematic units. Besides, thyrses are not considered as a basic inflorescence type, as their characteristic, the cymose branching, is a late ontogenetic step compared with the initial stages of branching (see also Endress, 2010). A thyrse is instead a derivate from each of the four basic inflorescences, explaining its diversity from compound to simple and from open to close examples.
Terminal flowers vs. terminal FUs. The identification of FUs as substitutes for flowers results in distinguishing, for example, racemes (developing from IMs) from heads (arising from FUMs), or compound heads (compound racemes or panicles with heads instead of flowers) from secondary heads (heads with heads instead of flowers). It also results in a restricted meaning of the terminal flower compared with a terminal unit. While open and closed inflorescences are found to develop differentially from the start and either maintain the indeterminate meristem or finally merge into a determinate stage (see Bull-Hereñu and Claßen-Bockhoff, 2011a, b, 2013), open and closed FUs both originate from determinate meristems. These meristems share main qualities with FMs and only differ from them by passing through fractionation before floral organ production. On the molecular level, this means that the expression of floral identity genes is suppressed in favour of meristem identity genes. However, little is known about the molecular regulation in FUMs. Data mainly gained from studies in Gerbera (Asteraceae) indicate that the genetic regulation of FUMs is different from that in the IMs of the model organisms Arabidopsis and Antirrhinum (e.g. Teeri et al., 2006; Wang and Li, 2008). First ontogenetic findings point to possible spatial constraints allowing or hindering terminal flower formation (Bull-Hereñu and Claßen-Bockhoff, 2010, 2011a).
It is evident that equating FUs with flowers will have implications for homology and the interpretation of character transformation. For instance, a panicle is a closed inflorescence irrespective of ending in a terminal flower or in a head (see discussion in Kusnetzova, 1988; Kunze, 1989). The evolutionary transformation from the assumed ancestral thyrsoid in the Menyanthaceae–Goodeniaceae–Calyceraceae–Asteraceae clade of Asterales into the head of Asteraceae (Pozner et al., 2012) need not comprise suppression of branching, internodes and the terminal flower but could be easily explained by a simple change in meristem condition substituting the terminal flower by a head. Likewise, the evolution of secondary heads may not need the steps of enrichment, racemization and truncation demanded by Maresquelle (1970; see also Pozner et al., 2012), but could be the mere consequence of fractionation (Claßen-Bockhoff, 1992).
Creating formulas for FSSs and RUs
Considering the main steps of inflorescence analysis, i.e. the definition of the FSS and the identification of position, type and developmental pathways of RMs, each associated with a limited number of alternatives, it is possible to classify FSSs and RUs in a formulistic way. Based on the ontogenetic decisions compiled in Fig. 4, this is exemplarily shown for the pictures illustrated in Fig. 6 (Table 3).
Ontogenetic transitions and the transient model
The use of the proposed concept is limited by the existence of ontogenetic transitions. These appear between VMs and RMs, between indeterminate and determinate IMs and between FMs and FUMs. Ontogenetic transitions illustrate that different RUs are not distinct morphological categories but continuously changing entities. In this sense, Linné introduced the term ‘inflorescence’ in the continuous form (see also Hallé et al., 1978). For practical reasons only, we define certain stages as types. Having this in mind, the existence of transitional stages offers the opportunity to reconstruct the evolutionary transition from one RU to another.
Salvia (Lamiaceae) offers an example for the gradual transition from VMs to RMs (Czarny and Claßen-Bockhoff, 2010). In S. viridis (Fig. 6N), the shoot apical meristem does not show the significant enlargement of RMs and produces sterile leaves on top of the flowering part. The ‘inflorescence’ could thus be interpreted as a proliferating FSS with axillary cymose FUs. However, as paraclades appear, it can also be interpreted as a proliferating terminal thyrse. S. pratensis (Fig. 6M) and S. verticllata (Fig. 10L) show clear IMs associated with bracts and limited growth. They have thyrses (open spikes with cymes). Although different types of flowering systems among closely related species may be unlikely on first view, it is to be expected under a dynamic evolutionary view. The evolutionary change between S. viridis (and few proliferating species as S. leucantha) and other Salvia species might include the single step from passing the shoot apical meristem from the vegetative to the reproductive state.
