Abstract
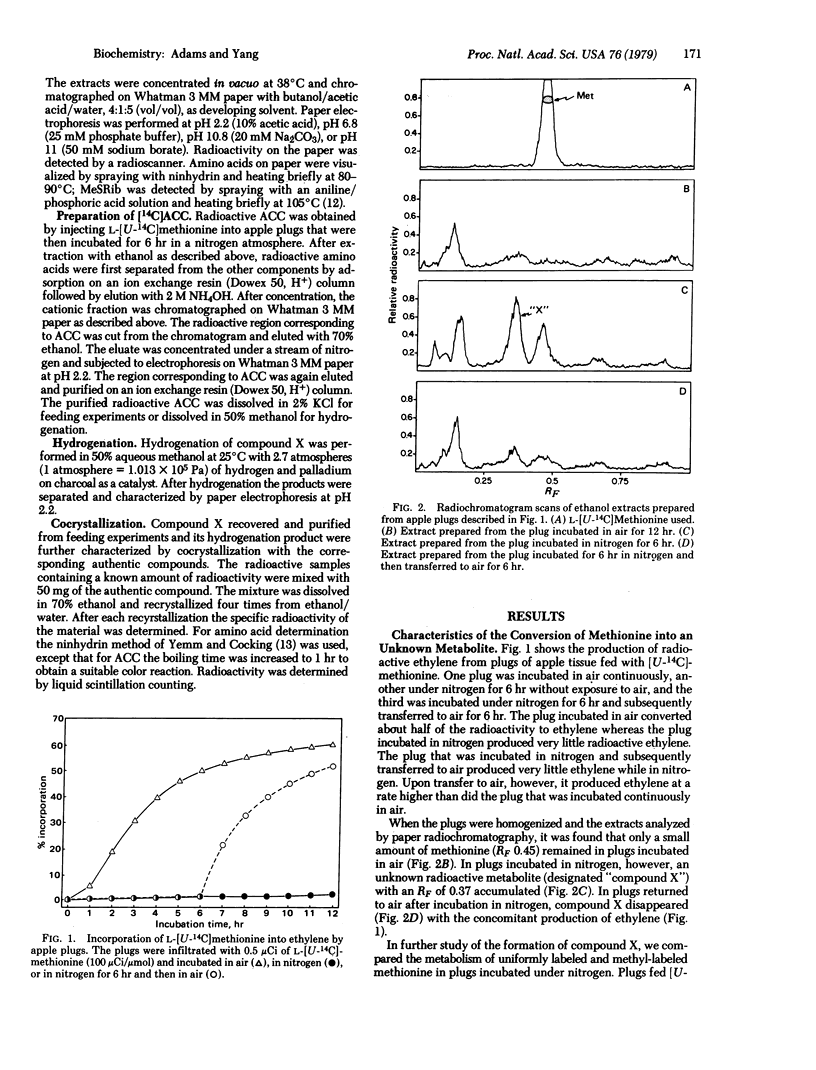
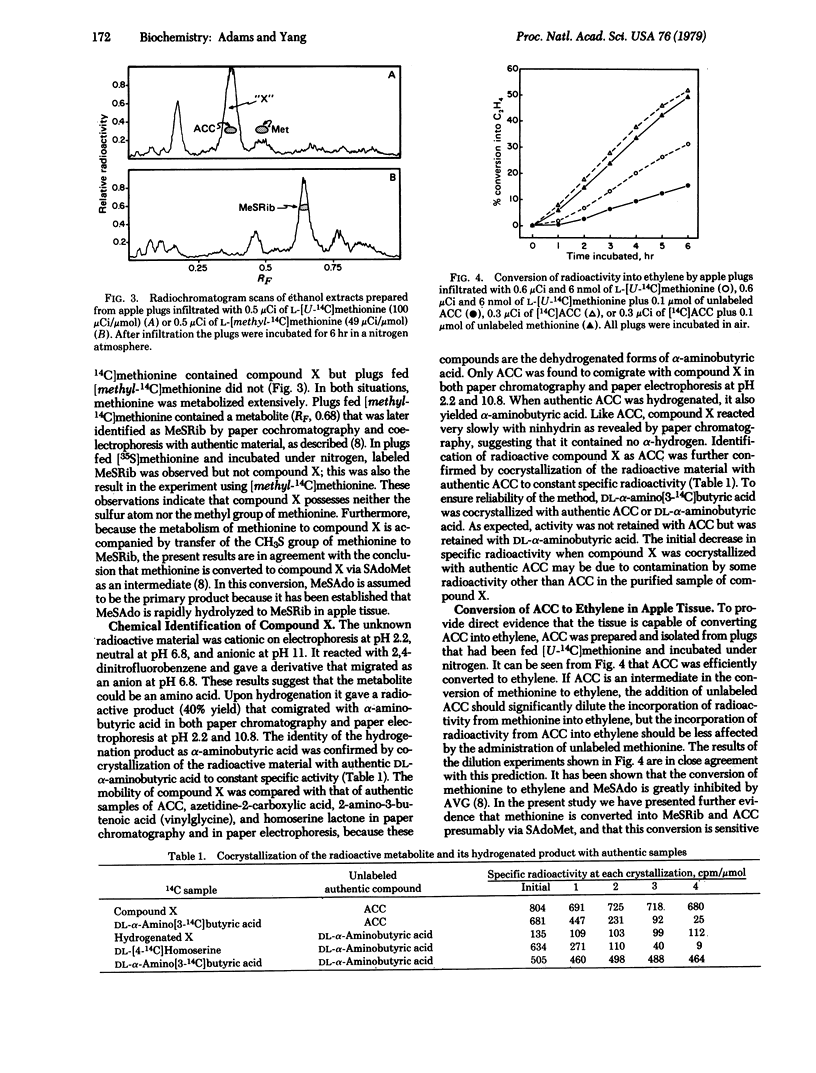
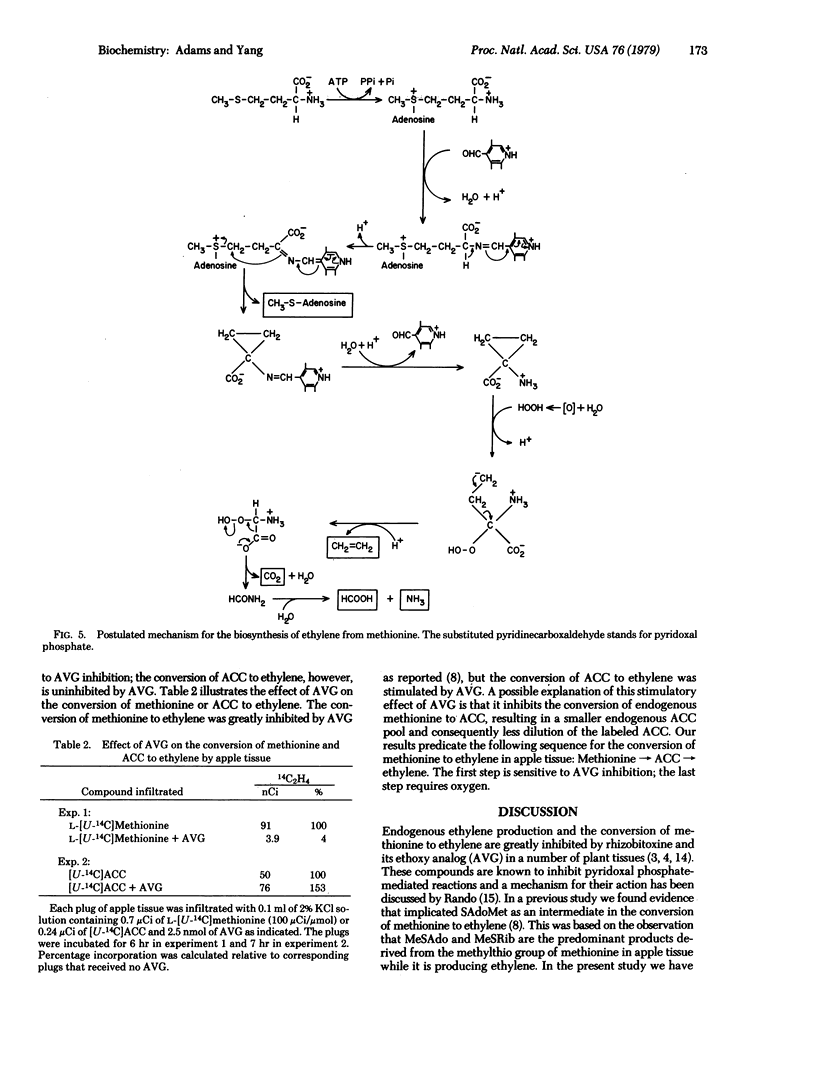
L-[U-14C]Methionine fed to apple tissue was efficiently converted to ethylene when the tissue was incubated in air. In nitrogen, however, it was not metabolized to ethylene but was instead converted to 1-aminocyclopropane-1-carboxylic acid (ACC). When apple tissues were fed with L-[methyl-14C]methionine or L-[35S]methionine and incubated in nitrogen, radioactivity was found subsequently in methylthioribose. This suggests that methionine is first converted to S-adenosylmethionine which is in turn fragmented to ACC and methylthioadenosine. Methylthioadenosine is then hydrolyzed to methylthioribose. The conclusion that ACC is an intermediate in the conversion of methionine to ethylene is based on the following observations: Labeled ACC was efficiently converted to ethylene by apple tissue incubated in air; the conversion of labeled methionine to ethylene was greatly decreased in the presence of unlabeled ACC, but the conversion of labeled ACC to ethylene was little affected by the presence of unlabeled methionine; and 2-amino-4-(2′-aminoethoxy)trans-3-butenoic acid, a potent inhibitor of