Abstract
Malignant gliomas are lethal cancers in the brain and heavily infiltrated by myeloid cells. Interleukin-4 receptor-α (IL-4Rα) mediates the immunosuppressive functions of myeloid cells, and polymorphisms in the IL-4Rα gene are associated with altered glioma risk and prognosis. In this study, we sought to evaluate an hypothesized causal role for IL-4Rα and myeloid suppressor cells in glioma development. In both mouse de novo gliomas and human glioblastoma cases, IL-4Rα was upregulated on glioma-infiltrating myeloid cells but not in the periphery or in normal brain. Mice genetically deficient for IL-4Rα exhibited a slower growth of glioma associated with reduced production in the glioma microenvironment of arginase, a marker of myeloid suppressor cells which is critical for their T cell inhibitory function. Supporting this result, investigations using bone marrow-derived myeloid cells showed that IL-4Rα mediates IL-13-induced production of arginase. Furthermore, glioma-derived myeloid cells suppressed T cell proliferation in an IL-4Rα-dependent manner, consistent with their identification as myeloid-derived suppressor cells. Granulocyte-macrophage colony-stimulating factor (GM-CSF) plays a central role for the induction of IL-4Rα expression on myeloid cells, and we found that GM-CSF is upregulated in both human and mouse glioma microenvironments compared with normal brain or peripheral blood samples. Together, our findings establish a GM-CSF-induced mechanism of immunosuppression in the glioma microenvironment via upregulation of IL-4Rα on myeloid-derived suppressor cells.
Keywords: MDSC, Glioma, IL-4Rα, Arginase, Immunosuppression
INTRODUCTION
Malignant gliomas represent approximately 80% of all malignant brain tumors accounting for as many as 26,000 U.S. and European deaths annually, making them a significant unmet medical need (1). Prognosis for malignant glioma patients remain dismal with a median survival of approximately 15 months for glioblastoma following surgery and chemo/radiation therapy (2). Despite extensive research, treatment options for malignant gliomas remain limited. While immunotherapeutic approaches have demonstrated safety and promising preliminary activities (3), their effectiveness can be improved by overcoming the immuno-suppressive mechanisms induced by these tumors (2).
Myeloid cells are the most abundant hematopoietic cells in the human body and have diverse functions. Mounting evidence indicates that the tumor microenvironment alters myeloid cells, and the concept of myeloid-derived suppressor cells (MDSCs) has emerged (4, 5). MDSCs represent a heterogenic population of immature myeloid cells (IMCs) with an impaired ability to fully develop into macrophages, granulocytes, or dendritic cells and have highly pleiotropic abilities to suppress a variety of T-cell functions and promote tumor growth through effector molecules including arginase (4, 5).
In mice, MDSCs are identified as cells that simultaneously express the two markers CD11b and Gr1 (6-8), and are subdivided into two different subsets based on their expression of the two molecules Ly6C and Ly6G (4). CD11b+Ly-6G−Ly6Chigh cells have monocytic-like morphology and are termed monocytic-MDSCs (M-MDSCs), while CD11b+Ly6G+Ly6Clow cells have granulocyte-like morphology and are termed granulocytic-MDSCs (G-MDSCs). In cancer patients, MDSCs are defined as cells that express the common myeloid marker CD33 but lack markers of mature myeloid cells, such as the human leukocyte antigen (HLA)-DR (9-13). Human MDSCs can be divided into at least two subsets that likely parallel those in the mouse model: the CD15+ G-MDSCs and the CD14+ M-MDSCs. IL-4Rα expression on MDSCs is known to play a role in their immunosuppressive functions (14-17).
With regard to the roles of myeloid cells in glioma environment [reviewed in (18)], although glioblastoma are highly infiltrated by microglia/macrophages (19), molecular mechanisms need to be elucidated as to how glioma-infiltrating myeloid cells influence the glioma growth. Recent epidemiology studies have reported that single nucleotide polymorphisms (SNPs) in IL-4Rα are associated with altered glioma risk and prognosis (20, 21), suggesting a possibility that IL-4Rα expression on myeloid cells may impact the glioma development. We therefore sought to determine whether IL-4Rα expression on myeloid cells plays a role in glioma development. Here we demonstrate, using a de novo glioma model and human malignant glioma tissues, GM-CSF, which is expressed at high levels in the glioma microenvironment, leads to up-regulation of IL-4Rα on CD11b+Gr1+ IMCs, thereby promoting the induction of arginase via IL-13. Our data demonstrate a novel immuno-suppressive mechanism in malignant glioma.
MATERIALS AND METHODS
Animals
BALB/c-background wild-type (WT) and Il4ra deficient mice were obtained from The Jackson Laboratory. Animals were maintained in the Animal Facility at the University of Pittsburgh per an Institutional Animal Care and Use Committee-approved protocol.
Bone Marrow (BM)-MDSC Generation
A similar procedure has been previously described (22, 23). Briefly, red blood cell (RBC) depleted BM cells were isolated from WT or Il4ra−/− mice. Granulocyte colony-stimulating factor (G-CSF) (100 ng/ml) and GM-CSF (250 U/ml) were added on days 0, 4 and 9 with IL-13 added (80 ng/ml) on days 4 and 9. All cytokines were purchased from Peprotech. CD11b+ cells were positively selected on day 10 and used in further experiments.
Arginase Activity Assay
The QuantiChrome™ arginase assay detection kit (DARG-200) was used according to the manufacturer’s instructions, optical density was determined at 430nm using a multiscan RC plate reader (Thermo).