Gradual transitions appear within determinate IMs initially starting from indeterminate ones. At present, little is known about the time at which the IM of a closed inflorescence changes its activity pattern. Gradual transitions can be also found between IMs of few-flowered botryoids and FUMs as both meristem types are indeterminate and do not elongate.
Finally, transitions may arise between FMs and FUMs which differ only in the degree of fractionation before merging into floral organ production. They are usually easy to distinguish by their one-flowered vs. several-flowered presentation. However, considering FM expansion (Ronse de Craene and Smets, 1991), dédoublement (Ronse de Craene and Smets, 1996) and fascicle formation (Rudall, 2008) on the flower side, and extreme flower reduction (Prenner and Rudall, 2007), fasciation (Sokoloff et al., 2007) and abnormal terminal structures (Rudall and Bateman, 2003; Sokoloff et al., 2006) on the FU side, it may be difficult to clearly recognize the boundary between flowers and FUs.
Regarding the transition from VMs via indeterminate and determinate IMs to FMs and FUMs, ontogeny largely supports the basic idea of the transient model introduced by Prusinkiewicz et al. (2007). The model is based on a factor of ‘vegetativeness’ (veg), decreasing from the VM to the IM and FM. At each time of development, the veg factor determines whether the meristem is still vegetative enough to remain indeterminate or weak enough to merge into a flower. The model easily explains the transition from VMs to RMs and from indeterminate to determinate IMs (referring to LFY/TFL), but not the relationship between FMs and FUMs. As FUMs are not known from basal angiosperms, we conclude that FUMs evolved from FMs by expansion.
CONCLUSIONS
In the present paper, the diversity of reproductive systems is investigated from the ontogenetic perspective. The most important results are the distinction of hierarchical levels of flowering (FSS, inflorescence, FU) and the identification of two different flower-producing meristems (IMs and FUMs). They provide a definite reference framework for all levels of flowering and clear definitions and terms. By reducing diversity it is even possible to formularize RUs (Table 3).
Equally important is the dynamic view of the flowering plant that produces RUs on different hierarchical levels. Passing from the vegetative to the flowering stage, the continuous decrease of vegetative qualities is reflected in the decrease of foliage, internode elongation and meristem tip activity and the increase in branching rate, meristem expansion and responses to spatial constraints due to density. On each level, i.e. the VM, IM and FUM, the same basic morphological and physiological conditions affect ontogeny and produce similar patterns but which are analogous. By discriminating FSSs from inflorescences and FUs, the concept enables us to identify and compare the same parts of the plant which is the precondition for homology.
In future, the ontogeny-based approach may contribute to disentangle those conflicts which presently preclude us from answering basic questions as to the origin and evolution of angiosperm inflorescences, their status as transitional stages between vegetative growth and flower production, and their homologies within phylogenetic lineages.
ACKNOWLEDGEMENTS
Our data were supplemented by data coming from student research projects and diploma theses. We particularly thank Melanie Arndt, Torsten Collet, Silke Czarny and Eileen Wasner (all Mainz) for their help and Stefan Gleissberg (Athens, OH) for providing us with Fig. 1A. We are very grateful to Barbara Dittmann and Madeleine Junginger for producing the histological sections and to the designers of our institute, Doris Franke and Anne Korek, for illustration and schemes.
