Abstract
We report the case of an adolescent girl with anti-N-methyl-D-aspartate-receptor (NMDAR) encephalitis who presented with focal seizures and hemichorea, followed by agitation, speech disturbance, mutism, and autonomic dysfunction. The institution of immunotherapy and removal of an ovarian cystadenofibroma led to full resolution of her symptoms with disappearance of serum NMDAR antibodies. This is the first report linking ovarian cystadenofibroma to anti-NMDAR encephalitis.
Introduction
Anti-N-methyl-D-aspartate-receptor (NMDAR) encephalitis is characterized by psychiatric symptoms at presentation, followed by episodes of hypoventilation, seizures, and movement disorders such as choreoathetoid, dystonia, and rigidity [1]. The clinical presentation of anti-NMDAR encephalitis can be highly variable, which may pose a diagnostic challenge. For example, seizures and movement disorders can be the presenting symptoms in children [2]. The most common underlying tumor in young adult women is ovarian teratoma, with limited reports on alternative ovarian disease. We report the case of a 19-year-old female with anti-NMDAR encephalitis who was found to have a cystic ovarian fibroadenoma and had full resolution of her symptoms with immune therapy and laparoscopic unilateral oopherectomy.
Case Report
A 19-year-old female was admitted to the Children’s Hospital at Montefiore Medical Center with acute right-sided focal motor seizures with secondary generalization. Five days after the onset of seizures, right arm and leg choreiform movements developed. Electroencephalography (EEG) revealed diffuse background slowing without any focal slowing or epileptiform discharges. The patient did not have a history of any concurrent medical problems. The seizures were controlled with intravenous fosphenytoin administration and long-term administration of carbamazepine, which resulted in worsening of the choreiform movements and a switch to levetiracetam. Evaluation for infectious, post-infectious, toxic, metabolic, and vasculitic causes was negative (Table 1). Initial magnetic resonance imaging (MRI) of the brain with and without contrast was normal. Cerebrospinal fluid (CSF) showed 57/mm3 white blood cells, with 88% lymphocytic predominance; protein was 91 mg/dL, and glucose was 75 mg/dL with positive oligoclonal bands.
Table 1.
Differential diagnosis and laboratory results for an adolescent female with acute-onset seizures and chorea
| Differential Diagnosis | Laboratory Test and Results |
|---|---|
| Infectious encephalitis | CSF: white cell 57/mm3, 88% lymphocytic predominance, protein 91 mg/dL, glucose 75 mg/dL, positive oligoclonal bands, negative HSV PCR |