MDSC-mediated T-cell Inhibition
CD8+ T-cells were isolated from WT BALB/c splenocytes (SPCs) using magnetic bead negative separation (Miltenyi Biotec), labeled with 100nM CFDA SE (Invitrogen) and incubated with varying amounts of day 10 cultured BM- or glioma-derived MDSCs for 5 days in the presence of anti-CD3/anti-CD28 Dynabeads (Invitrogen) and 30U/ml of hIL-2 (Peprotech). Cells were then analyzed by flow cytometry on an AccuriC6 (BD biosciences).
Antibody-mediated Immune Cell Depletion
The procedure has been described previously (8). Anti-Gr1 (RB6-8C5), anti-CD4 (GK1.5) and anti-CD8 (TIB105) monoclonal antibodies (mAbs) were obtained from Taconic; control IgG was obtained from Sigma-Aldrich. Mice with developing gliomas received i.p. injections of anti-Gr1 (0.25 mg/dose) 3x/week or anti-CD4 and anti-CD8 (0.5mg/dose) 2x/week starting on day 21 after induction of de novo glioma.
Real-time (RT)-PCR
The procedure has been described previously (7, 8). Primers and probes were obtained from Applied Biosystems. Human or mouse GAPDH was used as an internal control. All reactions were done in triplicate and relative expressions of RNAs compared to control samples were calculated using the ΔΔCT method.
Induction of de novo Gliomas by Intraventricular Transfection of Sleeping Beauty-Transposon-flanked Proto-Oncogenes
The procedure has been described previously (24). Briefly, DNA transfection reagent (In vivo-JetPEI) was obtained from Polyplus Transfection. The following DNA plasmids were used for glioma induction: pT2/C-Luc//PGK-SB13, pT/CAGGS-NRASV12, pT2/shP53 and PT3.5/CMV-EGFRvIII (0.125 μg for each). For immunological evaluation of WT and Il4ra−/− tumors, we conducted bioluminescence imaging (BLI) using an IVIS200 (Caliper Life Sciences) and evaluated tumors of comparable size (BLI of 2×108 luciferase units).
BM Chimera
BM chimera experiments were conducted as previously described (25). Briefly, RBC depleted BM cells were isolated from donor WT or Il4ra−/− mice. Host BALB/c-background WT mice received 10 Gy of total body irradiation followed by tail vein injection of 1 × 106 viable BM cells. The efficiency of our BM chimera protocol was confirmed to be >96% using donor BM cells derived from enhanced green fluorescent protein (EGFP)-transgenic mice (Supplementary Figure 1).
Isolation of Murine Brain Infiltrating Leukocytes (BILs)
BILs were isolated using methods described previously (7, 26) using the Percoll (Sigma-Aldrich) isolation method. Due to the small number of BILs obtained per mouse, BILs obtained from all mice in a given group (5 mice/group) were pooled and then evaluated for the relative number and phenotype of the BILs between groups.
Isolation of Human Glioma-infiltrating Leukocytes and peripheral blood mononuclear cells (PBMC)
De-identified fresh glioma tissues were obtained from the operating room per IRB-approved protocol, mechanically minced, resuspended in 70% Percoll (Sigma-Aldrich), overlaid with 37% and 30% Percoll, and centrifuged for 20 min at 500 × g. Enriched leukocyte populations were recovered at the 70%-37%. PBMC were isolated from whole blood using a standard Ficoll procedure (Stemcell Technologies).
Statistical Analyses
Statistical significance of differences between two groups was determined by Student’s t-test. The Log-rank test was used to determine significant differences in survival curves on Kaplan Meier plots among groups. All data were analyzed by GraphPad Prism (v5.0), P<0.05 was considered to be statistically significant.
RESULTS
Il4rα−/− mice exhibit delayed growth of de novo glioma compared with WT mice
To evaluate the role of IL-4Rα on glioma growth, we induced de novo gliomas by Sleeping Beauty (SB) transposon-mediated intraventricular transfection of the oncogenes EGFRvIII, NRas, and short hairpin (sh)P53 in neonatal BALB/c-background WT and Il4rα−/− mice (hereby SB glioma). While the median symptom-free survival (SFS) for WT mice was 55.5 days, Il4rα−/− mice exhibited prolonged survival with a median SFS of 90 days (p <0.001) (Figure 1A). As IL-4Rα is expressed on some MDSCs (15-17), we next evaluated whether the genetic deletion of Il4rα impacts the glioma infiltration of myeloid cells, such as CD11b+Gr1+ cells, which are likely MDSC (Figure 1B and 1C). In WT mice, SB glioma-bearing brains demonstrated higher numbers of CD11b+Gr-1+ cells compared with non-tumor bearing brains. Further, in the presence of the SB gliomas, WT CD11b+Gr-1+ contained a higher percentage of IL-4Rα expressing cells than those in non-tumor bearing animals. Compared with WT animals, non-tumor bearing brains of Il4ra−/− hosts had significantly fewer numbers of CD11b+Gr-1+ cells which did not increase significantly in the presence of the SB tumor (Figure 1B and 1C).
Figure 1. Effects of IL-4Rα on glioma development.