LITERATURE CITED
- Arber EAN, Parkin J. The origin of Angiosperms. Botanical Journal of the Linnean Society. 1907;38:29–80. [Google Scholar]
- Ajani Y, Claßen-Bockhoff R. Inflorescence architecture and sex distribution in the endemic Apiaceae Dorema aucheri Boiss. from Iran. Proceedings of the 21st International Symposium ‘Biodiversity and Evolutionary Biology’. 2012;85 Mainz: [Google Scholar]
- Bäurle I, Laux T. Apical meristems: the plant's fountain of youth. Bioessays. 2003;25:961–970. doi: 10.1002/bies.10341. [DOI] [PubMed] [Google Scholar]
- Bartholomew DP. Inflorescence development of pineapple (Ananas comosus [L.] Merr.) induced to flower with ethephon. Botanical Gazette. 1977;138:312–320. [Google Scholar]
- Benlloch R, Berbel A, Serrano-Mislata A, Madueño F. Floral initiation and inflorescence architecture: a comparative view. Annals of Botany. 2007;100:659–676. doi: 10.1093/aob/mcm146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernbeck F. Vergleichende Morphologie der Urticaceen- und Moraceen-Infloreszenzen. Botanische Abhandlungen. 1932;19:1–100. [Google Scholar]
- Bernhard A. Flower structure, development, and systematics in Passifloraceae and in Abatia (Flacourtiacae) International Journal of Plant Sciences. 1999;160:135–150. [Google Scholar]
- Bradley D, Carpenter R, Copsey L, Vincent C, Rothstein S, Coen E. Control of inflorescence architecture in Antirrhinum. Nature. 1996;379:791–797. doi: 10.1038/379791a0. [DOI] [PubMed] [Google Scholar]
- Briggs B, Johnson L. Evolution in the Myrtaceae – evidence from inflorescence structure. Proceedings of the Linnean Society of New South Wales. 1979;192:157–272. [Google Scholar]
- Bull-Hereñu K, Claßen-Bockhoff R. Developmental conditions for terminal flower production in apioid umbellets. Plant Diversity and Evolution. 2010;128:221–232. [Google Scholar]
- Bull-Hereñu K, Claßen-Bockhoff R. Open and closed inflorecences: more than simple opposites. Journal of Experimental Botany. 2011a;62:79–88. doi: 10.1093/jxb/erq262. [DOI] [PubMed] [Google Scholar]
- Bull-Hereñu K, Claßen-Bockhoff R. Ontogenetic course and spatial constraints in the appearance and disappearance of the terminal flower in inflorescences. International Journal of Plant Sciences. 2011b;172:471–498. [Google Scholar]
- Bull-Hereñu K, Claßen-Bockhoff R. The ontogenetic base for the transient model of inflorescence development. Annals of Botany. 2013;112:1543–1551. doi: 10.1093/aob/mct022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carles CC, Fletcher JC. Shoot apical meristem maintenance: the art of a dynamic balance. Trends in Plant Science. 2003;8:394–401. doi: 10.1016/S1360-1385(03)00164-X. [DOI] [PubMed] [Google Scholar]
- Castel R, Kusters E, Koes R. Inflorescence development in petunia: through the maze of botanical terminology. Journal of Experimental Botany. 2010;61:2235–2246. doi: 10.1093/jxb/erq061. [DOI] [PubMed] [Google Scholar]
- Claßen-Bockhoff R. Pattern analysis in pseudanthia. Plant Systematics and Evolution. 1990;171:57–88. [Google Scholar]
- Claßen-Bockhoff R. Anthodien, Pseudanthien und Infloreszenzblumen. Beiträge zur Biologie der Pflanzen. 1991a;66:221–240. [Google Scholar]
- Claßen-Bockhoff R. Untersuchungen zur Konstruktion des Bestäubungsapparates von Thalia geniculata L. (Marantaceae) Botanica Acta. 1991b;104:183–193. [Google Scholar]
- Claßen-Bockhoff R. Florale Differenzierung in komplex organisierten Asteraceenköpfen. Flora. 1992;186:1–22. [Google Scholar]
