Summary
Activation of the FMS-like tyrosine kinase 3 (FLT3) by its ligand, FLT3 ligand (FL), strongly augments development of natural killer (NK) cells from human CD34+ hematopoietic progenitor cells (HPCs) in the presence of interleukin-15 (IL-15), compared to IL-15 alone. In this study, we observed that blocking the receptor tyrosine kinase Axl/Gas6 pathway with a soluble Axl fusion protein (Axl-Fc) in the presence of FL significantly diminished the absolute number of CD3−CD56+ NK cells derived from human CD34+ HPCs. Axl-Fc reduced the expression level of the IL-2/15 receptor β chain (CD122) and γ chain (CD132) induced by activation of FLT3 and consequently reduced the frequency of NK precursor cells responding to IL-15. Furthermore, Axl-Fc diminished FL-induced FLT3 phosphorylation and impeded the physical interaction between Axl and FLT3 in CD34+ HPCs. Collectively, our data suggest that the Axl/Gas6 pathway contributes to normal human NK cell development at least in part via its positive regulatory effect on FLT3 signaling in CD34+ HPCs.
Keywords: NK cell, FLT3, Axl, CD34+ HPC
Introduction
The FMS-like tyrosin kinase 3 (FLT3) is a receptor tyrosine kinase (RTK) and plays an important role in hematopoiesis[1]. FLT3 is mainly expressed in hematopoietic stem and early progenitor cells[2], and its biological significance in hematopoietic development has been underscored by creating mice with a genetic disruption at the FLT3 locus. FLT3−/− mice show hematopoietic defects that include reduced B cell numbers and defective development of T cells[3]. Mice deficient in FLT3 ligand (FL) also show reduced numbers of B-lymphoid progenitors, dendritic cells, and natural killer (NK) cells[4]. Although FL alone cannot drive NK cell differentiation from CD34+ hematopoietic progenitor cells (HPCs), FL can enhance differentiation of NK cells from human CD34+ HPCs in the presence of interleukin-15 (IL-15) when compared to IL-15 alone[5]. Mechanistically, exogenous FL appears to increase expression of the IL-15 receptor beta (IL-15Rβ or CD122) on the surface of CD34+FLT3+ HPCs, thereby increasing the NK cell precursor frequency among total CD34+FLT3+ cells[5]. However, the mechanism(s) by which FLT3 itself is regulated in the context of CD34+ HPCs and human NK cell development is unknown.
The Axl/Gas6 pathway refers to the RTK Axl family proteins which is composed of Axl, Tyro3 (or Sky), and Mer[6], and two natural and highly homologous ligands for the Axl family, growth arrest-specific gene 6 (Gas6) and protein S[7, 8]. The Axl/Gas6 pathway has been shown to be important in a variety of cellular responses, such as cell migration[6], proliferation[9], and NK cell development[10, 11]. In our previous study[11] we showed that we could block the Axl/Gas6 pathway in human CD34+ HPCs with an excess of the soluble chimeric protein Axl-Fc, which is composed of the extracellular domain of Axl and Fc portion of human immunoglobulin G1 (IgG1). In doing so, Axl-Fc diminished STAT5 phosphorylation in CD34+ HPCs following their exposure to IL-15, and diminished the phosphorylation of c-Kit in CD34+ HPCs following their exposure to c-Kit ligand (KL), thereby impeding human NK cell development[11]. In the current study we explored the effects of impeding Axl activation on the RTK FLT3 which is highly homologous to c-Kit[12] and also involved in both murine and human NK cell development.
Results and Discussion
Although the FLT3/FL pathway cannot direct NK cell differentiation from CD34+ HPCs by itself, it can greatly augment NK cell development at least in part by increasing the NK cell precursor frequency, i.e., the number of CD34+ HPCs responsive to IL-15[5].