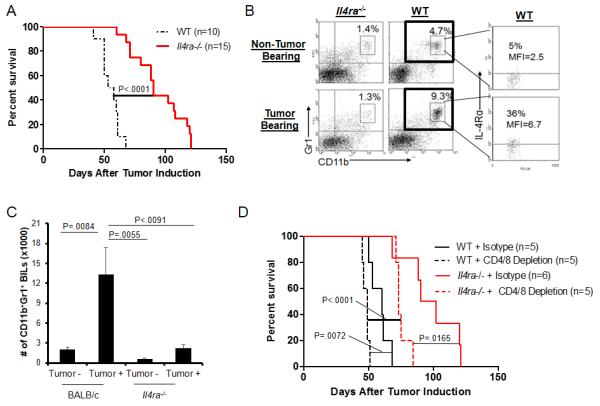
De novo SB gliomas were induced in neonatal mice. (A) SFS following the induction of gliomas. SFS of Il4ra−/− mice was significantly longer than WT mice. (B) Brains with or without de novo gliomas were harvested from WT and Il4ra−/− mice. BILs were analyzed for the percentage of CD11b+Gr1+ populations in leukocyte-gated cells and IL-4Rα expression on CD11b+Gr1+ cells. (C) Absolute numbers of CD11b+Gr1+ BILs in tumor-bearing or non-tumor bearing mice. Bars represent the mean and the standard deviation (SD) of results from 3-independent experiments. (D) Glioma-bearing WT and Il4ra−/− mice were depleted for CD4+ and CD8+ cells. Control mice received isotype control rat IgG. SFS was evaluated.
To determine whether the different expression levels of IL-4Rα and numbers of CD11b+Gr-1+ cells in tumor-bearing hosts are also seen in periphery, we analyzed SPCs derived from SB glioma-bearing mice (Supplementary Figure 2). Similar to our observation in the brain, WT but not Il4rα−/− hosts demonstrated an increase of CD11b+Gr1+ cells in the spleen following induction of SB glioma. However, unlike the brain, IL-4Rα expression levels on peripheral CD11b+Gr1+ cells remained low even in tumor-bearing animals. Thus IL-4Rα expression on CD11b+Gr1+ cells appears to be relatively limited to the cells infiltrating in the gliomas.
T-cells deficient of IL-4R or its major signaling molecule signal transducer and activator of transcription (STAT)-6 are typically skewed towards type-1 immune response, which is known to promote anti-tumor immunity (2, 27-29). To exclude a possibility that the prolonged survival of Il4rα−/− mice is due solely to enhanced anti-tumor T-cell response, we induced SB gliomas in WT and Il4rα−/− hosts in which CD4+ and CD8+ T-cells were depleted (Figure 1D) and Supplementary Figure 3). Although depletion of T-cells significantly accelerated the growth of gliomas in both WT and Il4ra−/− mice, Il4ra−/− mice still demonstrated improved SFS over WT mice when both were depleted of T-cells. These data demonstrate that the improved survival of Il4ra−/− mice is at least partially independent of T-cells.
Il4rα−/− tumor tissue and tumor-derived CD11b+Gr1+ myeloid cells express decreased levels of inhibitory molecules
To examine the impact of IL-4Rα on the glioma microenvironment, total RNA was extracted from WT or Il4ra−/− de novo gliomas of similar size, and inflammation-associated genes were evaluated by RT-PCR (Figure 2A). The gliomas in WT mice demonstrated significantly higher levels of immunosuppressive Tgfb and Arg1 than those in Il4ra−/− mice. Notably, while similar levels of IL-13 were detected in WT and Il4ra−/− tumors, IL-4 expression was undetectable.
Figure 2. Effects of IL-4Rα on the glioma microenvironment and glioma-infiltrating myeloid cells.
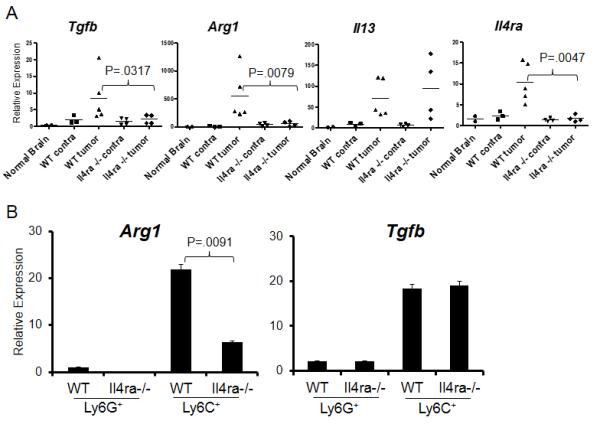
(A) Total RNA was isolated from brains of non-tumor-bearing mice (normal brain), the contralateral (contra) or tumor-bearing (tumor) hemispheres of brains derived from WT and Il4ra−/− mice. mRNA expression levels of Tgfb, Arg1, Il13 and Il4ra were analyzed by RT-PCR, relative to normal brain. (B) BILs from WT or Il4ra−/− mice were sorted for cell populations double-positive for CD11b and Ly6C or CD11b and Ly6G. Arg1 and Tgfb mRNA levels were evaluated by RT-PCR in each of these populations. Samples were analyzed relative to WT Ly6G+ sorted cells. Bars represent the mean and SD of results from triplicates.
To better understand the significance of IL-4Rα expression in CD11b+Gr1+ cells in the glioma, we isolated two subsets of CD11b+Gr1+ cells by fluorescence-activated cell sorting (FACS), CD11b+Ly6Chigh monocytic cells and CD11b+Ly6Ghigh granulocytic cells, and analyzed MDSC-associated genes by RT-PCR (Figure 2B). While CD11b+Ly6Chigh cells expressed higher levels of both Tgfb and Arg1 than CD11b+Ly6Ghigh cells, Arg1 expression was significantly lower in Il4ra−/− CD11b+Ly6Chigh cells than WT counterparts. These data suggest a significant role of IL-4Rα expression on MDSCs in the glioma microenvironment, especially though Arg1.