- Claßen-Bockhoff R. Functional units beyond the level of the capitulum and cypsela in Compositae. In: Cagliari PDS, Hind DJN, editors. Compositae: biology & utilization. Vol. 2. Kew: Royal Botanic Gardens; 1996. pp. 129–160. [Google Scholar]
- Claßen-Bockhoff R. Inflorescences in Bruniaceae. With general comments on inflorescences in woody plants. Opera Botanica Belgica. 2000;12:5–310. [Google Scholar]
- Claßen-Bockhoff R, Frankenhäuser H. The male flower in Ricinus communis – a pseudanthium? Proceedings from the 19th International Symposium ‘Biodiversity and Evolutionary Biology’. 2008;51 Göttingen: [Google Scholar]
- Claßen-Bockhoff R, Heller A. Floral synorganisation and secondary pollen presentation in four Marantaceae from Costa Rica. International Journal of Plant Sciences. 2008;169:745–760. [Google Scholar]
- Claßen-Bockhoff R, Armstrong JA, Ohligschläger M. The inflorescences of the Australian genera Diplolaena R. Br. and Chorilaena Endl. (Rutaceae) Australian Journal of Botany. 1991;39:31–42. [Google Scholar]
- Claßen-Bockhoff R, Froebe HA, Langerbeins D. Die Infloreszenzstruktur von Gundelia tournefortii L. (Asteraceaea) Flora. 1989;182:463–479. [Google Scholar]
- Claßen-Bockhoff R, Ruonala R, Bull-Hereñu K, Marchant N, Albert V. The unique pseudanthium of Actinodium (Myrtaceae) – morphological reinvestigation and possible regulation by CYCLOIDEA-like genes. EvoDevo. 2013 doi: 10.1186/2041-9139-4-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes FAL. Apical meristems. Oxford: Blackwell; 1961. [Google Scholar]
- Czarny S, Claßen-Bockhoff R. Cyme diversity in the genus Salvia (Lamiaceae) – an ontogenetic approach. Proceedings from the 20th International Symposium ‘Biodiversity and Evolutionary Biology’. 2010;81 Vienna: [Google Scholar]
- Diggle PK. Architectural effects on floral form and function: a review. In: Stuessy TF, Mayer V, Hörandl E, editors. Deep morphology. toward a renaissance of morphology in plant systematics. Koeltz: Königstein; 2003. pp. 63–80. [Google Scholar]
- Douglas AW, Tucker SC. Inflorescence ontogeny and floral organogenesis in Grevilleoideae (Proteaceae), with emphasis on the nature of the flower pairs. International Journal of Plant Sciences. 1996;157:341–371. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical plants. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Endress PK. Disentangling confusions in inflorescence morphology: patterns and diversity of reproductive shoot ramification in angiosperms. Journal of Systematics and Evolution. 2010;48:225–239. [Google Scholar]
- Endress PK, Doyle JA. Reconstructing the ancestral angiosperm flower and its initial specializations. American Journal Botany. 2009;96:22–66. doi: 10.3732/ajb.0800047. [DOI] [PubMed] [Google Scholar]
- Feng CM, Xiang QY, Franks RG. Phylogeny-based developmental analyses illuminate evolution of inflorescence architectures in dogwoods (Cornus s.l., Cornaceae) New Phytologist. 2011;191:850–869. doi: 10.1111/j.1469-8137.2011.03716.x. [DOI] [PubMed] [Google Scholar]
- Goebel K. Blütenbildung und Sproßgestaltung (Anthokladien und Infloreszenzen). Suppl. Vol. 2. Jena: Fischer; 1931. Organographie der Pflanzen. [Google Scholar]
- Hallé F, Oldeman RAA, Tomlinson PB. Tropical trees and forests. An architectural analysis. Berlin: Springer; 1978. [Google Scholar]
- Harder LD, Jordan CY, Gross E, Routley MB. Beyond floricentrism: the pollination function of inflorescences. Plant Species Biology. 2004;19:137–148. [Google Scholar]
