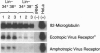Abstract
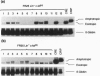
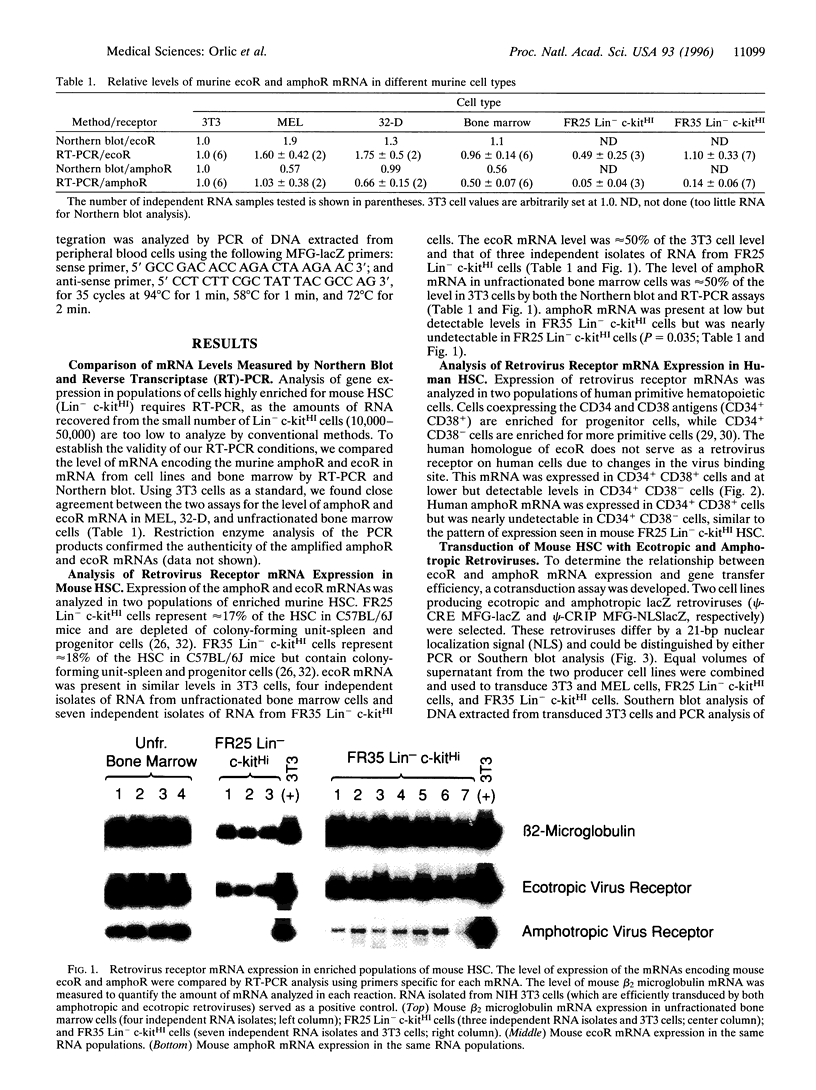
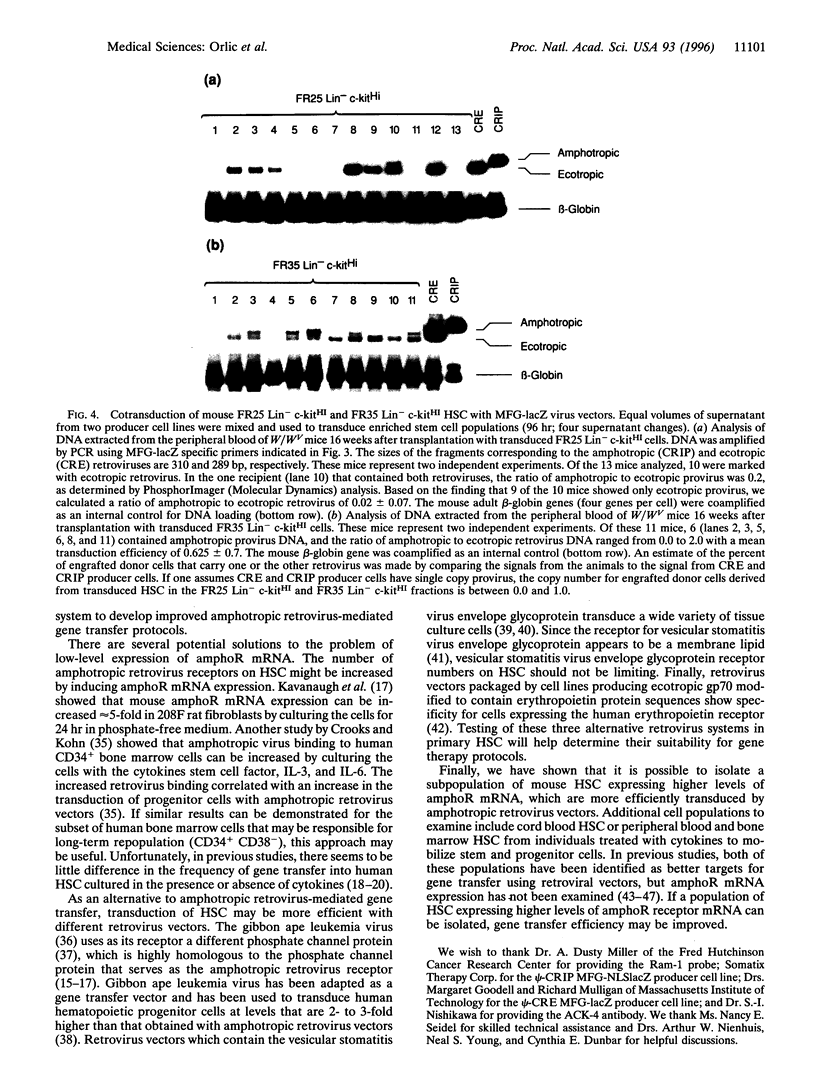
The low level of amphotropic retrovirus-mediated gene transfer into human hematopoietic stem cells (HSC) has been a major impediment to gene therapy for hematopoietic diseases. In the present study, we have examined amphotropic retrovirus receptor (amphoR) and ecotropic retrovirus receptor mRNA expression in highly purified populations of mouse and human HSC. Murine HSC with low to undetectable levels of amphoR mRNA and relatively high levels of ecotropic retrovirus receptor mRNA were studied. When these HSC were analyzed simultaneously for ecotropic and amphotropic retrovirus transduction, ecotropic provirus sequences were detected in 10 of 13 long-term repopulated animals, while amphotropic proviral sequences were detected in only one recipient. A second distinct population of murine HSC were isolated that express 3-fold higher levels of amphoR mRNA. When these HSC were analyzed simultaneously for ecotropic and amphotropic retrovirus transduction, 11 of 11 repopulated mice contained ecotropic provirus and 6 of 11 contained amphotropic provirus sequences, a significant increase in the amphotropic retrovirus transduction (P = 0.018). These results indicate that, among the heterogeneous populations of HSC present in adult mouse bone marrow, the subpopulation with the highest level of amphoR mRNA is more efficiently transduced by amphotropic retrovirus. In a related study, we found low levels of human amphoR mRNA in purified populations of human HSC (CD34+ CD38-) and higher levels in committed progenitor cells (CD34+ CD38+). We conclude that the amphoR mRNA level in HSC correlates with amphotropic retrovirus transduction efficiency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Kim J. W., Tseng L., Cunningham J. M. Envelope-binding domain in the cationic amino acid transporter determines the host range of ecotropic murine retroviruses. J Virol. 1993 Apr;67(4):2091–2096. doi: 10.1128/jvi.67.4.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Baum C. M., Weissman I. L., Tsukamoto A. S., Buckle A. M., Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont J. W., MacGregor G. R., Wager-Smith K., Fletcher F. A., Moore K. A., Hawkins D., Villalon D., Chang S. M., Caskey C. T. Expression of human adenosine deaminase in murine hematopoietic cells. Mol Cell Biol. 1988 Dec;8(12):5116–5125. doi: 10.1128/mcb.8.12.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., Moritz T., Donahue R. E., Luskey B. D., Kessler S. W., Martin D. I., Orkin S. H., Nienhuis A. W., Williams D. A. Long-term in vivo expression of a murine adenosine deaminase gene in rhesus monkey hematopoietic cells of multiple lineages after retroviral mediated gene transfer into CD34+ bone marrow cells. Blood. 1993 Oct 1;82(7):1975–1980. [PubMed] [Google Scholar]