Depletion of CD11b+Gr1+ cells prolongs survival of mice bearing de novo gliomas
We next examined whether depletion of CD11b+Gr1+ cells prolongs survival in mice bearing the de novo gliomas. While there are multiple methods to deplete CD11b+Gr1+ cells, such as with chemotherapeutics Sunitinib or Gemcitabine (30, 31), these also could have direct anti-tumor activities. Thus, we used anti-Gr1 (RB6-8C5) monoclonal mAb which efficiently depleted CD11b+Gr1+ cells in de novo glioma models in our previous studies (7, 8). To maintain complete depletion, we administered 50 mg/dose anti-Gr1 mAb 3x/week starting at day 23 (Figure 3A) (7, 8). Mice depleted of CD11b+Gr1+ cells experienced significantly prolonged SFS (Figure 3B) with 3 of 7 animals surviving past day 120 (median survival of 74 days), while all control mice treated with control isotype IgG died by day 68 (median survival of 55.5 days). BLI revealed that some (n=3) mice treated with anti-Gr1 mAb even experienced tumor regression below the level of detection (Figure 3C). These data demonstrate the importance of CD11b+Gr1+ cells in the development of de novo glioma.
Figure 3. Depletion of CD11b+Gr1+ cells in mice bearing de novo gliomas.
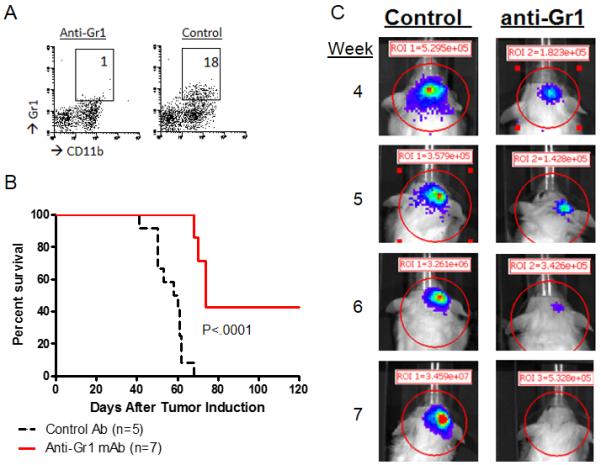
Mice bearing de novo gliomas received anti-Gr1 mAb (A) CD11b+Gr1+ BILs from mice receiving control rat IgG or anti-Gr1 mAb. The number in each panel indicates the percentage of CD11b+Gr1+ cells in leukocyte-gated populations. (B) SFS was monitored in glioma-bearing mice treated with anti-Gr1 mAb or control IgG until day 120. (C) Representative of BLI showing complete regression of glioma following anti-Gr1 mAb treatment and rapid progression with control IgG treatment at Weeks 4-7 following the tumor induction.
BM chimeric mice reveal that Il4rα on BM cells is critical for accumulation of CD11b+Gr1+ cells in the brain
As we demonstrated in Figure 1, gliomas in Il4ra−/− mice had fewer infiltrating CD11b+Gr1+ cells than in WT controls. To assess whether the difference was specifically due to intrinsic factors in BM cells, we evaluated the impact of Il4ra status on glioma-infiltration of CD11b+Gr1+ cells using a BM chimera system. Because induction of de novo gliomas is possible in neonatal mice only but not adult mice (24), as an alternative glioma model, BM chimera mice received stereotactic injections of cultured glioma cells established from syngeneic de novo glioma. In both SPCs and BILs, mice with WT BM displayed greater numbers of CD11b+Gr1+ cells but lower numbers of CD4+ and CD8+ T-cells compared with ones with Il4ra−/− BM (Figure 4). These data suggest that IL-4Rα expression on BM-derived cells promotes the systemic distribution of CD11b+Gr1+ cells but may inhibit that of T-cells.
Figure 4. Critical role of IL-4Rα on BM cells in the immunological environment of glioma.
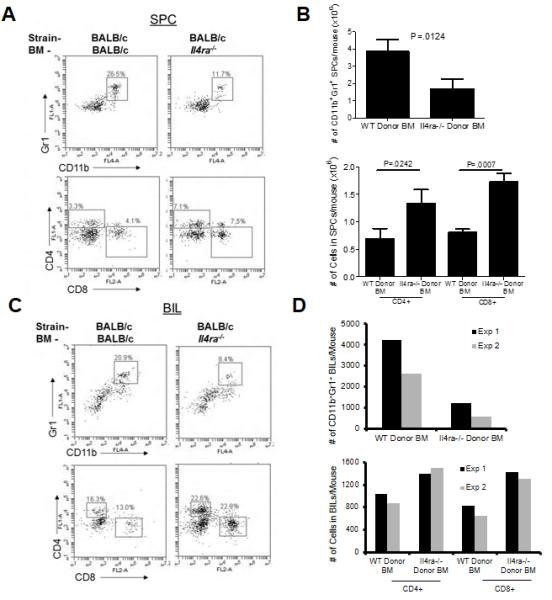
WT mice chimeric with either WT or Il4ra−/− mouse-derived BM received intracranial injections of syngeneic glioma cells. At 3 weeks after glioma cell inoculation, SPCs and BILs were harvested and analyzed by flow cytometry. (A and C) CD11b+Gr1+ double-positive cells (top) and CD4+ and CD8+ cells (bottom) in SPCs (A) and BILs (C). The numbers in each histogram indicate the % of gated populations in leukocyte- (CD11b+Gr1+ cell analysis) or lymphocyte-gated (CD4+ and CD8+ cell analysis) cells. Data represent results from one of five spleens or a pooled BIL sample in one of two experiments with similar results. (B and D) Absolute numbers of CD11b+Gr1+ cells (top) and CD4+ or CD8+ cells (bottom). Bars represent the mean and SD in five spleens (B). In pooled BILs (D), bars represent the mean from each of 2 independent experiments.