- Hempel FD, Feldman LJ. Bi-directional inflorescence development in Arabidopsis thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta. 1994;192:276–286. [Google Scholar]
- Judd WS, Campbell CS, Kellogg EA, Steves PF. Plant systematics. A phylogenetic approach. Sunderland, MA: Sinauer; 1999. [Google Scholar]
- Kirchoff BK. Floral organogenesis in five genera of the Marantaceaae and in Canna (Cannaceae) American Journal of Botany. 1983;70:508–523. [Google Scholar]
- Kirchoff BK. Inflorescence structure and development in the Zingiberales: Thalia geniculata (Marantaceae) Canadian Journal of Botany. 1986;64:859–864. [Google Scholar]
- Kunze H. Vergleichend-morphologische Untersuchungen an komplexen Compositen-Blütenständen. Beiträge zur Biologie der Pflanzen. 1969;46:97–154. [Google Scholar]
- Kunze H. Die Infloreszenzen der Marantaceen und ihr Zusammenhang mit dem Typus der Zingiberales-Synfloreszenz. Beiträge zur Biologie der Pflanzen. 1985;60:93–140. [Google Scholar]
- Kunze H. Probleme der Infloreszenztypologie von W. Troll. Plant Systematics and Evolution. 1989;163:187–199. [Google Scholar]
- Kusnetzova TV. Angiosperm inflorescences and different types of their structural organization. Flora. 1988;181:1–17. [Google Scholar]
- Kwiatkowska D. Surface growth at the reproductive shoot apex of Arabidopsis thaliana: pin-formed 1 and wild type. Journal of Experimental Botany. 2004;55:1021–1032. doi: 10.1093/jxb/erh109. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska D. Flowering and apical meristem growth dynamics. Journal of Experimental Botany. 2008;59:187–201. doi: 10.1093/jxb/erm290. [DOI] [PubMed] [Google Scholar]
- Leins P, Erbar C. Flower and fruit. Morphology, ontogeny, phylogeny, function and ecology. Stuttgart: Schweizerbart; 2008. [Google Scholar]
- Leins P, Gemmeke V. Infloreszenz- und Blütenentwicklung bei der Kugeldistel Echinops exaltatus (Asteraceaea) Plant Systematics and Evolution. 1979;132:189–204. [Google Scholar]
- Lloyd DG, Webb CJ. The avoidance of interference between the presentation of pollen abd stigmas in angiosperms. I. Dichogamy. New Zealand Journal of Botany. 1986;24:135–162. [Google Scholar]
- Maresquelle HJ. Le thème évolutif des complexes d'inflorescences. Son aptitude susciter des problèmes noveaux. Bulletin de la Societé Botanique de France. 1970;117:1–4. [Google Scholar]
- Narbona E, Ortiz PL, Arista M. Dichogamy and sexual dimorphism in floral traits in the andromonoecious Euphorbia boetica. Annals of Botany. 2005;95:779–787. doi: 10.1093/aob/mci077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, Palmer JH. Changes in mitotic activity and cell size in the apical meristem of Helianthus annuus L. during the transition to flowering. American Journal of Botany. 1982;69:768–775. [Google Scholar]
- Parkin J. The evolution of the inflorescence. Linnean Society of Botany London. 1914;42:511–563. [Google Scholar]
- Pilger R. Bericht der Freien Vereinigung für Pflanzengeographie und systematische Botanik für das Jahr 1919. Berlin: 1921. Bemerkungen zur phylogenetischen Entwicklung der Blütenstände; pp. 9–77. [Google Scholar]
- Pilger R. Über Verzweigung und Blütenstandbildung bei den Holzgewächsen. Bibliotheca Botancia. 1922;90:1–38. [Google Scholar]
- Pozner R, Zanotti C, Johnson LA. Evolutionary origin of the Asteraceae capitulum: insights from Calyceraceae. American Journal of Botany. 2012;99:1–13. doi: 10.3732/ajb.1100256. [DOI] [PubMed] [Google Scholar]
