Abstract
Background
Inflorescences are complex structures with many functions. At anthesis they present the flowers in ways that allow for the transfer of pollen and optimization of the plant's reproductive success. During flower and fruit development they provide nutrients to the developing flowers and fruits. At fruit maturity they support the fruits prior to dispersal, and facilitate effective fruit and seed dispersal. From a structural point of view, inflorescences have played important roles in systematic and phylogenetic studies. As functional units they facilitate reproduction, and are largely shaped by natural selection.
Scope
The papers in this Special Issue bridge the gap between structural and functional approaches to inflorescence evolution. They include a literature review of inflorescence function, an experimental study of inflorescences as essential contributors to the display of flowers, and two papers that present new methods and concepts for understanding inflorescence diversity and for dealing with terminological problems. The transient model of inflorescence development is evaluated in an ontogenetic study, and partially supported. Four papers present morphological and ontogenetic studies of inflorescence development in monophyletic groups, and two of these evaluate the usefulness of Hofmeister's Rule and inhibitory fields to predict inflorescence structure. In the final two papers, Bayesian and Monte-Carlo methods are used to elucidate inflorescence evolution in the Panicoid grasses, and a candidate gene approach is used in an attempt to understand the evolutionary genetics of inflorescence evolution in the genus Cornus (Cornaceae). Taken as a whole, the papers in this issue provide a glimpse of contemporary approaches to the study of the structure, development, and evolution of inflorescences, and suggest fruitful new directions for research.
Keywords: Inflorescence; development; morphology; pollination; Hofmeister's rule; terminology; evolution, flowers; monocots; eudicots; repetitive units; conceptual frameworks; sexual systems; genetic regulation; reproductive meristems
INTRODUCTION
Inflorescences directly influence the reproductive success of a plant by presenting flowers in space and time. They connect the vegetative stages in a plant's life cycle with the flowers, providing the context in which effective pollen transfer and fruit set take place. Their enormous phenotypic diversity raises questions about their functional and evolutionary significance. Their production initiates reproductive growth, and requires extensive changes to the vegetative meristem and to the underlying developmental program of the plant body. All of these aspects of structure and function have been shaped, at least to some extent, by natural selection.
Recent studies have continued the investigation of inflorescence structure and function through a broad range of disciplines, including developmental genetics, computer simulation, pollination ecology, experimental reproductive biology, phylogeny and evolutionary biology. This Special Issue brings together 11 of these studies, covering some of the many existing aspects of inflorescence biology. Two papers deal with inflorescence function, either in the form of a literature survey (Harder and Prusinkiewicz, 2013), or as an experimental study of inflorescence architecture (Reuther and Claßen-Bockhoff, 2013). Three explore the conceptual framework in which we understand inflorescence structure and deal with questions of terminology (Bull-Hereñu and Claßen-Bockhoff, 2013; Claßen-Bockhoff and Bull-Hereñu, 2013; Stützel and Trovuó, 2013). One of these papers introduces a new conceptual framework for the classification of inflorescences based on meristem structure and development (Claßen-Bockhoff and Bull-Hereñu, 2013). Four papers deal with structural and developmental aspects of inflorescences in specific lineages (Bello et al., 2013; Prenner, 2013; Remizowa et al., 2013; Weber, 2013), and one paper uses modern statistical techniques to investigate character evolution in grass inflorescences (Reinheimer et al., 2013). The final paper deals with the genetic control of inflorescence form (Liu et al., 2013).
INFLORESCENCES AS FLOWER PRESENTERS IN SPACE AND TIME
With respect to function, the branching pattern of the inflorescence, which has played such a large role in systematic studies, will most likely have little importance unless it affects the manner in which fertilization is accomplished, or nutrients are supplied to the developing flowers and fruits. Changes that affect fertilization could occur through changes to the scaffold (the mature branch system that supports the floral display), or the pattern of when flowers open and how long they remain open (the display dynamics). Changes to either of these factors will affect the three-dimensional arrangement of flowers over time, which will affect pollinator behaviour (in animal-pollinated systems), the dynamics of pollen availability (in abiotic systems) and pollen transfer with respect to the plant's reproductive success. Selective influences on the floral display vary with the pollination system, and within a pollination system with the specific actions of the pollen vector. Inflorescence function is thus something that occurs in specific taxa, on a specific temporal scale, and with specific pollen vectors. For these reasons a better understanding of inflorescence function may only be achieved by comprehensive field studies conducted in monophyletic groups, combined with ecological models and computer simulation.
