Abstract
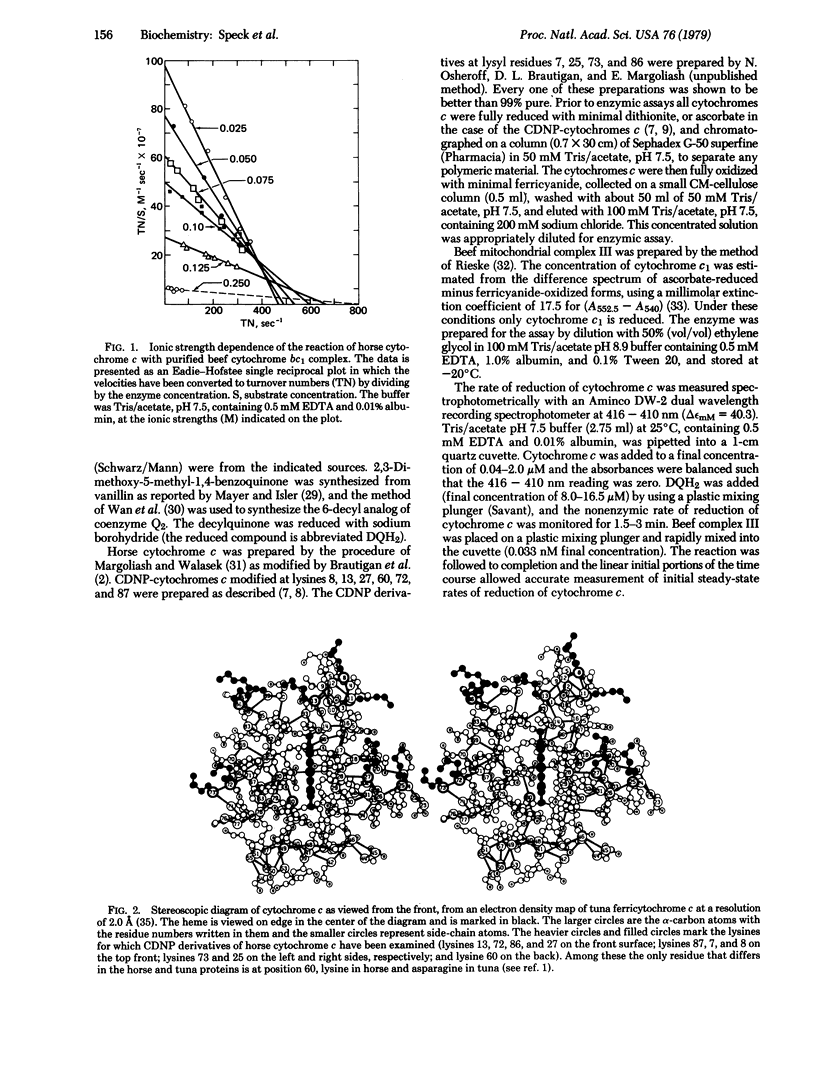
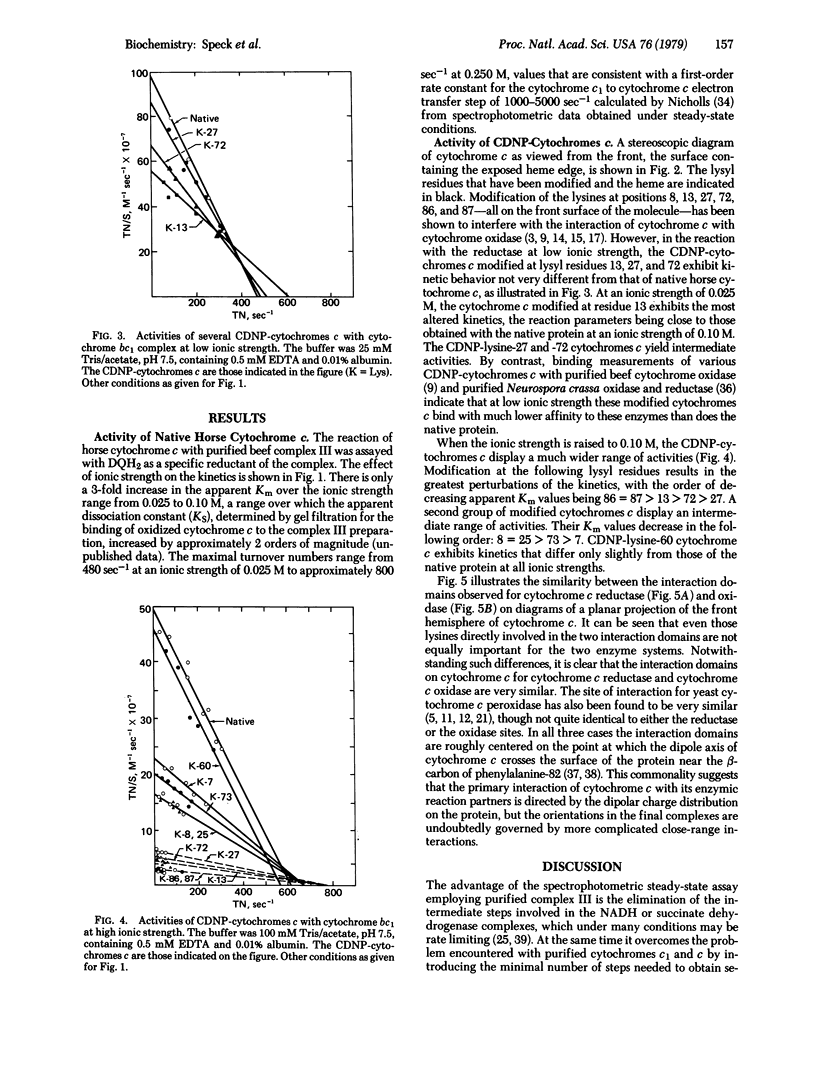
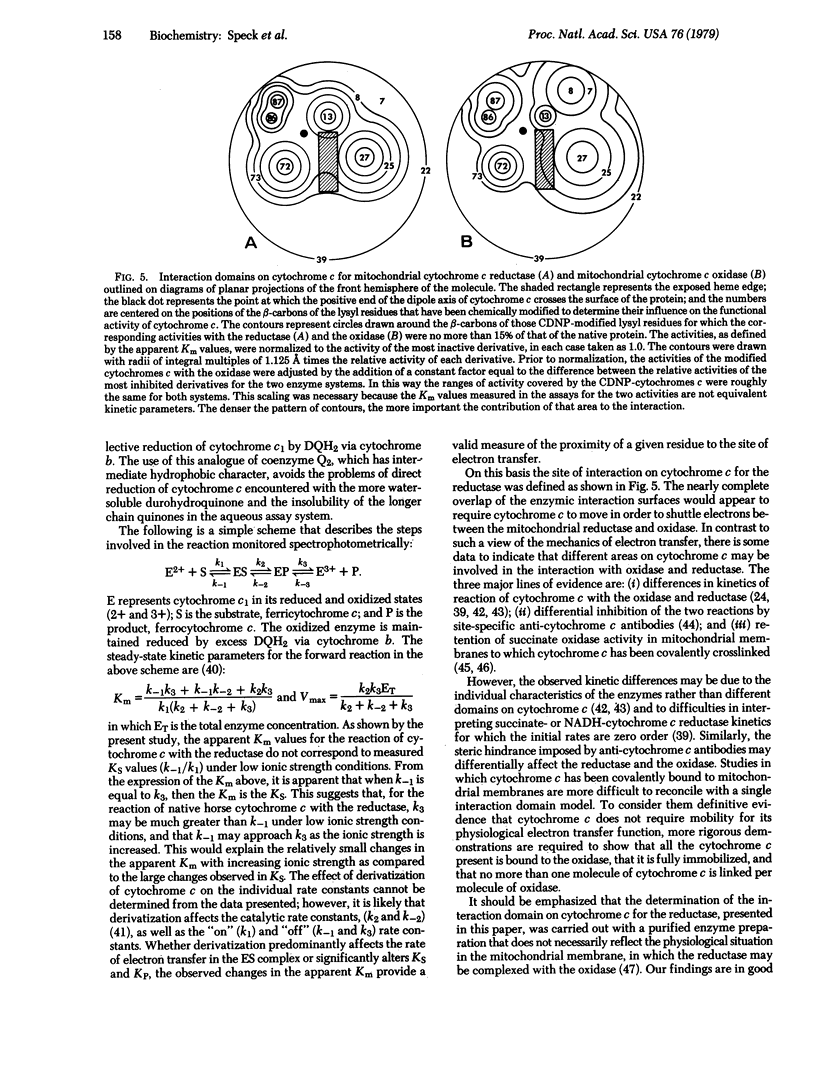
An assay has been developed to study the steady-state kinetics of the reduction of cytochrome c by purified beef heart mitochondrial cytochrome c reductase (cytochrome bc1 complex, complex III). An analogue of coenzyme Q2 (2,3-dimethoxy-5-methyl-6-decylhydroquinone) was employed as an antimycin-sensitive reductant. The kinetics of reaction of ten different mono(4-carboxy-2,6-dinitrophenyl) derivatives of horse cytochrome c were determined. The modified proteins showed higher apparent Km values than the native protein and greater sensitivity to ionic strength, defining an interaction domain on cytochrome c for purified cytochrome c reductase. This interaction site is located on the front surface of the molecule (which contains the exposed heme edge) and surrounds the point at which the positive end of the dipole axis crosses the surface of the protein. The site is similar to that previously determined for mitochondrial cytochrome c oxidase and yeast cytochrome c peroxidase, suggesting that the primary interaction with redox partners is directed by the dipolar charge distribution on cytochrome c. The extensive overlapping of the interaction domains for the mitochondrial cytochrome c oxidase and reductase indicates that cytochrome c must be mobile in order to transfer electrons between them, depending on their relative positions in the membrane. Whether such mobility is necessary in intact mitochondria depends on whether the interactions with the complete membrane-bound system are the same as with the purified components.
Keywords: mono(carboxydinitrophenyl) cytochromes c; cytochrome bc1 complex; 2,3-dimethoxy-5-methyl-6-decylhydroquinone; ionic strength effects; steady-state kinetics