pyridoxal phosphate-mediated enzyme reactions, greatly inhibited the conversion of methionine to ethylene but did not inhibit conversion of ACC to ethylene. These data indicate the following sequence for the pathway of ethylene biosynthesis in apple tissue: methionine → S-adenosylmethionine → ACC → ethylene. A possible mechanism accounting for these reactions is presented.
Keywords: S-adenosylmethionine, aminoethoxyvinylglycine, fruit ripening, apple
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Methionine metabolism in apple tissue: implication of s-adenosylmethionine as an intermediate in the conversion of methionine to ethylene. Plant Physiol. 1977 Dec;60(6):892–896. doi: 10.1104/pp.60.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROUGHS L. F. 1-Aminocyclopropane-1-carboxylic acid: a new amino-acid in perry pears and cider apples. Nature. 1957 Feb 16;179(4555):360–361. doi: 10.1038/179360a0. [DOI] [PubMed] [Google Scholar]
- Baur A. H., Yang S. F. Precursors of ethylene. Plant Physiol. 1969 Sep;44(9):1347–1349. doi: 10.1104/pp.44.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T., Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977 Mar;59(3):411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Clagett C. O. Conversion of methionine to ethylene in vegetative tissue and fruits. Biochem Biophys Res Commun. 1967 Apr 20;27(2):125–130. doi: 10.1016/s0006-291x(67)80050-0. [DOI] [PubMed] [Google Scholar]
- Burg S. P. Ethylene in plant growth. Proc Natl Acad Sci U S A. 1973 Feb;70(2):591–597. doi: 10.1073/pnas.70.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Thimann K. V. THE PHYSIOLOGY OF ETHYLENE FORMATION IN APPLES. Proc Natl Acad Sci U S A. 1959 Mar;45(3):335–344. doi: 10.1073/pnas.45.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Methionine metabolism and ethylene biosynthesis in senescent flower tissue of morning-glory. Plant Physiol. 1976 Apr;57(4):528–537. doi: 10.1104/pp.57.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD S. H. Enzymatic cleavage of S-adenosylmethionine. J Biol Chem. 1959 Jan;234(1):87–92. [PubMed] [Google Scholar]
- Owens L. D., Lieberman M., Kunishi A. Inhibition of ethylene production by rhizobitoxine. Plant Physiol. 1971 Jul;48(1):1–4. doi: 10.1104/pp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. R. Chemistry and enzymology of kcat inhibitors. Science. 1974 Jul 26;185(4148):320–324. doi: 10.1126/science.185.4148.320. [DOI] [PubMed] [Google Scholar]