Harder and Prusinkiewicz (2013) provide a literature review of the relationship between inflorescence structure and reproductive function. They address the need for a functional interpretation of inflorescence structure by considering architectural components with recognizable ecological implications. Of the many factors important in inflorescence function, the authors give primary attention to the floral display, the display dynamics, scaffold structure, the sequence of flower opening, and the display geometry (the three-dimensional arrangement of flowers over time). Given that these components are largely subject to continuous variation, inflorescence evolution can be expected to proceed along a multidimensional continuum. The authors make a compelling case for the necessity of studying inflorescence structure in a functional context. For instance, the importance of the floral display is supported by evidence such as that of Bradford and Barnes (2001), who found that changes in branching pattern were more frequent than changes in flower maturation pattern in the inflorescences of the Cunoniaceae. At least in this family, selection seems to preserve inflorescence features important in pollination, at the expense of architectural features.
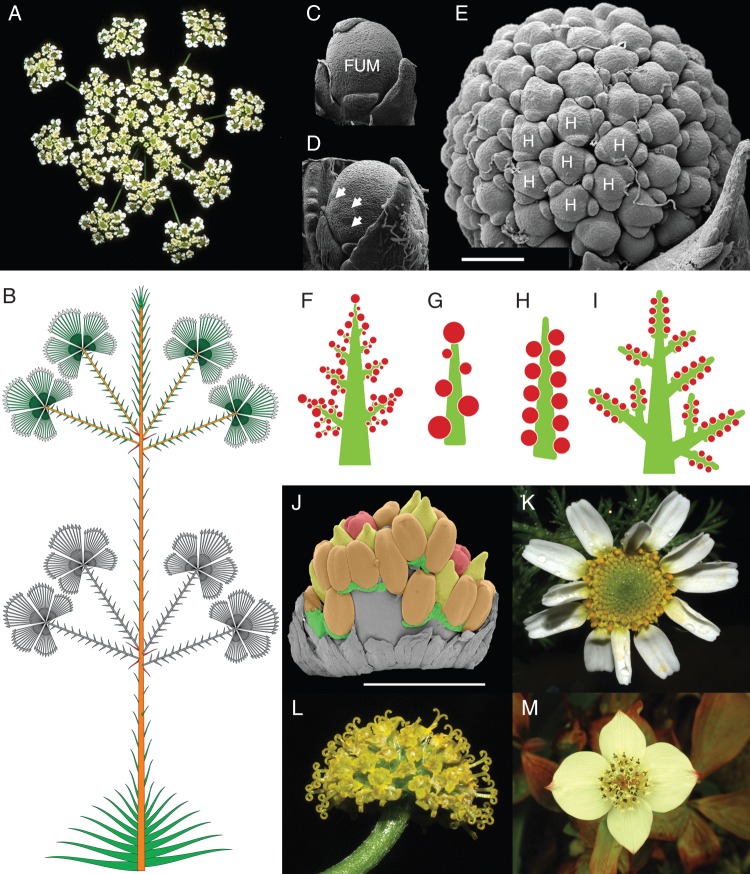
Reuther and Claßen-Bockhoff (2013) experimentally test the influence of plant architecture and flowering sequence on the reproductive success in Chaerophyllum bulbosum (Apiaceae). Chaerophyllum bulbosum is andromonoecious (it possesses both hermaphrodite and functionally male flowers; Fig. 1A). Each plant possesses up to three branch orders of umbels with an increasing percentage of male flowers in each order. The authors performed a series of pollination, bagging and removal experiments to test whether this andromonoecious arrangement of flowers is induced by changes in resource allocation, or whether it is genetically fixed. They find that andromonoecy is inherited in C. bulbosum, but that the proportion of hermaphrodite and (functionally) male flowers responds plastically to the environment. The study clearly illustrates how the interplay of architectural constraints, flowering dynamics, pollinator availability and population size results in a self-regulating sexual system that saves resources and optimizes fruit set.
Fig. 1.