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. J., Smith H. T., Smith M. B., Millett F. S. Effect of specific lysine modification on the reduction of cytochrome c by succinate-cytochrome c reductase. Biochemistry. 1978 Jun 27;17(13):2479–2483. doi: 10.1021/bi00606a003. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Definition of cytochrome c binding domains by chemical modification. I. Reaction with 4-chloro-3,5-dinitrobenzoate and chromatographic separation of singly substituted derivatives. J Biol Chem. 1978 Jan 10;253(1):130–139. [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Margoliash E. Mitochondrial cytochrome c: preparation and activity of native and chemically modified cytochromes c. Methods Enzymol. 1978;53:128–164. doi: 10.1016/s0076-6879(78)53021-8. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Ferguson-Miller S., Tarr G. E., Margoliash E. Definition of cytochrome c binding domains by chemical modification. II. Identification and properties of singly substituted carboxydinitrophenyl cytochromes c at lysines 8, 13, 22, 27, 39, 60, 72, 87, and 99. J Biol Chem. 1978 Jan 10;253(1):140–148. [PubMed] [Google Scholar]
- Erecińska M., Vanderkooi J. M., Wilson D. F. Cytochrome c interactions with membranes. A photoaffinity-labeled cytochrome c. Arch Biochem Biophys. 1975 Nov;171(1):108–116. doi: 10.1016/0003-9861(75)90013-2. [DOI] [PubMed] [Google Scholar]
- Errede B., Kamen M. D. Comparative kinetic studies of cytochromes c in reactions with mitochondrial cytochrome c oxidase and reductase. Biochemistry. 1978 Mar 21;17(6):1015–1027. doi: 10.1021/bi00599a012. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Chance B., Waring A., Margoliash E. Low-temperature studies of electron transfer between different cytochromes c and cytochrome c oxidase. Biochemistry. 1978 May 30;17(11):2246–2249. doi: 10.1021/bi00604a036. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J Biol Chem. 1978 Jan 10;253(1):149–159. [PubMed] [Google Scholar]