Inhibition of the Axl/Gas6 pathway decreases human NK cell development induced by FL
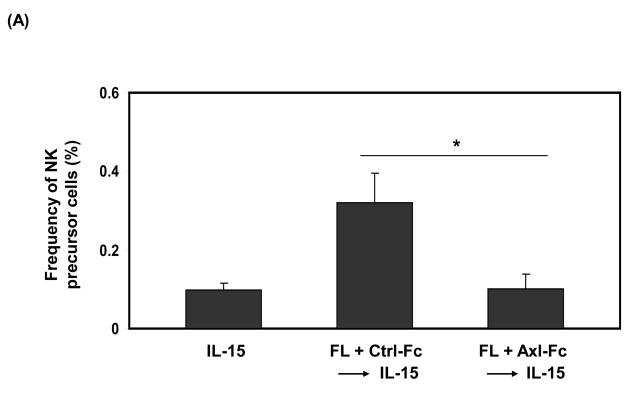
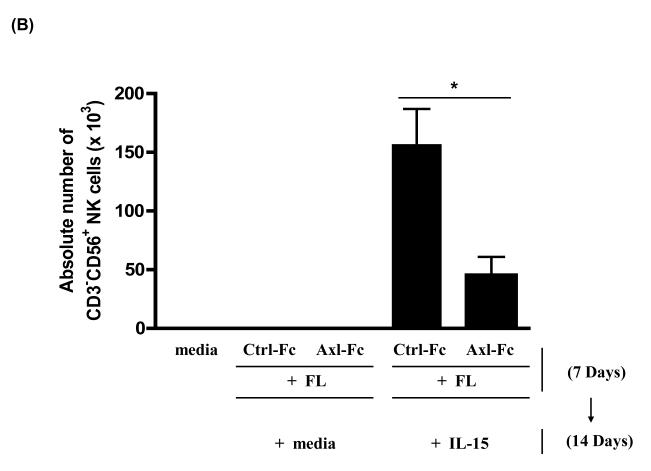
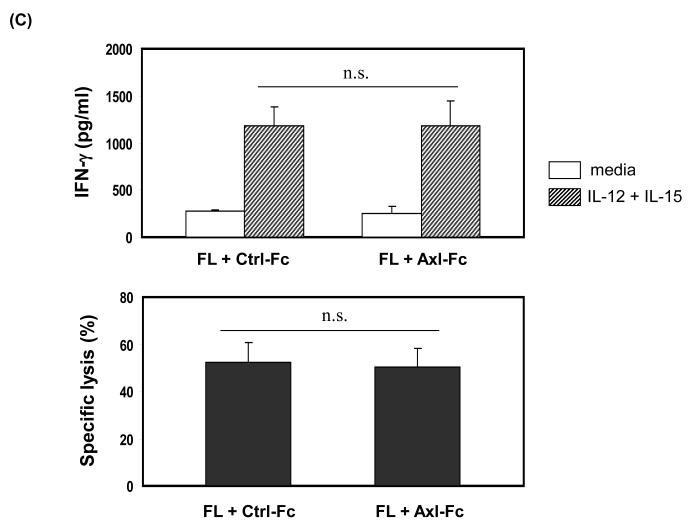
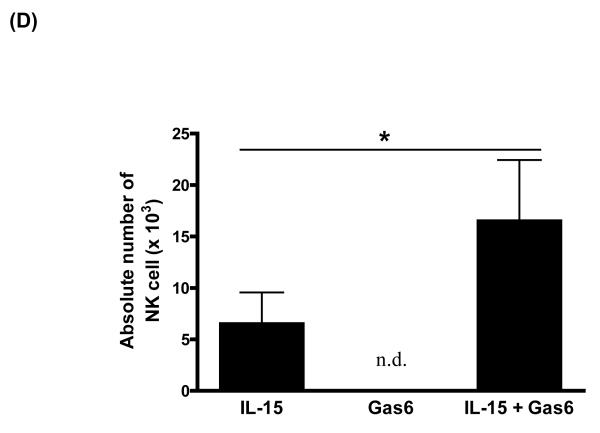
To determine if the Axl/Gas6 pathway regulated NK cell precursor frequency, we cultured human CD34+ HPCs with FL in the presence of control Fc (Ctrl-Fc) or Axl-Fc for 7 days, which was followed by washings to remove Axl-Fc, and subsequent culture with IL-15 alone for another 2 weeks. Interrupting the Axl/Gas6 pathway with Axl-Fc under these conditions significantly reduced the frequency of CD34+ NK precursor cells induced by FL when compared to cultures with Ctrl-Fc (Figure 1A). Consistent with this, the absolute number of CD3−CD56+ NK cells resulting from the differentiation of CD34+ HPCs under these culture conditions was significantly lower in the presence of Axl-Fc when compared to identical cultures in the presence of Ctrl-Fc (Figure 1B). The inhibitory effect of Axl-Fc on the development of NK cells is not due to its direct effect on NK cells as the Axl ligand, Gas6, is not expressed by NK cells [11]. On the other hand, the fewer NK cells that did differentiate from CD34+ HPCs in the presence of Axl-Fc had normal IFN-γ production and cytotoxicity per se when compared to NK cells differentiated from CD34+ HPCs in the presence of Ctrl-Fc (Figure 1C). It is consistent with the previous report showing that addition of FL into the culture of CD34+ HPCs did not alter either IFN-γ production or cytotoxicity by differentiated NK cells[5]. Furthermore, the absolute number of differentiated NK cells was significantly higher when CD34+ HPCs were cultured with IL-15 plus recombinant Gas6 protein, compared to IL-15 only (Figure 1D). Gas6 alone, however, did not lead to NK cell development. Collectively, these results suggest that the Axl/Gas6 pathway is required in order to obtain an optimal number of CD34+ NK cell precursors generated by the FLT3/FL pathway, as well as for the optimal number of NK cells differentiated from CD34+ NK precursors in the presence of IL-15. However the Axl/Gas6 pathway does not appear to have any impact on the function of the mature NK cells generated in these culture conditions with regard to IFN-γ production and cytotoxic activity.