Illustrations of different aspects of inflorescence research. (A) Compound umbel of Chaerophyllum bulbosum (Apiaceae) with umbellets composed of hermaphrodite and small (functionally) male flowers. (B) The branching pattern of Actinocephalus bongardii (A. St.-Hil.) Sano (Eriocaulaceae). The grey branches are decaying or had already fallen at the time this diagram was made. Graphics in this manor facilitate comparisons between species and allow visualization of morphological and developmental patterns. (C–E) Development of a floral unit meristem (FUM) producing the compound head of Echinops bannaticus Rochel ex Schrad (Asteraceae). Scale bar = 200 µm and applies to all three images. (C) Young FUM with characteristic apex lacking primoridia. (D) Small head primordia (arrowheads) are produced by meristem fractionation at the base of the FUM. (E) Young double head after fractionation into heads ‘H’ and before flower production. (F–I) Basic inflorescence types according to the ontogenetic concept of inflorescences: (F) panicle; (G) botryoid; (H) raceme; and (I) compound raceme. (J) Young inflorescence of Posidonia (Posidoniaceae). Green = flower-subtending bracts (FSBs); yellow = stamen connective; orange = thecae; red = carpel. The FSBs are delayed in development in this species, and become clearly visible only at anthesis. Scale bar = 1 mm. (K) Top view of a heterogamous inflorescence of Anacyclus clavatus (Asteraceae) with zygomorphic ray flowers and tubular flowers. (L) Lateral view of a homogamous inflorescence of Anacylus monanthos (Asteraceae) with tubular flowers in anthesis. (M) Inflorescence of Cornus canadensis L. f. (Cornaceae).
CONCEPTS AND TERMS: DEALING WITH INFLORESCENCE DIVERSITY
The inflorescence characteristics that are important at a functional level may not be the same as those that allow us to trace the broad patterns of evolutionary change in inflorescence structure, although there has been so little work in this area that it is hard to draw definitive conclusions. Papers that deal with fine-scale changes in inflorescence characteristics are only beginning to appear (Doust and Kellogg, 2002; Bröderbauer et al., 2013; Landrein and Prenner, 2013). Up to now, branching patterns have been used for the identification of homologies because they are thought to be more evolutionarily conservative than floral displays. Selective pressures probably shape the appearance of the floral display, but are likely to have less effect on early inflorescence development. For example, racemes may look rather different depending on whether their flowers are pollinated by wind, beetles or hummingbirds, and yet have similar underlying developmental patterns.
Attempts to deal with the diversity of inflorescence branching patterns and create a standardized terminology have led to different conceptual approaches with different, and often antithetical, elaborate and confusing terminologies (see reviews by Claßen-Bockhoff, 2000; Prenner et al., 2009; Endress, 2010). Although there has been a recent attempt to model the development of some basic inflorescence types, and to determine their position in an adaptive landscape (Prusinkiewicz et al., 2007), there is as yet no comprehensive theory that addresses the complexities of inflorescence structure and function, and thus no comprehensive terminology that can satisfy all needs. The following three papers in this Special Issue contribute to a solution of these problems.
Stützel and Trovuó (2013) deal with the problem of repetitive units in the inflorescences of the Eriocaulaceae. Like the Asteraceae, the flowers of the Eriocaulaceae are arranged in capitula, which can be further aggregated into richly branched flowering systems (Fig. 1B; see also the cover image of this issue). In some species the reproductive units terminate the main stem, while in others they are lateral and the main stem remains vegetative. Instead of trying to name all types of these highly branched systems, the authors describe the basic reproductive unit that is common to all systems, and then describe the levels of repetition in each system. In this way, they are able to describe the morphology of the branching pattern in a way that is useful for systematic studies, while at the same time preserving information on the commonalities of appearance that may be important for ecological studies. To deal with the problem of the delimitation of reproductive units in plants that grow in non-seasonal environments, the authors use the time lag between the appearance of successive components to capture a sense of seasonal growth units, sensu Briggs and Johnson (1979). Separating the description of the repetitive units from their positions within the shoot system, along with the use of temporal sequences, is a novel approach that simplifies the comparison of inflorescence structures among species.
Claßen-Bockhoff and Bull-Hereñu (2013) present a new conceptual model of inflorescence structure using meristem types and morphogenetic processes as reference frameworks. They point out that reproductive meristems differ from vegetative meristems in relative size, phyllome development and pattern of meristematic activity. Based on these differences, the authors distinguish three types of reproductive meristems: inflorescence, flower and the newly introduced floral unit meristems. While inflorescence meristems share more characters with vegetative meristems (e.g. acropetal primordial production), floral unit meristems have more in common with flower meristems (e.g. the process of fractionation; Fig. 1C–E). The heads of the Asteraceae and umbels of the Apiaceae are examples of floral units. According to this finding, inflorescences in the traditional sense are split into three groups: vegetative shoot systems bearing reproductive units, inflorescences sensu strictu originating from inflorescence meristems, and floral units originating from floral unit meristems. As a consequence of this new grouping, processes such as truncation, homogenization and pattern repetition have to be reconsidered. The new concept allows a comprehensive treatment of the diversity of flowering systems and provides a new developmental basis for homology hypotheses and for the reconstruction of character transformations in evolution.