- Kang C. H., Brautigan D. L., Osheroff N., Margoliash E. Definitaion of cytochrome c binding domains by chemical modification. Reaction of carboxydinitrophenyl- and trinitrophenyl-cytochromes c with baker's yeast cytochrome c peroxidase. J Biol Chem. 1978 Sep 25;253(18):6502–6510. [PubMed] [Google Scholar]
- Koppenol W. H., Vroonland C. A., Braams R. The electric potential field around cytochrome c and the effect of ionic strength on reaction rates of horse cytochrome c. Biochim Biophys Acta. 1978 Sep 7;503(3):499–508. doi: 10.1016/0005-2728(78)90149-4. [DOI] [PubMed] [Google Scholar]
- Ng S., Smith M. B., Smith H. T., Millett F. Effect of modification of individual cytochrome c lysines on the reaction with cytochrome b5. Biochemistry. 1977 Nov 15;16(23):4975–4978. doi: 10.1021/bi00642a006. [DOI] [PubMed] [Google Scholar]
- Nicholls P. Catalytic activity of cytochromes c and c1 in mitochondria and submitochondrial particles. Biochim Biophys Acta. 1976 Apr 9;430(1):30–45. doi: 10.1016/0005-2728(76)90219-x. [DOI] [PubMed] [Google Scholar]
- ORII Y., SEKUZU I., OKUNUKI K. Studies on cytochrome cl. II. Oxidation mechanism of cytochrome c1 in the presence of cytochromes a and c1. J Biochem. 1962 Mar;51:204–215. doi: 10.1093/oxfordjournals.jbchem.a127522. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. Mapping an electron transfer site on cytochrome c. FEBS Lett. 1978 Feb 1;86(1):14–16. doi: 10.1016/0014-5793(78)80087-8. [DOI] [PubMed] [Google Scholar]
- Rieder R., Bosshard H. R. Cytochrome bc1 and cytochrome oxidase can bind to the same surface domain of the cytochrome c molecule. FEBS Lett. 1978 Aug 15;92(2):223–226. doi: 10.1016/0014-5793(78)80759-5. [DOI] [PubMed] [Google Scholar]
- Rieder R., Bosshard H. R. The cytochrome c oxidase binding site on cytochrome c. Differential chemical modification of lysine residues in free and oxidase-bound cytochrome c. J Biol Chem. 1978 Sep 10;253(17):6045–6053. [PubMed] [Google Scholar]
- Smith H. T., Staudenmayer N., Millett F. Use of specific lysine modifications to locate the reaction site of cytochrome c with cytochrome oxidase. Biochemistry. 1977 Nov 15;16(23):4971–4974. doi: 10.1021/bi00642a005. [DOI] [PubMed] [Google Scholar]