Figure 1. The Axl/Gas6 pathway is important for human NK cell development by FLT3/FL.
(A) Isolated CD34+ HPCs from human peripheral blood were plated using serial dilution (from 6,000 to 94 cells/well × 12 wells/condition). CD34+ HPCs were cultured in one of three conditions: 1) IL-15 (100 ng/ml) for 2 weeks (IL-15); 2) FL (100 ng/ml) plus Ctrl-Fc (1 μg/ml) for 7 days followed by culture in IL-15 (100 ng/ml) alone for 2 weeks (FL + Ctrl-Fc → IL-15); 3) FL (100 ng/ml) plus Axl-Fc (1 μg/ml) for 7 days followed by culture in IL-15 (100 ng/ml) alone for 2 weeks (FL + Axl-Fc → IL-15). Cells cultured in medium alone are non-viable within 7 days. At the completion of culture in IL-15, cells were stained with anti-CD3 and anti-CD56 antibodies and NK precursor frequency was determined as described in the Materials and Methods. Results are from 5 donors and show the mean + standard error of the mean (S.E.M.). * p < 0.05. (B) Purified CD34+ HPCs from human peripheral blood were cultured for 7 days with FL (100 ng/ml) in the presence of Ctrl-Fc or Axl-Fc (1 μg/ml). Cells were then washed twice and then resuspended in culture medium along with IL-15 (100 ng/ml) for the following 2 weeks. Harvested cells were stained with conjugated anti-CD3 and anti-CD56 antibodies or conjugated isotype control antibody and analyzed by flow cytometry. Graph shows the absolute numbers of CD3−CD56+ NK cells (calculated by multiplying total number of cells counted in each well times the percentage of NK cells). Result is from 5 separate donors and the bar showing the S.E.M. * p < 0.05. (C) Differentiated NK cells were prepared as described in (B), isolated, and plated in equivalent numbers to measure IFN-γ production (top) or cytotoxicity (bottom). For the quantification of IFN-γ, NK cells generated from human CD34+ HPCs under each culture condition were treated with recombinant human IL-12 (10 ng/ml) and IL-15 (100 ng/ml) for 24 hours after which the concentration of IFN-γ in the culture supernatant was measured by ELISA. For cytotoxicity, NK cells prepared from (B) were cultured for 4 hours with 51Cr-labeled NK-sensitive K562 target cells at an effector to target ratio of 5 to 1. Graphs show the data from 3 donors and error bar shows S.E.M. n.s., not significant. (D) Isolated CD34+ HPCs from human peripheral blood were cultured with IL-15 alone (100 ng/ml), recombinant Gas6 protein alone (1 μg/ml), or IL-15 plus Gas6 for 14 days. Cells were then stained with anti-CD3 and anti-CD56 antibodies, analyzed with flow cytometry, and the absolute number of differentiated NK cells was calculated. The graph shows the data from 3 separate donors (mean + S.D). * p < 0.05 (paired t-test). n.d., not detected.