IL-4Rα signaling promotes the T-cell-suppressing function of BM-derived and glioma-infiltrating CD11b+Gr1+ cells
Using mouse CD11b+Gr1+ cells derived from BM of WT or Il4ra-/- mice, we first confirmed previously reported observations (6, 14) that the IL-13-IL-4Rα signaling mediates T-cell suppressing activities of IMCs via induction of arginase (Supplementary Figure 4). Notably, WT CD11b+Gr1+ cells suppressed T-cell proliferation and IFN-γ levels at lower BM-CD11b+Gr1+:T-cell ratios than Il4ra−/− CD11b+Gr1+ cells. Furthermore, supplementation with an arginase inhibitor Nw-hydroxy-nor-arginine or L-arginine inhibited the T-cell suppressing activity of WT CD11b+Gr1+ cells. When we isolated CD11b+ cells from gliomas growing in the brain of WT or Il4ra-/- mice, WT mouse-derived myeloid cells demonstrated more profound levels of inhibition on CD8+ T-cell proliferation compared with ones derived from Il4ra-/- mice (Supplementary Figure 5). These data indicate that CD11b+Gr1+ BILs are indeed capable of suppressing T-cell proliferation in an IL-4Rα-dependent manner. We hereby term CD11b+Gr1+ BILs MDSCs in the glioma environment.
GM-CSF up-regulates IL-4Rα on BM cells and is overexpressed in gliomas
As IL-4Rα expression on CD11b+Gr1+ cells is increased in de novo gliomas, we next examined the factors in the glioma microenvironment that lead to the up-regulation of IL-4Rα. BM-derived cells were cultured with G-CSF (100 ng/ml), GM-CSF (250 U/ml), IL-13 (80 ng/ml) or tumor-conditioned media (TCM) from the culture of a de novo glioma-derived cell line for 4 days, and IL-4Rα expression was then measured by flow cytometry (Figure 5A). While G-CSF, GM-CSF and TCM treatment all up-regulated IL-4Rα expression in three independent experiments, GM-CSF treatment had the most pronounced effect. We thus evaluated our hypothesis that the glioma microenvironment exhibits elevated levels of GM-CSF compared with normal brains or peripheral blood cells. Indeed, in both mouse de novo (Figure 5B) and human (Figure 5C) glioma tissues displayed higher GM-CSF expression levels compared with normal brains, contralateral brains (tested in mice only) and PBMC. When we evaluated protein levels of GM-CSF in patient-derived glioblastoma tissues by ELISA, we found that the levels (mean of 2.85 ng/gram tissue, n=8) (Figure 5D) were very similar to those we found effective to promote IL-4Rα up-regulation in mouse BM-derived MDSCs in vitro [experiments in Figure 5A using 250 U/ml (≈2.5 ng/ml)], suggesting that glioma-derived GM-CSF may be sufficient to induce IL-4Rα on myeloid cells in the glioma microenvironment.
Figure 5. GM-CSF promotes IL-4Rα expression on myeloid cells and is up-regulated in human and mouse glioma tissues.
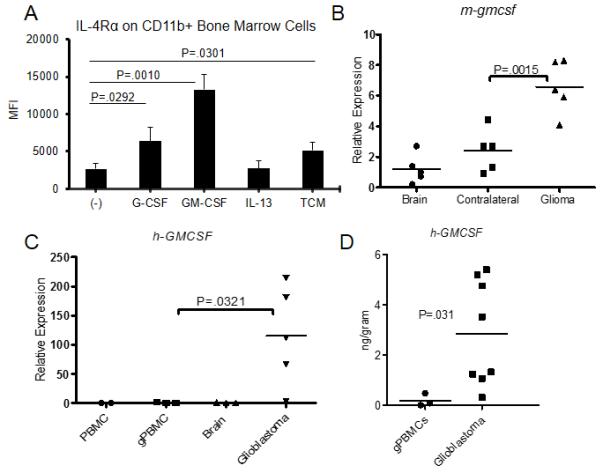
BM cells were cultured in the presence of G-CSF (100 ng/ml), GM-CSF (250 U/ml), IL-13 (80 ng/ml), or TCM for 4 days. (A) IL-4Rα expression was determined by flow cytometry. Bars represent the mean and SD of mean fluorescence intensity (MFI) from 3 independent experiments. (B) Mouse gmcsf (m-gmcsf) was evaluated from brains of non-tumor-bearing mice (Brain), or the contralateral (Contralateral) or tumor-bearing (Glioma) hemispheres of WT mice bearing de novo glioma, relative to Brain. (C), Human GMCSF (h-GMCSF) expression was evaluated in total RNA isolated from healthy donor-derived PBMCs (PBMCs; n=2), glioma patient-derived PBMCs (gPBMCs; n=3), normal human brain tissue (Brain; n=3) or glioblastoma tissue (Glioblastoma; n=5), relative to PBMCs. (D) GM-CSF levels were evaluated by ELISA in protein extracts of gPBMCs and glioblastoma tissues. Values are adjusted to one gram of blood (for gPBMCs) or tumor tissue (for glioblastoma).
Human Glioma-infiltrating CD14+HLA-DR− monocytes express IL-4Rα associated with suppressor functions
As murine M-MDSCs (CD11b+Ly6Chigh), but not G-MDSCs (CD11b+Ly6Ghigh) express enhanced levels of Arg1 (Figures 2B) and as it has been reported that human monocytic CD33+CD14+HLA-DR− cells have T-cell suppressive (i.e., MDSC) functions (32-34), we next evaluated IL-4Rα expression on human glioblastoma-infiltrating (n=7) and glioblastoma patient PBMC-derived (n=5) CD14+HLA-DR− cells by flow cytometry (Figure 6A). Although glioblastoma tumor-infiltrating leukocytes (TILs) and PBMC cannot be isolated by the same method (see Materials and Methods), using identical forward and side scatter gating on CD14+HLA-DR− monocytes in both types of samples, IL-4Rα was detected on 20-30% of CD14+HLA-DR− TIL while IL-4Rα was barely detectable on corresponding populations in the PBMC. We further examined IL-4Rα expression on frozen glioblastoma TILs (n=13) and glioblastoma-patient derived PBMC (n=10) (Figure 6B left). Consistently, all glioblastoma-infiltrating CD33+CD14+HLA-DR− cells, but not peripheral CD14+HLA-DR− cells had detectable IL-4Rα+ cell populations, including those in 4 matched patient samples (Figure 6B right).