- Smith L., Davies H. C., Nava M. Oxidation and reduction of soluble cytochrome c by membrane-bound oxidase and reductase systems. J Biol Chem. 1974 May 10;249(9):2904–2910. [PubMed] [Google Scholar]
- Smith L., Davies H. C., Reichlin M., Margoliash E. Separate oxidase and reductase reaction sites on cytochrome c demonstrated with purified site-specific antibodies. J Biol Chem. 1973 Jan 10;248(1):237–243. [PubMed] [Google Scholar]
- Staudenmayer N., Ng S., Smith M. B., Millett F. Effect of specific trifluoroacetylation of individual cytochrome c lysines on the reaction with cytochrome oxidase. Biochemistry. 1977 Feb 22;16(4):600–604. doi: 10.1021/bi00623a007. [DOI] [PubMed] [Google Scholar]
- Staudenmayer N., Smith M. B., Smith H. T., Spies F. K., Jr, Millett F. An enzyme kinetics and 19F nuclear magnetic resonance study of selectively trifluoroacetylated cytochrome c derivatives. Biochemistry. 1976 Jul 27;15(15):3198–3205. doi: 10.1021/bi00660a007. [DOI] [PubMed] [Google Scholar]
- Swanson R., Trus B. L., Mandel N., Mandel G., Kallai O. B., Dickerson R. E. Tuna cytochrome c at 2.0 A resolution. I. Ferricytochrome structure analysis. J Biol Chem. 1977 Jan 25;252(2):759–775. [PubMed] [Google Scholar]
- Utsumi K., Packer L. Glutaraldehyde-fixed mitochondria. I. Enzyme activity, ion translocation, and conformational changes. Arch Biochem Biophys. 1967 Sep;121(3):633–640. doi: 10.1016/0003-9861(67)90048-3. [DOI] [PubMed] [Google Scholar]
- Wan Y. P., Williams R. H., Folkers K., Leung K. H., Racker E. Low molecular weight analogs of coenzyme Q as hydrogen acceptors and donors in systems of the respiratory chain. Biochem Biophys Res Commun. 1975 Mar 3;63(1):11–15. doi: 10.1016/s0006-291x(75)80003-9. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Yu L., King T. E. Kinetics of electron transfer between cardiac cytochrome c 1 and c. J Biol Chem. 1973 Jan 25;248(2):528–533. [PubMed] [Google Scholar]