- Bodine D. M., Seidel N. E., Gale M. S., Nienhuis A. W., Orlic D. Efficient retrovirus transduction of mouse pluripotent hematopoietic stem cells mobilized into the peripheral blood by treatment with granulocyte colony-stimulating factor and stem cell factor. Blood. 1994 Sep 1;84(5):1482–1491. [PubMed] [Google Scholar]
- Bregni M., Magni M., Siena S., Di Nicola M., Bonadonna G., Gianni A. M. Human peripheral blood hematopoietic progenitors are optimal targets of retroviral-mediated gene transfer. Blood. 1992 Sep 15;80(6):1418–1422. [PubMed] [Google Scholar]
- Brenner M. K., Rill D. R., Holladay M. S., Heslop H. E., Moen R. C., Buschle M., Krance R. A., Santana V. M., Anderson W. F., Ihle J. N. Gene marking to determine whether autologous marrow infusion restores long-term haemopoiesis in cancer patients. Lancet. 1993 Nov 6;342(8880):1134–1137. doi: 10.1016/0140-6736(93)92122-a. [DOI] [PubMed] [Google Scholar]
- Burns J. C., Friedmann T., Driever W., Burrascano M., Yee J. K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel A., Cottler-Fox M., Doren S., Dunbar C. E. Retroviral-mediated gene transfer into CD34-enriched human peripheral blood stem cells. Exp Hematol. 1993 Apr;21(4):585–591. [PubMed] [Google Scholar]
- Crooks G. M., Kohn D. B. Growth factors increase amphotropic retrovirus binding to human CD34+ bone marrow progenitor cells. Blood. 1993 Dec 1;82(11):3290–3297. [PubMed] [Google Scholar]
- Dunbar C. E., Cottler-Fox M., O'Shaughnessy J. A., Doren S., Carter C., Berenson R., Brown S., Moen R. C., Greenblatt J., Stewart F. M. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood. 1995 Jun 1;85(11):3048–3057. [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Hanley M. E., Nolta J. A., Parkman R., Kohn D. B. Umbilical cord blood cell transduction by retroviral vectors: preclinical studies to optimize gene transfer. Blood Cells. 1994;20(2-3):539–546. [PubMed] [Google Scholar]