Figure 6. IL-4Rα expression on human tumor-infiltrating monocytes.
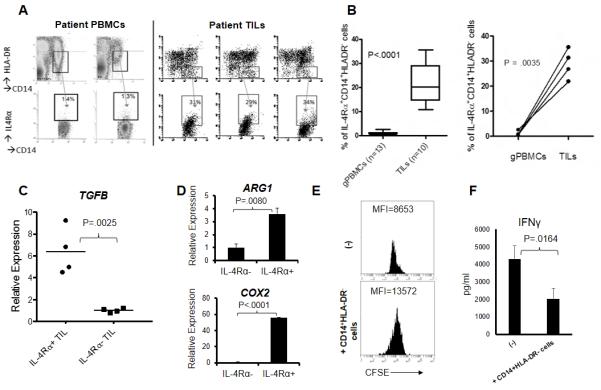
TILs were isolated from fresh glioblastoma tissues by Percoll density separation; and glioblastoma patient-derived PBMCs were collected by Ficoll method. (A) IL-4Rα was analyzed on CD14+HLA-DR− cells. (B) Percentages of CD14+HLA-DR− cells expressing IL-4Rα in the PBMCs (n=13) and GBM tissues (n=10) (Left). Percentages of IL-4Rα+ cells among CD14+HLA-DR− cells in the paired patient PBMCs and GBM tissues (n=4) (Right). (C) CD14+HLA-DR− populations of TILs were sorted for two separate populations based on IL-4Rα+ or IL-4Rα−, and evaluated for expression levels of TGFB in relative to those in PBMCs which are negative for IL-4Rα. (D) CD14+HLA-DR− populations of a GBM patient-derived leukapheresis sample were sorted for two separate populations based on IL-4Rα+ or IL-4Rα−, and evaluated by RT-PCR for expression levels of ARG1 and COX2, relative to the IL-4Rα− sample. (E) CD14+HLA-DR− cells were sorted from glioblastoma-derived TILs and cultured with PBMC-derived, CFSE-labeled CD8+ T-cells for 5 days at a ratio of 1:4 (CD14+HLA-DR− cells:T-cells). Proliferation was analyzed by flow cytometry gating on live lymphocytes; and (F) IFN-γ levels were assessed by ELISA.
We next addressed whether IL-4Rα expression on glioblastoma-infiltrating CD33+CD14+HLA-DR− cells was associated with immune suppressor functions. Using FACS, we isolated IL-4Rα-positive and -negative sub populations of TILs, extracted total RNA and analyzed TGFB (Figure 6C). IL-4Rα+ cells had higher TGFB expression levels than their IL-4Rα− counterparts. Although we also analyzed expression of ARG1 and COX2, possibly due to limited numbers of human glioblastoma-infiltrating cells, expression of these molecules was below our limit of detection in both IL-4Rα positive and negative CD14+HLA-DR− monocytes. We therefore examined IL-4Rα-positive and negative populations in a leukapheresis-derived PBMC obtained from a glioblastoma patient (Figure 6D). Although there was a much smaller percentage (about 5% of CD14+HLA-DR− cells) of IL-4Rα+ cells compared with those in TILs, CD14+HLA-DR− IL-4R+ cells had higher levels of ARG1 and COX2 expression than their IL-4R− counterpart. Importantly, glioblastoma-derived CD14+HLA-DR− cells suppressed proliferation of autologous PBMC-derived CD8+ T-cells (Figure 6E) and IFN-γ production (Figure 6F), indicating that these cells are indeed MDSCs in glioblastoma. These data strongly suggest that IL-4Rα on CD14+HLA-DR− cells in the tumor microenvironment is important for the immunosuppressive activity of these cells.
DISCUSSION
An ideal immunotherapy for gliomas would maximize the therapeutic index by both improving anti-tumor effector immune cell-functions and inhibiting the immune suppressor cells. Our data demonstrate for the first time, in glioblastoma patients and the de novo murine glioma model, that IL-4Rα is up-regulated on myeloid cells specifically in the tumor environment but not in the periphery. While we addressed our main focus on CD11b+Gr1+/high cells as the most abundant BIL population in the brain (Figure 1B), we have also noted that all CD11b+Gr1+ cells, including CD11b+Gr1low cells, express up-regulated IL-4Rα in the glioma microenvironment compared with those cells in the non-tumor bearing brain (Supplementary Figure 2).
Our studies using in vitro cultured cells and cells isolated from glioma-bearing hosts collectively suggest that GM-CSF, which is uniquely up-regulated in the glioma microenvironment, induces IL-4Rα expression on myeloid cells, thereby facilitating IL-13-induced arginase expression and resulting T-cell suppression. Our data are consistent with a previous report that GM-CSF and the GM-CSF-receptor on gliomas correlates with advanced tumor stage (35). While we did not examine mechanism of GM-CSF up-regulation in human and mouse gliomas, it is possible that GM-CSF may be up-regulated through an oncogenic Ras-dependent mechanism (36, 37), which was expressed in our de novo gliomas. Although Ras mutations are uncommon in human gliomas, activation of the Ras pathway is typical via signaling from receptor tyrosine kinases that are often overexpressed in human gliomas.