Bull-Hereñu and Claßen-Bockhoff (2013) evaluate the transient model of inflorescence formation proposed by Prusinkiewicz et al. (2007). This model unites three basic inflorescence patterns (panicle, raceme, cyme) into a common developmental framework. The framework is based on the presence of a hypothesized quality of the apical meristem called vegetativeness (veg). In the simplest version of the model veg declines in the apical meristem until it reaches some threshold, at which point the meristem transforms into a terminal flower. In the case of a raceme, veg also declines in the lateral meristems until the threshold is reached, at which point they also transform into flowers. Bull-Hereñu and Claßen-Bockhoff (2013) investigate the validity of the veg model through a study of transformations in the size of the inflorescence apex in panicles and compound racemes (Fig. 1F–I). Since veg declines uniformly in the main axis of compound racemes and panicles, they predict that apex size will also decline uniformly. This prediction is verified for panicles, but not for compound racemes, a finding that only partially validates the transient model.
INFLORESCENCE DEVELOPMENT IN MONOPHYLETIC LINEAGES
While conceptual frameworks aim to provide a general reference system for homology hypotheses and for the application of a clear and universal terminology, developmental studies in monophyletic lineages provide important information on the evolution of developmental patterns. The specificity of inflorescence modification is clearly seen in the following four studies in this Special Issue, which address phyllotaxis, unusual flower arrangements and patterns of meristem fractionation.
Remizowa et al. (2013) use the early-diverging and lilioid monocots to investigate the relationship between inflorescence and floral morphology. Although the flowers of these taxa are characterized by stability in organ number and position, the presence of floral subtending bracts and prophylls is variable across taxa (Fig. 1J). The occasional absence of these phyllomes means that models of organ position based on Hofmeister's Rule (Kirchoff, 2000, 2003) cannot easily be extended to all of these taxa. Hofmeister's Rule postulates that new organs are initiated in the largest gap between those already present on the apex. The problem presented by the early-diverging and lilioid monocots is that organ position remains stable even when the requisite phyllomes for determining organ position are lacking. To address these issues, Remizowa et al. (2013) postulate the existence of inhibitory zones that may or may not be associated with existing organs, but which have the same effect on organ placement as extant organs. Based on detailed studies describing the course of vascular bundles, the authors offer two possible explanations for the absence of subtending bracts. The formation of the organs may be suppressed, or they may exist but in the form of a hybrid bract/floral primordium. The existence of hybrid organs is one way in which inhibitory fields could be maintained in taxa that lack subtending bracts.
Prenner (2013) describes aspects of inflorescence development in several species of the Papilionoideae (Leguminosae). In Swainsona formosa (Galegeae), racemes are formed in the axils of semi-distichous leaves, which are positioned more toward one side of the shoot than the other in a form of pendulum symmetry (see fig. 2A in Prenner, 2013). Prenner explains this pattern of development based on spatial constraints exerted by the developing inflorescences. The remarkable oscillating developmental pattern of flower initiation in Abrus precatorius (Abreae) is explained by the existence of an inhibitory field centered on main inflorescence axis. Yet, despite the possible importance of inhibitory fields in these cases, they cannot explain all developmental phenomena. Prenner (2013) also re-evaluates the cases of Hardenbergia vilacea and Kennedia nigricans (both Phaseoleae–Kennediinae), which have three- and two-flowered axillary units, respectively. These axillary units may have evolved from lateral inflorescences similar to those of Abrus precatorius through a reduction in the number of flowers and developmental synchronization. Based on these results, Prenner reinterprets the papilionoid pseudoraceme as a compound raceme with condensed lateral axes.