Inhibition of the Axl/Gas6 pathway diminishes IL-15 responsiveness of CD34+ HPCs
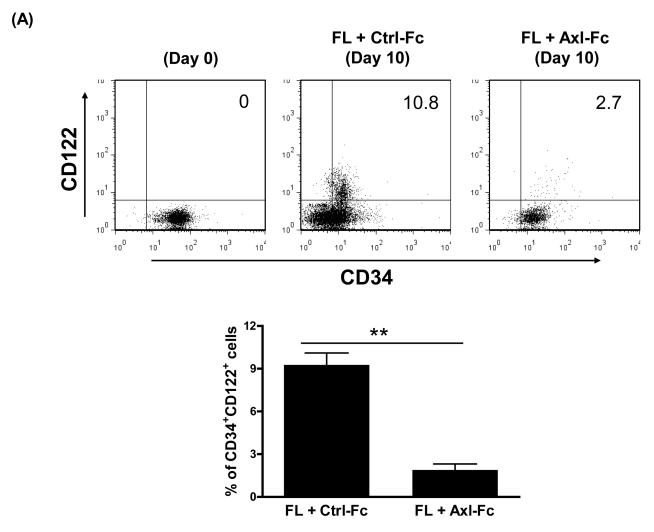
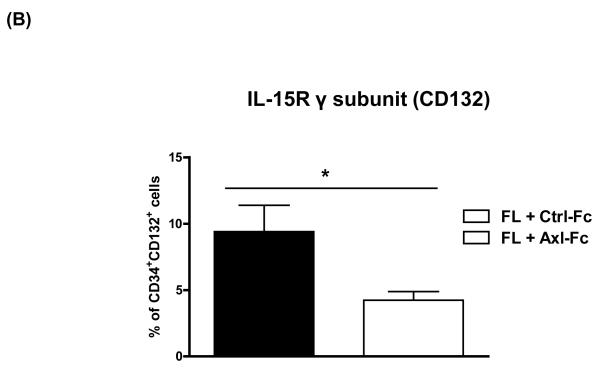
One mechanism by which FL enhances NK cell precursor frequency for FLT3+CD34+ HPCs is by upregulating their expression of IL-15Rβ (or CD122), thereby generating more HPCs responsive to IL-15 and thus NK cell differentiation[5]. We asked if blocking the Axl/Gas6 pathway in FLT3+CD34+ HPCs cultured with FL would affect the surface density expression of CD122. As shown in Figure 2A, freshly isolated human CD34+ HPCs showed little or no surface density expression of CD122 (top left, Figure 2A), yet this was augmented by 10 days of culture in FL plus Ctrl-Fc (top middle, Figure 2A). When identical CD34+ HPCs were cultured for 10 days in the presence of FL and Axl-Fc there was a significantly lower surface density expression of CD122 on the CD34+ HPCs (top right and bottom, Figure 2A). In addition, we tested whether Axl-Fc could regulate other components of the IL-15 receptor (IL-15R), i.e. IL-15R α subunit (IL-15Rα) and γ subunit (IL-15Rγ, CD132). Like CD122, neither receptor was detected by flow cytometry in freshly isolated CD34+ HPCs (data not shown). When CD34+ HPCs were cultured with FL, the expression level of IL-15Rγ was increased, and this increase was significantly inhibited in the presence of Axl-Fc (Figure 2B). On the other hand, IL-15Rα expression was not induced by FL (data not shown). This suggests that the adverse effects on human NK cell development from CD34+ HPCs caused by the interruption of the Axl/Gas6 pathway results, at least in part, from the latter’s impedance of signaling down the FLT3/FL pathway.
Figure 2. The Axl/Gas6 pathway positively regulates FLT3 activation.