Il4ra−/− gliomas are infiltrated by significantly fewer CD11b+Gr1+ cells than gliomas in WT mice. It remains elusive whether this is due to differential mobilization from BM, systemic expansion and/or survival of these cells. Based on a study evaluating anti-IL-4Rα aptamer treatment (38), blockade of IL-4Rα resulted in increased apoptosis of MDSCs, suggesting that Il4ra−/− MDSCs may be more prone to apoptosis. Our BM chimera experiments further revealed that the increased number of CD11b+Gr1+ cells in WT mice is intrinsic to hematopoietic cells as total body irradiated mice receiving Il4ra−/− BM had fewer CD11b+Gr1+ cells than mice receiving WT BM in both spleens and brains. More work is warranted to determine the precise mechanisms as to how Il4ra status impact the generation of CD11b+Gr1+ cells systemically.
Our findings are consistent with previous reports on IL-4Rα signaling for arginase expression (14, 16, 22). Interestingly, Arg1 expression levels were significantly higher in Ly6C+ monocytic MDSCs than those in Ly6G+ granulocytic MDSCs. Together with the fact that approximately 75% of CD11b+ BILs were Ly6C+ cells in our mouse model (data not shown), T-cell inhibition by MDSC in our model appears to be largely mediated by arginase.
Higher levels of Tgfb were detected in de novo gliomas in WT mice compared with those in Il4ra−/− mice. While CD11b+Ly6C+ cells expressed higher levels of Tgfb compared with CD11b+Ly6G+ populations in both WT and Il4ra−/− mice, there was not a significant difference in the Tgfb expression levels between WT and Il4ra−/− cells. This may mean that the higher Tgfb levels in the whole glioma tissue in WT mice may be attributed to the higher number of glioma-infiltrating myeloid cells in WT mice compared to Il4ra−/− mice. It is also possible that, as an indirect MDSC-mediated mechanism, other cells in the glioma environment, such as regulatory T-cells, which can be induced by MDSCs, may also contribute to the higher Tgfb expression in WT gliomas (39, 40). While the expression of Tgfb in murine MDSCs was not influenced by Il4ra expression, human IL-4Rα positive cells expressed elevated levels of TGFB compared with IL-4Rα negative cells. Our group and others have previously demonstrated the immune-suppressive role of TGFB and its impact on gliomas (41) and other cancers (16). Further studies are warranted to understand the differential regulation of TGF-β expression between murine and human MDSCs.
Our finding that Il4ra−/− mice have prolonged SFS compared with WT mice in the absence of T-cells suggests that cells other than T-cells are also important for the prolonged survival of Il4ra−/− mice bearing de novo gliomas. Thus, it is important to note that in the absence of T-cells, IL-4Rα+ myeloid cells may exert suppressive functions on other immune cells, such as NK cells, or possibly promotion of tumor cell growth via non-immunological mechanisms, such as enhancement of angiogenesis. It is also noteworthy that, although the median survival of mice treated with anti-Gr1 mAb was shorter than that of Il4ra-/- mice, 3 of 7 animals receiving anti-Gr1 mAb for depletion of Gr1+ cells survived for longer than 120 days. While IL-4Rα plays a critical role in MDSCs, based on the partial abrogation of the MDSC-mediated T-cell suppression in Il4ra-/- mice (Supplementary Figure 5), it is likely MDSCs have other non-IL-4Rα-dependent mechanisms of immune suppression, and thus the depletion of Gr1+ cells may have more robust impacts than the disruption of Il4ra.
In humans, healthy donor-derived human CD14+ monocytes exposed to glioma cells acquire MDSC-like properties, including increased production of IL-10, TGF-β, and B7-H1 and a heightened ability to induce apoptosis in activated lymphocytes (42). Patients with glioblastoma have more circulating CD33+HLA-DR− MDSCs in their peripheral blood than do normal donors (42, 43). Furthermore, significant increases in arginase 1 activity levels have been observed in plasma of glioblastoma patients (43, 44). Interestingly, T-cell suppression in glioblastoma was completely reversed through the pharmacologic inhibition of arginase 1 or with arginine supplementation (44). In regard to MDSC subpopulations, a recent study examining 6 MDSC subpopulations in renal cell cancer patients identified 2 subtypes, CD14+HLA-DR-/lo and CD11b+CD14−CD15+ cells, negatively associated with overall patients survival (45). In the current study, as the vast majority (approximately 75%) of the MDSCs in mouse SB gliomas are ly6C+ monocytic MDSCs expressing Arg1, we focused on the monocytic CD14+HLA-DR− population in human glioblastoma. Although glioblastoma is densely infiltrated by microglia/macrophages (19, 46), to our knowledge, our current study is one of the first to characterize the phenotype and function of MDSCs in human gliomas.
Our findings demonstrate a novel mechanism of immuno-suppression in the glioma microenvironment. GM-CSF, which is expressed at high levels in both human and mouse gliomas, promotes IL-4Rα expression on glioma-infiltrating myeloid cells with MDSC-properties, thereby leading to IL-13-mediated production of arginase. Arginase can then suppress anti-tumor immune cells, including T-cells, thereby promoting the development of glioma growth.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the following for their assistance: Dr. Masaki Terabe and Maria Sierra.
Grant Supports from: The NIH [2R01 NS055140, 2P01 NS40923, 1P01 CA132714] and Musella Foundation for Brain Tumor Research and Information. This project used UPCI shared resources (Animal Facility, Small Animal Imaging facility and Cytometry Facility) that are supported in part by NIH P30CA047904.
Footnotes
The authors have no conflict of interest to report.