- Prenner G, Rudall PJ. Comparative ontogeny of the cyathium in Euphorbia (Euphorbiaceae) and its allies: exploring the organ-flower-inflorescence boundary. American Journal of Botany. 2007;94:1612–1629. doi: 10.3732/ajb.94.10.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner G, Box MS, Cunniff J, Rudall PJ. The branching stamens of Ricinus and the homologies of the angiosperm stamen fascicle. International Journal of Plant Sciences. 2008;169:735–744. [Google Scholar]
- Prenner G, Vergara-Silva F, Rudall PJ. The key role of morphology in modelling inflorescence architecture. Trends in Plant Science. 2009;14:302–309. doi: 10.1016/j.tplants.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E. Evolution and development of inflorecence architecture. Science. 2007;316:1452–1456. doi: 10.1126/science.1140429. [DOI] [PubMed] [Google Scholar]
- Reuther K, Claßen-Bockhoff R. Diversity behind uniformity – inflorescence architecture and flowering sequence in Apiaceae-Apioideae. Plant Diversity and Evolution. 2010;128:181–220. [Google Scholar]
- Rijpkemaa AS, Vandenbusscheb M, Koes R, Heijmamsd K, Gerats T. Variations on a theme: changes in the floral ABCs in angiosperms. Seminars in Cell & Developmental Biology. 2010;21:100–107. doi: 10.1016/j.semcdb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Ronse de Craene LP. Floral diagrams. An aid to understaning flower morphology and evolution. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- Ronse de Craene LP, Smets E. The distriburtion and the systematic relevance of the androecial characters oligomery and polymery in the Magnoliophytina. Nordic Journal of Botany. 1987;7:239–253. [Google Scholar]
- Ronse de Craene LP, Smets E. The impact of receptacular growth on polyandry in the Myrtales. Botanical Journal of the Linnean Socitey. 1991;105:257–269. [Google Scholar]
- Ronse de Craene LP, Smets E. The morphological variation and systematic value of stamen pairs in the Magnoliatae. Feddes Repertorium. 1996;107:1–17. [Google Scholar]
- Rudall PJ. Monocot pseudanthia revisited: floral structure of the mycoheterotrophic family Triuridaceae. International Journal of Plant Sciences. 2003;164(Suppl.):S307–S320. [Google Scholar]
- Rudall PJ. Fascicles and filamentous structures: comparative ontogeny of morphological novelities in Triuridaceae. International Journal of Plant Sciences. 2008;169:1023–1037. [Google Scholar]
- Rudall PJ. All in a spin: centrifugal organ formation and floral patterning. Current Opinion in Plant Biology. 2010;13:108–114. doi: 10.1016/j.pbi.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Bateman RM. Evolutionary change in flowers and inflorescences: evidence from naturally occurring terata. Trends in Plant Science. 2003;8:76–82. doi: 10.1016/S1360-1385(02)00026-2. [DOI] [PubMed] [Google Scholar]
- Schlessman MA, Graceffa LM. Protogyny, pollination, and sex expression of andromonoecious Pseudocymopterus montanus (Apiaceae, Apioideae) International Journal of Plant Sciences. 2002;163:409–417. [Google Scholar]
- Schröder FG. Infloreszenzen, Synfloreszenzen und Moduln. Ein terminologischer Beitrag zur Infloreszenzmorphologie. Botanische Jahrbücher für Systematik. 1987;108:449–471. [Google Scholar]
- Sell Y. Tendances évolutives parmi les complexes inflorescentiels. Revue Générale de Botanique. 1976;83:247–267. [Google Scholar]
- Sell Y. Die komplexen racemösen Infloreszenzstrukturen bei einigen Myrtalen. Beiträge zur Biologie der Pflanzen. 1982;56:381–414. [Google Scholar]