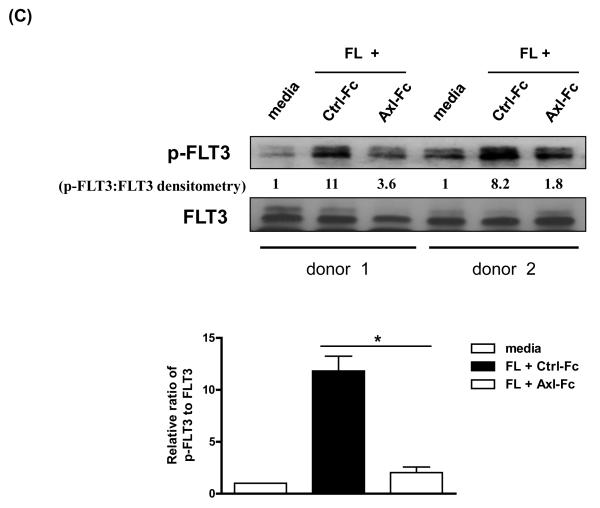
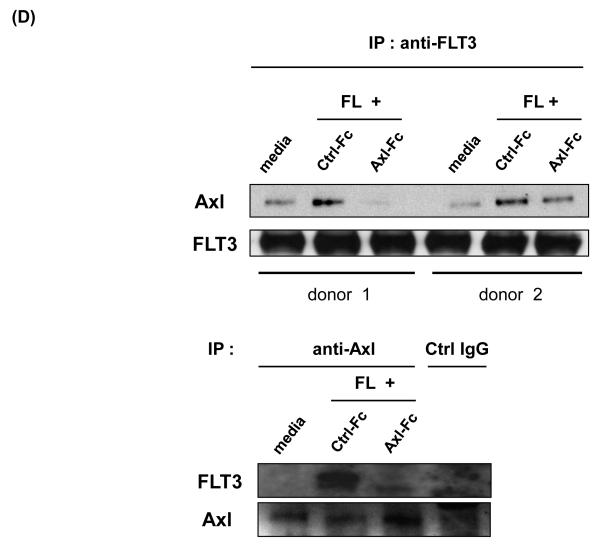
(A) Purified CD34+ HPCs from human peripheral blood were either analyzed on day 0 or cultured in FL for 10 days in the presence of Ctrl-Fc or Axl-Fc. Cells were stained with anti-CD34 and anti-IL-15Rβ subunit (CD122) antibodies. The top of the figure shows a representative flow cytometric plot. Numbers indicate the percentage of CD34+CD122+ cells (upper right quadrant). The bottom depicts a graph summarizing the data from 3 donors. ** p < 0.01. (B) Purified CD34+ HPCs from human peripheral blood were cultured with FL for 10 days in the presence of Ctrl-Fc or Axl-Fc. Cells were stained with anti-CD34 and anti-IL-15Rγ subunit (CD132) antibodies. The graph summarizes the data from 3 donors with 3 separate experiments. * p < 0.05. (C) (Top) Purified CD34+ HPCs from two different donors were pre-incubated with Ctrl-Fc or Axl-Fc for 30 min, which was followed by treatment with FL for 15 min. Immunoblot was performed to detect phospho-FLT3 (p-FLT3) and total FLT3. Band density was measured and numbers indicate the relative ratio of p-FLT3 to FLT3. The p-FLT3:FLT3 ratio of CD34+ HPCs treated with media only was arbitrarily set as one. (Bottom) The graph summarizes the data pooled from 4 donors (mean + S.E.M.). * p < 0.05 (D) Purified CD34+ HPCs from two different donors were pre-incubated with Ctrl-Fc or Axl-Fc for 30 min, which was followed by treatment with FL for 15 min. Thereafter, cell lysates were prepared, which was followed by immunoprecipitation using anti-FLT3 (top) or anti-Axl (bottom) antibody and protein A-agarose beads. Protein A-agarose beads were pelleted by centrifugation, washed four times, boiled, and subject to immunoblot to detect Axl and FLT3.
Axl/Gas6 pathway positively regulates FLT3 activation in CD34+ HPCs
It has been well known that the phosphorylation of FLT3 which follows the binding of its ligand FL is essential for biological activities of the FLT3/FL pathway[13, 14]. Furthermore, our previous report documented that the Axl/Gas6 pathway was crucial for optimal KL-induced phosphorylation of c-Kit[11] (which is highly homologous to FLT3[12]) in human CD34+ HPCs. Therefore we asked if impedance of the Axl/Gas6 pathway would result in a diminished FL-induced FLT3 phosphorylation in human CD34+ HPCs. As shown in Figure 2C, interruption of the Axl/Gas6 pathway resulted in a marked reduction of FLT3 phosphorylation in the presence of FL when compared to CD34+ HPCs incubated with Ctrl-Fc and FL. These data also support the notion that the Axl/Gas6 pathway is important for signaling via the FLT3/FL pathway and participates in FL-induced human NK cell development by positively regulating FLT3 signaling.