Weber (2013) reviews the structural and developmental evidence for the unusual paired-flowered cymes in the Gesneriaceae and related taxa of the Lamiales (Calceolariaceae, Sanango, two related tribes of Plantaginaceae). Pair-flowered cymes exhibit a normal cymose branching pattern, but instead of a single flower each unit bears two flowers. Each regular flower in the cyme is associated with a supernumerary flower in a frontal position (front flower). This pattern is repeated throughout the system. Developmental study of Sinningia bulbosa (Gesneriaceae) demonstrates that the front flower is actually produced in the axil of a bract, which is inserted higher on the axis than the two bracteoles of the cyme (see fig. S7 in Weber, 2013). This pattern, and the occurrence of this third bract in the mature inflorescence of some species, suggests that pair-flowered cymes originated from many-flowered, paniculate inflorescences. Just such inflorescences are found in Peltanthera floribunda, which is placed sister to the taxa with pair-flowered cymes in most molecular phylogenies.
Bello et al. (2013) investigate capitulum structure and development in the genus Anacyclus (Anthemideae, Asteraceae). Anacyclus possesses both heterogamous (tubular disk flowers, and female, zygomorphic ray flowers; Fig. 1K) and homogamous capitula (only disk flowers; Fig. 1L). The outermost primordia form involucral bracts, while those in the centre form disk flowers and their subtending bracts (paleae). The disk flowers and the paleae originate from a common primordium, as occurs in many taxa where the subtending bract remains small. In heterogamous capitula the ray flowers lack paleae and form in the axils of the involucral bracts, but only after a time lag during which disk flower formation begins. This delay in development and the lack of paleae suggests that the ray flowers are the remnants of a different order of branching than the disk flowers, and supports an origin of the capitula from a thyrsoid-like ancestor such as those of the sister-group Calyceraceae (Pozner et al., 2012).
CHARACTER EVOLUTION AND GENETIC REGULATION
Modern techniques for the study of evolution include the use of statistical techniques to investigate character evolution, and the use of a candidate gene approach to investigate the genetic regulation of development in a systematic context. The final two papers in this Special Issue apply these techniques to the Panicoid grasses (Reinheimer et al., 2013) and the genus Cornus (Liu et al., 2013), respectively.
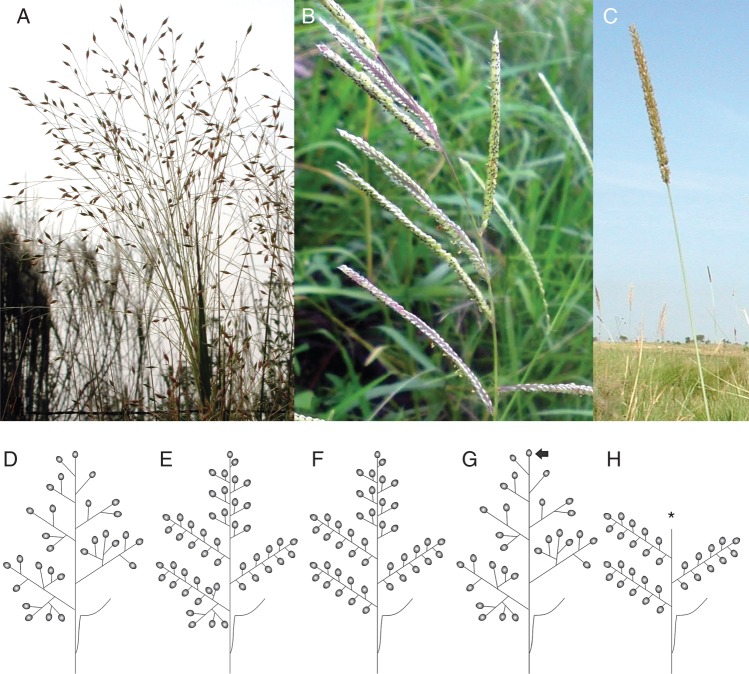
Reinheimer et al. (2013) use maximum likelihood and Bayesian Markov chain Monte-Carlo methods to test models of character evolution in the Panicoid grasses. They consider three traits that have been used in studies of grass evolution: degree of inflorescence condensation (Fig. 2A–C), degree of homogenization (Fig. 2D–F), and presence or absence of a terminal spikelet (Fig. 2G, H). In all of their reconstructions the authors show that the ancestor of the panicoid grasses had a partially or fully homogenized, lax inflorescence with a terminal spikelet. Despite many independent origins and reversals in these traits, some general evolutionary patterns are found. The processes of de-condensation (becoming lax), de-homogenization and loss of the terminal spikelet appear to be favoured over the reverse processes, and homogenization appears to be a prerequisite for loss of the terminal spikelet. There also appears to be a relationship between homogenization and condensation, although it is not possible to establish a temporal order in this relationship. The authors also checked for associations between the inflorescence traits, plant longevity and photosynthetic type (C3 versus C4). Neither plant longevity nor photosynthetic type are found to be strongly correlated with any of the inflorescence traits, although there is a weak correlation between photosynthetic type and inflorescence aspect.