Reference List
- 1.Vredenburgh JJ, Desjardins A, Reardon DA, Friedman HS. Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol. 2009;11:80–91. doi: 10.1215/15228517-2008-075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okada H, Kohanbash G, Zhu X, Kastenhuber ER, Hoji A, Ueda R, et al. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009;29:1–42. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-Cell Responses Against Novel Glioma-Associated Antigen Peptides and Clinical Activity by Vaccinations With {alpha}-Type 1 Polarized Dendritic Cells and Polyinosinic-Polycytidylic Acid Stabilized by Lysine and Carboxymethylcellulose in Patients With Recurrent Malignant Glioma. J Clin Oncol. 2011;29:330–6. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. Journal of Immunology. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youn J-I, Gabrilovich DI. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. European Journal of Immunology. 2010;40:2969–75. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–74. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita M, Scheurer ME, Decker SA, McDonald HA, Kohanbash G, Kastenhuber ER, et al. Role of Type 1 IFNs in Antiglioma Immunosurveillance—Using Mouse Studies to Guide Examination of Novel Prognostic Markers in Humans. Clinical Cancer Research. 2010;16:3409–19. doi: 10.1158/1078-0432.CCR-10-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almand B, Clark JI, Nikitina E, van BJ, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. The Journal of Immunology. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 10.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 11.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 12.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J ClinOncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 13.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. The Journal of clinical investigation. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. JImmunol. 2009;182:6562–8. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 16.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohanbash G, Okada H. Myeloid-derived suppressor cells in gliomas and glioma-development. Immunol Invest. 2012;41:658–79. doi: 10.3109/08820139.2012.689591. [DOI] [PubMed] [Google Scholar]
- 19.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. Journal of Clinical Neuroscience. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartzbaum JA, Ahlbom A, Lonn S, Malmer B, Wigertz A, Auvinen A, et al. An international case-control study of interleukin-4Ralpha, interleukin-13, and cyclooxygenase-2 polymorphisms and glioblastoma risk. Cancer EpidemiolBiomarkers Prev. 2007;16:2448–54. doi: 10.1158/1055-9965.EPI-07-0480. [DOI] [PubMed] [Google Scholar]
- 21.Scheurer ME, Amirian E, Cao Y, Gilbert MR, Aldape KD, Kornguth DG, et al. Polymorphisms in the interleukin-4 receptor gene are associated with better survival in patients with glioblastoma. ClinCancer Res. 2008;14:6640–6. doi: 10.1158/1078-0432.CCR-07-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–47. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Wiesner SM, Decker SA, Larson JD, Ericson K, Forster C, Gallardo JL, et al. De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res. 2009;69:431–9. doi: 10.1158/0008-5472.CAN-08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epperly MW, Shields D, Niu Y, Carlos T, Greenberger JS. Bone marrow from CD18−/− (MAC-1−/−) homozygous deletion recombinant negative mice demonstrates increased longevity in long-term bone marrow culture and decreased contribution to irradiation pulmonary damage. In Vivo. 2006;20:431–8. [PubMed] [Google Scholar]
- 26.Zhu X, Fallert-Junecko BA, Fujita M, Ueda R, Kohanbash G, Kastenhuber ER, et al. Poly-ICLC promotes the infiltration of effector T cells into intracranial gliomas via induction of CXCL10 in IFN-alpha and IFN-gamma dependent manners. Cancer Immunol Immunother. 2010;59:1401–9. doi: 10.1007/s00262-010-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita M, Zhu X, Ueda R, Sasaki K, Kohanbash G, Kastenhuber ER, et al. Effective Immunotherapy against Murine Gliomas Using Type 1 Polarizing Dendritic Cells--Significant Roles of CXCL10. Cancer Res. 2009;69:1587–95. doi: 10.1158/0008-5472.CAN-08-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada H. Brain tumor immunotherapy with type-1 polarizing strategies. Ann N Y Acad Sci. 2009;1174:18–23. doi: 10.1111/j.1749-6632.2009.04932.x. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki K, Pardee AD, Qu Y, Zhao X, Ueda R, Kohanbash G, et al. IL-4 suppresses very late antigen-4 expression which is required for therapeutic Th1 T-cell trafficking into tumors. J Immunother. 2009;32:793–802. doi: 10.1097/CJI.0b013e3181acec1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 31.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–9. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117:872–81. doi: 10.1182/blood-2010-05-283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, et al. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443–55. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–45. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 35.Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE. Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol. 1999;155:1557–67. doi: 10.1016/S0002-9440(10)65472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–47. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Liu Y, Li Z, Du J, Ryu MJ, Taylor PR, et al. Endogenous oncogenic Nras mutation promotes aberrant GM-CSF signaling in granulocytic/monocytic precursors in a murine model of chronic myelomonocytic leukemia. Blood. 2010;116:5991–6002. doi: 10.1182/blood-2010-04-281527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-Mediated Blockade of IL4Ralpha Triggers Apoptosis of MDSCs and Limits Tumor Progression. Cancer Res. 2012;72:1373–83. doi: 10.1158/0008-5472.CAN-11-2772. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueda R, Fujita M, Zhu X, Sasaki K, Kastenhuber ER, Kohanbash G, et al. Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Clin Cancer Res. 2009;15:6551–9. doi: 10.1158/1078-0432.CCR-09-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12:351–65. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro-Oncology. 2011;13:591–9. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, et al. Neutrophil Degranulation and Immunosuppression in Patients with GBM: Restoration of Cellular Immune Function by Targeting Arginase I. Clinical Cancer Research. 2011 doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- 45.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nature medicine. 2012 doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 46.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. JNeurosciRes. 2005;81:447–55. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