- Sell Y. Recherches de critères pour determiner la limite entre les domains vegetative et floral. Feddes Repertorium. 1995;106:371–390. [Google Scholar]
- Seybold S. Schmeil – Fitschen. Die Flora Deutschlands und der angrenzenden Länder. 95th edn. Wiebelsheim: Quelle & Meyer; 2011. [Google Scholar]
- Shannon S, Meeks-Wagner DR. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell. 1991;3:877–892. doi: 10.1105/tpc.3.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S, Sollinger J, Maki S, et al. Inflorescence architecture: a developmental genetics approach. Botanical Review. 1999;65:385–410. [Google Scholar]
- Sokoloff DD, Rudall PJ, Remizowa M. Flower-like terminal structures in racemose inflorescences: a tool in morphogenetic and evolutionary research. Journal of Experimental Botany. 2006;57:3517–3530. doi: 10.1093/jxb/erl126. [DOI] [PubMed] [Google Scholar]
- Sokoloff DD, Oskolski AA, Remizowa MV, Nuraliev MS. Flower structure and development in Tupidanthus calyptratus (Araliaceae): an extreme case of polymery among asteroids. Plant Systematics and Evolution. 2007;268:209–234. [Google Scholar]
- Stauffer HU. Gestaltwandel bei Blütenständen von Dicotyledonen. Botanische Jahrbücher Systematik. 1963;82:216–251. [Google Scholar]
- Stebbins GL. Evolutionary trends in the inflorescence of angiosperms. Flora. 1973;162:501–528. [Google Scholar]
- van Steenis CGGJ. Definition of the concept ‘inflorescence’ with special reference to ligneous plants. Flora Malesiana Bulletin. 1963;18:1005–1007. [Google Scholar]
- Teeri TH, Uimari A, Kotilainen R, Help H, Elomaa P, Albert V. Reproductive meristem fates in Gerbera. Journal of Experimnetal Botany. 2006;57:3445–3455. doi: 10.1093/jxb/erl181. [DOI] [PubMed] [Google Scholar]
- Troll W. Organisation und Gestalt im Bereich der Blüte. Berlin: Springer; 1928. [Google Scholar]
- Troll W. Die Infloreszenzen. Typologie und Stellung im Aufbau des Vegetationskörpers. I. Jena: Fischer; 1964. [Google Scholar]
- Troll W, Weberling F. Infloreszenzuntersuchungen an monotelen Familien. Materialien zur Infloreszenzmorphologie. Stuttgart: Fischer; 1989. [Google Scholar]
- Tucker SC. Ontogeny of the inflorescence of Saururus cernuus (Saururaceae) American Journal of Botany. 1979;88:227–236. [Google Scholar]
- Tucker SC. Inflorescence and floral development in Houttuynia cordata (Saururceae) American Journal of Botany. 1981;68:1017–1032. [Google Scholar]
- Tucker SC, Grimes J. The inflorescence: introduction. The Botanical Review. 1999;65:303–316. [Google Scholar]
- von Uexküll-Gyllenband M. Phylogenie der Blütenform und der Geschlechterverteilung bei den Compositen. Bibliotheca Botanica. 1901;52:1–80. 2 plates. [Google Scholar]
- Wagenitz G. Wörterbuch der Botanik. Morphologie, Anatomie, Taxonomie, Evolution. 2nd edn. Hamburg: Nikol; 2008. [Google Scholar]
- Wang Y, Li J. Molecular basis of plant architecture. Annual Review of Plant Biology. 2008;59:253–279. doi: 10.1146/annurev.arplant.59.032607.092902. [DOI] [PubMed] [Google Scholar]
- Wasner E, Claßen-Bockhoff R. ‘False resupination’ of flower-pairs – the example of Thalia geniculata L. (Marantaceae) Proceedings from the 20th International Symposium ‘Biodiversity and Evolutionary Biology’. 2010;125 Vienna: [Google Scholar]
- Weberling F. Inflorescence structure in primitive angiosperms. Taxon. 1988;37:657–690. [Google Scholar]
- Weberling F. Morphology of flowers and inflorescences. Cambridge: Cambridge University Press; 1989. [Google Scholar]