- Jordan C. T., Lemischka I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990 Feb;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Kasahara N., Dozy A. M., Kan Y. W. Tissue-specific targeting of retroviral vectors through ligand-receptor interactions. Science. 1994 Nov 25;266(5189):1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Miller D. G., Zhang W., Law W., Kozak S. L., Kabat D., Miller A. D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B., Apperley J. F., Orkin S. H., Williams D. A. Long-term expression of human adenosine deaminase in mice transplanted with retrovirus-infected hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8892–8896. doi: 10.1073/pnas.86.22.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Xiao M., Clapp D. W., Li Z. H., Broxmeyer H. E. High efficiency retroviral mediated gene transduction into single isolated immature and replatable CD34(3+) hematopoietic stem/progenitor cells from human umbilical cord blood. J Exp Med. 1993 Dec 1;178(6):2089–2096. doi: 10.1084/jem.178.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Garcia J. V., von Suhr N., Lynch C. M., Wilson C., Eiden M. V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991 May;65(5):2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. G., Edwards R. H., Miller A. D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- O'Hara B., Johann S. V., Klinger H. P., Blair D. G., Rubinson H., Dunn K. J., Sass P., Vitek S. M., Robins T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990 Mar;1(3):119–127. [PubMed] [Google Scholar]
- Orlic D., Anderson S., Biesecker L. G., Sorrentino B. P., Bodine D. M. Pluripotent hematopoietic stem cells contain high levels of mRNA for c-kit, GATA-2, p45 NF-E2, and c-myb and low levels or no mRNA for c-fms and the receptors for granulocyte colony-stimulating factor and interleukins 5 and 7. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4601–4605. doi: 10.1073/pnas.92.10.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D., Bodine D. M. Pluripotent hematopoietic stem cells of low and high density can repopulate W/Wv mice. Exp Hematol. 1992 Dec;20(11):1291–1295. [PubMed] [Google Scholar]
- Orlic D., Fischer R., Nishikawa S., Nienhuis A. W., Bodine D. M. Purification and characterization of heterogeneous pluripotent hematopoietic stem cell populations expressing high levels of c-kit receptor. Blood. 1993 Aug 1;82(3):762–770. [PubMed] [Google Scholar]
- Osborne W. R., Hock R. A., Kaleko M., Miller A. D. Long-term expression of human adenosine deaminase in mice after transplantation of bone marrow infected with amphotropic retroviral vectors. Hum Gene Ther. 1990 Spring;1(1):31–41. doi: 10.1089/hum.1990.1.1-31. [DOI] [PubMed] [Google Scholar]
- Richardson C., Ward M., Podda S., Bank A. Mouse fetal liver cells lack functional amphotropic retroviral receptors. Blood. 1994 Jul 15;84(2):433–439. [PubMed] [Google Scholar]