Physical interaction between Axl and FLT3
Previous studies showed that Axl family receptors functionally and physically interact with a variety of signaling molecules, such as PI3K and Ras/Erk, and in turn induce activation of those signaling pathways[15]. We therefore examined whether Axl and FLT3 would physically interact with each other and, if so, if abrogation of Axl activation would diminish the interaction between two molecules. While we did indeed observe a low, basal level of constitutive binding of Axl with FLT3, physical association between Axl and FLT3 was substantially increased when FLT3 is stimulated by FL in CD34+ HPCs. When Axl-Fc was added, however, that physical association, as documented by immunoblot analysis, was significantly decreased. These results suggest that activation of both Axl and FLT3 is necessary for optimal physical association between the two molecules. As Axl-Fc was able to decrease FL-induced FLT3 phosphorylation, it seems likely that an activated Axl positively regulates FL-induced FLT3 phosphorylation through physical interaction with activated FLT3.
Concluding Remarks
In summary, we have demonstrated here that impedance of the Axl/Gas6 pathway adversely impacts FL-induced FLT3 phosphorylation in CD34+ HPCs. Diminished FLT3 phosphorylation in turn decreases the expression of IL-15Rβ (CD122) and IL-15Rγ (CD132) on CD34+ NK precursor cells and consequently decreases the frequency of their differentiation into mature human NK cells in the presence of IL-15. Our study provides mechanistic insight as to how two RTKs, Axl and FLT3, interact and eventually promote optimal NK cell development. In conjunction with our earlier study[11] the work presented here indicates that impedance of the Axl/Gas6 pathway reduces optimal signaling of two receptor tyrosine kinases, c-Kit and FLT3. This raises the possibility that the Axl/Gas6 pathway could be important for the normal development and growth of multiple cell types under the regulatory control of these and other RTKs.
Materials and Methods
Isolation of CD34+ HPCs from human peripheral blood and generation of NK cells
Human CD34+ HPCs were isolated from human leukopaks (obtained from American Red Cross, Columbus, OH) with approval from The Ohio State University Institutional Review Board. After the leukopak was incubated with a Rosette NK enrichment cocktail (StemCell Technologies, Canada) for 30 min at room temperature, mononuclear cells were obtained using Ficoll-Paque™ Plus (GE Healthcare, UK). CD34+ HPCs were then isolated using human Indirect CD34 MicroBead kit (Miltenyi Biotec, Germany) (> 95% purity). Purified CD34+ progenitor cells were plated (10,000 to 20,000 cells per well) on a 96-well plate with α-minimal essential medium (MEM) (Cambrex, Walkersville, MD) containing 20% fetal bovine serum (GIBCO, Grand Island, NY), and FL (100 ng/ml) (R&D Systems, Minneapolis, MN). After culturing for 7 days, cells were washed and cultured with IL-15 (100 ng/ml) (R&D Systems) alone for another 2 weeks. Harvested cells were then stained with anti-CD3 and anti-CD56 antibodies (BD Biosciences, Franklin Lakes, NJ) and analyzed by the FACSCalibur flow cytometer (BD Biosciences).
Assessment of functional activities of differentiated NK cells
Following culture of human CD34+ HPCs with the cytokines and reagents as described in the figures, cells were harvested and differentiated NK cells were isolated using CD56 microbeads (Miltenyi Biotec). For measuring IFN-γ production, NK cells generated from human CD34+ HPCs under each culture condition were plated in equivalent numbers (10,000 cells/well) in a round-shape 96-well cell culture plate in RPMI 1640 (GIBCO) containing 10% fetal bovine serum (GIBCO). Cells were then treated with recombinant human IL-12 (10 ng/ml) and IL-15 (100 ng/ml) for 24 hours, and culture supernatant was collected. IFN-γ concentration was measured by the ELISA using anti-IFN-γ capture antibody and biotin-labeled detection antibody from Pierce Endogen (Rockford, IL). For cytotoxicity, NK cells prepared as described for assessment of IFN-γ production were plated with equivalent numbers in a V-shape 96-well culture plate and cultured for 4 hours with 51Cr (Perkin Elmer, Waltham, MA)-labeled NK-sensitive K-562 target cells (5,000 cells/well) at an effector to target ratio of 5 to 1. Supernatants were collected and percentage of specific lysis was measured as previously described[16].
Limiting dilution assay
NK precursor frequency was determined by limiting dilution assay as previously described[5, 17]. Briefly, isolated human CD34+ HPCs from peripheral blood were plated onto a 96-well plate (6,000 cells per well to 94 cells per well with serial dilution) and cultured with FL (100 ng/ml) (R&D Systems) in the presence of control Fc (Ctrl-Fc) or Axl-Fc (each 1 μg/ml) for 7 days. After washing the cells twice, the cells were further cultured with IL-15 (100 ng/ml) alone for another 2 weeks. Separately, CD34+ HPCs from the same donor were cultured with IL-15 alone for 2 weeks. After culture, cells were harvested and analyzed for expression of CD56 and CD3 by flow cytometry to score positive (CD3−CD56+) or negative by comparison to staining with isotype control antibody. NK precursor frequency was calculated as the reciprocal of the number of cells that resulted in 37% negative wells as described previously[5, 17].