Fig. 2.
(A–C) Degree of inflorescence condensation in the Poaceae. (A) Panicum olyroides Kunth. Lax. (B) Paspalum dilatatum Poir. Lax to condensed. (C) Sacciolepis vilvoides (Trin.) Chase. Condensed. (D–F) Degree of inflorescence homogenization. (D) Non-homogenized. (E) Partially homogenized. (F) Completely homogenized. (G, H) Presence or absence of a terminal spikelet (truncation). (G) Present (non-truncated; arrow). (H) Absent (truncated; star).
Liu et al. (2013) investigate the role of the LEAFY homolog CorLFY in the genus Cornus (Cornaceae). LEAFY homologs have been implicated in controlling inflorescence architecture in a number of species. Effects such as internodal compression (Arabidopsis, Malus) and repressed pedicel elongation (Arabidopsis) are associated with or brought about by modified LEAFY expression. Similar changes in internode and pedicel elongation have occurred in the various lineages of Cornus, resulting in head-like and umbel-like inflorescence forms (Fig 1M). LEAFY is thus a logical candidate for gene expression studies in Cornus. Non-quantitative PCR analysis of early and late inflorescence developmental stages, and in situ hybridization at early developmental stages, shows that CorLFY is present in all six species, but no differences in expression level are detected. Based on work in Petunia and Nicotiana, lower levels of expression were expected in the species with elongated internodes, but no evidence for this is found. The expression of CorLFY is, however, consistent with the expectation that CorLFY is required for normal inflorescence and floral development, as has been found in other species.
SUMMARY AND OUTLOOK
The 11 papers in this Special Issue provide an overview of contemporary work on inflorescence morphology, function and development. Morphological and developmental work continues to provide valuable insights, and is being extended through the use of statistical and genetic techniques for the study of inflorescence evolution. The great diversity in inflorescence architecture continues to be explored through new conceptual schemes that hold the potential for understanding the genesis of inflorescence diversity, and for simplifying terminology. Studies of inflorescence function are beginning to link morphological and ecological aspects, although a comprehensive understanding of inflorescence structure and function yet eludes us. Future functional studies may continue to bridge the gap between ecology and morphology by relating the appearance of the floral display to the branching pattern of the inflorescence, and by elucidating the constraints on inflorescence form from both functional and structural aspects. Up to now, inflorescence structure and function have usually been investigated separately. We hope that this Special Issue will serve as a stimulus for studies that unite these aspects.
ACKNOWLEDGEMENTS
The authors thank the Chief Editor of the Annals of Botany, Prof. Heslop-Harrison, and the Managing Editor, Dr David Frost, for their support in bringing this project to fruition. We also thank the authors who participated in this project and submitted their manuscripts for inclusion in this Special Issue. The following authors provided the illustrations for the plates: Regine Claßen-Bockhoff (Fig. 1A), Thomas Stützel (Fig. 1B), Kester Bull Hereñu (Fig. 1C–I), Margarita Remizowa (Fig. 1J), M. Angélica Bello (Fig. 1K, L), Qiu-Yun (Jenny) Xiang (Fig. 1M), Renata Reinheimer (Fig. 2A–H).