- Schlegel R., Tralka T. S., Willingham M. C., Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983 Feb;32(2):639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Sorrentino B. P., Brandt S. J., Bodine D., Gottesman M., Pastan I., Cline A., Nienhuis A. W. Selection of drug-resistant bone marrow cells in vivo after retroviral transfer of human MDR1. Science. 1992 Jul 3;257(5066):99–103. doi: 10.1126/science.1352414. [DOI] [PubMed] [Google Scholar]
- Sorrentino B. P., McDonagh K. T., Woods D., Orlic D. Expression of retroviral vectors containing the human multidrug resistance 1 cDNA in hematopoietic cells of transplanted mice. Blood. 1995 Jul 15;86(2):491–501. [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Terstappen L. W., Huang S., Safford M., Lansdorp P. M., Loken M. R. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38- progenitor cells. Blood. 1991 Mar 15;77(6):1218–1227. [PubMed] [Google Scholar]
- Thomas E. D., Storb R., Clift R. A., Fefer A., Johnson L., Neiman P. E., Lerner K. G., Glucksberg H., Buckner C. D. Bone-marrow transplantation (second of two parts). N Engl J Med. 1975 Apr 24;292(17):895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Danos O., Grossman M., Raulet D. H., Mulligan R. C. Expression of human adenosine deaminase in mice reconstituted with retrovirus-transduced hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):439–443. doi: 10.1073/pnas.87.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J. H., Sands M. S., Barker J. E., Gwynn B., Rowe L. B., Vogler C. A., Birkenmeier E. H. Reversal of pathology in murine mucopolysaccharidosis type VII by somatic cell gene transfer. Nature. 1992 Dec 24;360(6406):749–753. doi: 10.1038/360749a0. [DOI] [PubMed] [Google Scholar]
- Yee J. K., Miyanohara A., LaPorte P., Bouic K., Burns J. C., Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusechem V. W., Kukler A., Heidt P. J., Valerio D. Long-term expression of human adenosine deaminase in rhesus monkeys transplanted with retrovirus-infected bone-marrow cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7640–7644. doi: 10.1073/pnas.89.16.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeijl M., Johann S. V., Closs E., Cunningham J., Eddy R., Shows T. B., O'Hara B. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kalle C., Kiem H. P., Goehle S., Darovsky B., Heimfeld S., Torok-Storb B., Storb R., Schuening F. G. Increased gene transfer into human hematopoietic progenitor cells by extended in vitro exposure to a pseudotyped retroviral vector. Blood. 1994 Nov 1;84(9):2890–2897. [PubMed] [Google Scholar]