Immunoblot and immunoprecipitation
In order to measure the level of phosphorylated FLT3 and total FLT3, anti-phospho-FLT3, anti-FLT3 rabbit monoclonal antibodies (both antibodies from Cell Signaling, Danvers, MA), and anti-rabbit secondary antibody conjugated with horseradish peroxidase (GE Healthcare, UK) were used, which was followed by detection using enhanced chemiluminescence system (GE Healthcare). For immunoprecipitation, cells were lysed with RIPA buffer containing phosphatase inhibitor cocktail (Sigma, St. Louis, MO) and immunoprecipitated using anti-FLT3 rabbit antibody (Cell Signaling) or anti-Axl goat antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and Protein A-agarose beads (Santa Cruz Biotechnology). Protein A-agarose beads were pelleted by centrifugation and washed four times with RIPA buffer containing phosphatase inhibitor cocktail, which was followed by immunoblot to detect Axl and FLT3.
Statistical Analysis
Data from experimental groups were compared using the Student’s t-test and it was considered statistically significant when p value is less than 0.05.
Acknowledgement
We thank Ms. Mao Charlene for flow cytometry analysis. This work was supported by the grants from the National Institutes of Health (CA16058, CA95426, CA68458, and CA09338 to M.A.C.).
Abbreviations
- FLT3
FMS-like tyrosine kinase 3
- FL
FLT3 ligand
- NK
natural killer
- HPC
hematopoietic progenitor cell
Footnotes
Conflict of Interest Statement The authors declare no competing financial interests
References
- 1.Naoe T, Kiyoi H. Normal and oncogenic FLT3. Cell Mol Life Sci. 2004;61:2932–2938. doi: 10.1007/s00018-004-4274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosnet O, Schiff C, Pebusque MJ, Marchetto S, Tonnelle C, Toiron Y, Birg F, Birnbaum D. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993;82:1110–1119. [PubMed] [Google Scholar]
- 3.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 4.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 5.Yu H, Fehniger TA, Fuchshuber P, Thiel KS, Vivier E, Carson WE, Caligiuri MA. Flt3 ligand promotes the generation of a distinct CD34(+) human natural killer cell progenitor that responds to interleukin-15. Blood. 1998;92:3647–3657. [PubMed] [Google Scholar]
- 6.Hafizi S, Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. Febs J. 2006;273:5231–5244. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- 7.Evenas P, Dahlback B, Garcia de Frutos P. The first laminin G-type domain in the SHBG-like region of protein S contains residues essential for activation of the receptor tyrosine kinase sky. Biol Chem. 2000;381:199–209. doi: 10.1515/BC.2000.027. [DOI] [PubMed] [Google Scholar]
- 8.Godowski PJ, Mark MR, Chen J, Sadick MD, Raab H, Hammonds RG. Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell. 1995;82:355–358. doi: 10.1016/0092-8674(95)90424-7. [DOI] [PubMed] [Google Scholar]
- 9.Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–4453. doi: 10.1128/mcb.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caraux A, Lu Q, Fernandez N, Riou S, Di Santo JP, Raulet DH, Lemke G, Roth C. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006;7:747–754. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- 11.Park IK, Giovenzana C, Hughes TL, Yu J, Trotta R, Caligiuri MA. The Axl/Gas6 pathway is required for optimal cytokine signaling during human natural killer cell development. Blood. 2009;113:2470–2477. doi: 10.1182/blood-2008-05-157073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masson K, Ronnstrand L. Oncogenic signaling from the hematopoietic growth factor receptors c-Kit and Flt3. Cell Signal. 2009;21:1717–1726. doi: 10.1016/j.cellsig.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan JF, Kastelein R, Hudak S, Wagner J, Mattson J, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368:643–648. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- 14.Lyman SD, Brasel K, Rousseau AM, Williams DE. The flt3 ligand: a hematopoietic stem cell factor whose activities are distinct from steel factor. Stem Cells. 1994;12(Suppl 1):99–107. discussion 108-110. [PubMed] [Google Scholar]
- 15.Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- 17.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]