LITERATURE CITED
- Bello MA, Álvarez I, Torices R, Fuertes-Aguilar J. Floral development and evolution of capitulum structure in Anacyclus (Anthemideae, Asteraceae) Annals of Botany. 2013;112:1597–1612. doi: 10.1093/aob/mcs301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford JC, Barnes RW. Phylogenetics and classification of Cunoniaceae (Oxalidales) using chloroplast DNA sequences and morphology. Systematic Botany, 2001;26:354–385. [Google Scholar]
- Briggs BG, Johnson LAS. Evolution in the Myrtaceae - evidence from inflorescence structure. Proceedings of the Linnean Society of New South Wales, 1979;102:1–256. [Google Scholar]
- Bröderbauer D, Weber A, Diaz A. The design of trapping devices in pollination traps of the genus Arum (Araceae) is related to insect type. Botanical Journal of the Linnean Society, 2013;172:385–397. doi: 10.1111/boj.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull-Hereñu K, Claßen-Bockhoff R. Testing the ontogenetic base for the transient model of inflorescence development. Annals of Botany. 2013;112:1543–1551. doi: 10.1093/aob/mct022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claßen-Bockhoff R. Inflorescences in Bruniaceae. Opera Botanica Belgica, 2000;12:1–310. [Google Scholar]
- Claßen-Bockhoff R, Bull-Hereñu K. Towards an ontogenetic understanding of inflorescence diversity. Annals of Botany. 2013;112:1523–1542. doi: 10.1093/aob/mct009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust AN, Kellogg EA. Integrating phylogeny, developmental morphology and genetics: A case study of inflorescence evolution in the “bristle grass” clade (Panicoideae: Poaceae) In: Cronk QCB, Bateman RM, Hawkins JA, editors. Developmental Genetics and Plant Evolution. London: Taylor & Francis; 2002. [Google Scholar]
- Endress PK. Disentangling confusions in inflorescence morphology: Patterns and diversity of reproductive shoot ramification in angiosperms. Journal of Systematics and Evolution, 2010;48:225–239. [Google Scholar]
- Harder LD, Prusinkiewicz P. The interplay between inflorescence development and function as the crucible of architectural diversity. Annals of Botany. 2013;112:1477–1493. doi: 10.1093/aob/mcs252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchoff BK. Hofmeister's rule and primordium shape: Constraints on organ position in Hedychium coronarium (Zingiberaceae) In: Wilson KL, Morrison DA, editors. Monocots: Systematics and Evolution. Collingwood, Australia: CSIRO Publishing; 2000. [Google Scholar]
- Kirchoff BK. Shape matters: Hofmeister's rule, primordium shape, and flower orientation. International Journal of Plant Sciences, 2003;164:505–517. [Google Scholar]
- Landrein S, Prenner G. Unequal twins? Inflorescence evolution in the twinflower tribe Linnaeeae (Caprifoliaceae s.l.) International Journal of Plant Sciences, 2013;174:200–233. [Google Scholar]
- Liu J, Franks RG, Feng C-M, Liu X, Fu C-X, Xiang Q-Y. Characterization of the sequence and expression pattern of LFY homologues from dogwood species (Cornus) with divergent inflorescence architectures. Annals of Botany. 2013;112:1629–1641. doi: 10.1093/aob/mct202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozner R, Zanotti C, Johnson LA. Evolutionary origin of the Asteraceae capitulum: Insights from Calyceraceae. American Journal of Botany, 2012;99:1–13. doi: 10.3732/ajb.1100256. [DOI] [PubMed] [Google Scholar]
- Prenner G, Vergara-Silva F, Rudall PJ. The key role of morphology in modelling inflorescence architecture. Trends in Plant Science. 2009;14:302–309. doi: 10.1016/j.tplants.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Prenner G. Papilionoid inflorescences revisited (Leguminosae-Papilionoideae) Annals of Botany. 2013;112:1567–1576. doi: 10.1093/aob/mcs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinkiewicz P, Erasmus Y, Lane B, Harder LD, Coen E. Evolution and development of inflorescence architectures. Science, 2007;316:1452–1456. doi: 10.1126/science.1140429. [DOI] [PubMed] [Google Scholar]
- Reinheimer R, Vegetti AC, Rua GH. Macroevolution of panicoid inflorescences: a history of contingency and order of trait acquisition. Annals of Botany. 2013;112:1613–1628. doi: 10.1093/aob/mct027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remizowa MV, Rudall PJ, Choob VV, Sokoloff DD. Racemose inflorescences of monocots: structural and morphogenetic interaction at the flower/inflorescence level. Annals of Botany. 2013;112:1553–1566. doi: 10.1093/aob/mcs246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther K, Claßen-Bockhoff R. Andromonoecy and developmental plasticity in Chaerophyllum bulbosum (Apiaceae–Apioideae) Annals of Botany. 2013;112:1495–1503. doi: 10.1093/aob/mct073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stützel T, Trovó T. Inflorescences in Eriocaulaceae: taxonomic relevance and practical implications. Annals of Botany. 2013;112:1505–1522. doi: 10.1093/aob/mct234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. Pair-flowered cymes in the Lamiales: structure, distribution and origin. Annals of Botany. 2013;112:1577–1595. doi: 10.1093/aob/mct156. [DOI] [PMC free article] [PubMed] [Google Scholar]