| EEG: diffuse background slowing, no focal slowing or epileptiform discharges | |
| MRI of the brain with contrast: normal | |
| Encephalopathy due to systemic infection | Lyme antibody, ACE, syphilis and HIV: negative |
| Systemic encephalopathy | Serum leukocytes, ESR, CRP: normal |
| Thyroid | Thyroid function test and anti thyroid antibodies: normal |
| SLE | ANA, anti-DNAse B, C3, C4, anti-Ro, anti-La, rheumatoid factor and antiphospholipid antibody: negative |
| Sydenham chorea | Normal ASO titer and negative group A streptococcus throat swab culture |
| Toxicologic | Urine toxicology: negative |
| Chorea gravidarum | Urine pregnancy test: negative |
| Wilson disease | Serum ceruloplasmin and urine copper: normal |
| Acanthocytosis | Blood acanthocytes: negative |
| Primary CNS vasculitis | Cerebral angiogram and brain biopsy: negative |
| Immune-mediated encephalitis | NMDAR antibody: positive |
| Anti-YO, anti-HU, anti-RI, anti-voltage-gated calcium channel and anti-voltage-gated potassium channel: negative |
Abbreviations:
ACE = Angiotensin-converting enzyme
ANA = Antinuclear antibody
anti-DNAse B = Antideoxyribonuclease B
ASO = Antistreptolysin O titer
C = Complement
CRP = C-reactive protein
CSF = Cerebrospinal fluid
EEG = Electroencephalogram
ESR = Erythrocyte sedimentation rate
HIV = Human immunodeficiency virus
HSV = Herpes simplex virus
MRI = Magnetic resonance imaging
NMDA = N-methyl-D-aspartate
PCR = Polymerase chain reaction
SLE = Systemic lupus erythematosus
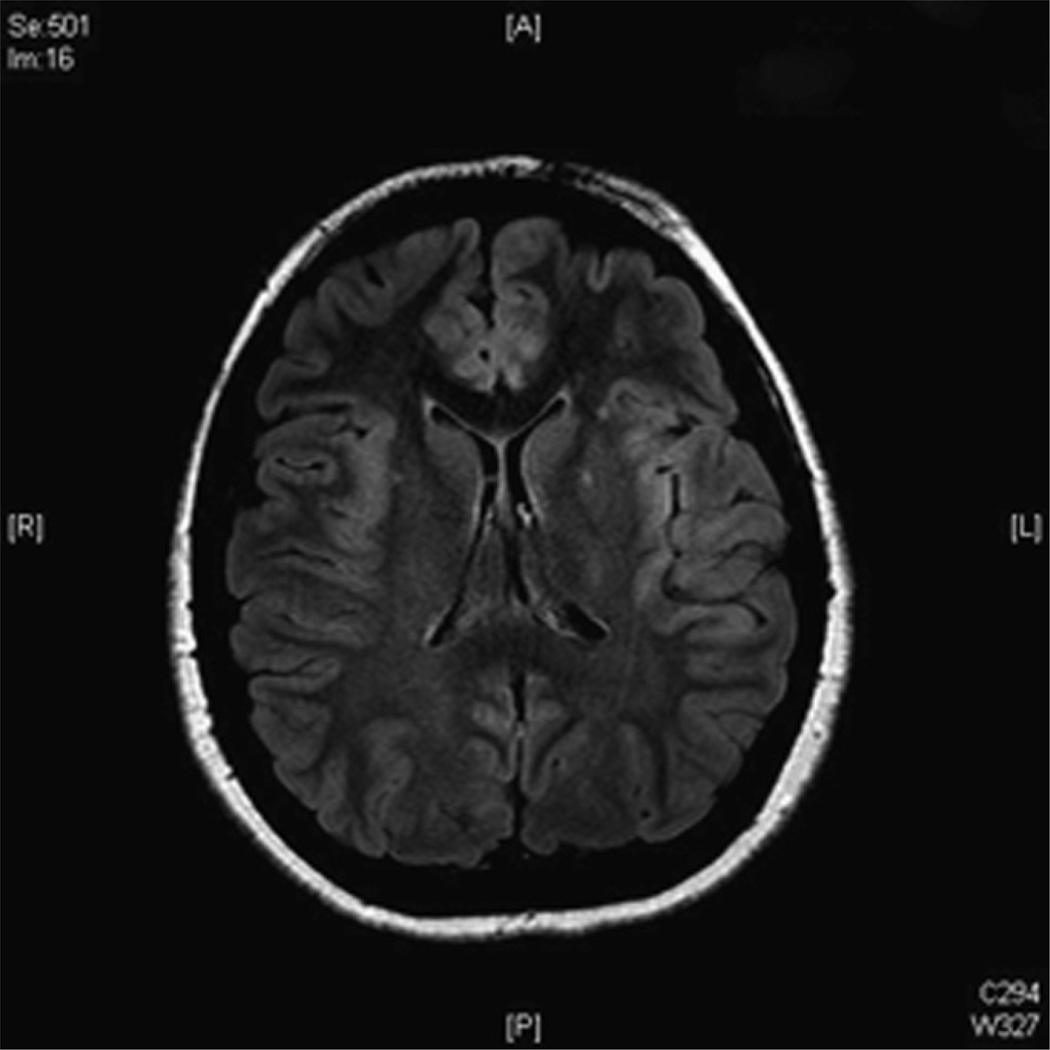
During the second week, worsening chorea, dystonia, and orofacial dyskinesia developed. The patient was lethargic with intermittent agitation. Her speech became slurred and slow. A repeat MRI revealed subtle areas of abnormality involving the right medial frontal lobe, the left frontotemporal cortex, and the insular cortex bilaterally (Fig 1). Repeated EEG showed continuous rhythmic delta slowing over the left frontal and temporal areas, intermittent polymorphic delta and theta slowing over the right temporal area, less well-sustained posterior rhythm over the left occipital area, and frontal intermittent rhythmic delta activity. Conventional cerebral angiography showed normal vessels. The result of single photon emission computed tomography was normal.
Figure 1.
Cranial magnetic resonance imaging (MRI) taken 14 days into clinical course revealed subtle areas of asymmetric increased signal intensity on fluid-attenuated inversion recovery (FLAIR) images involving the bilateral insular cortex.
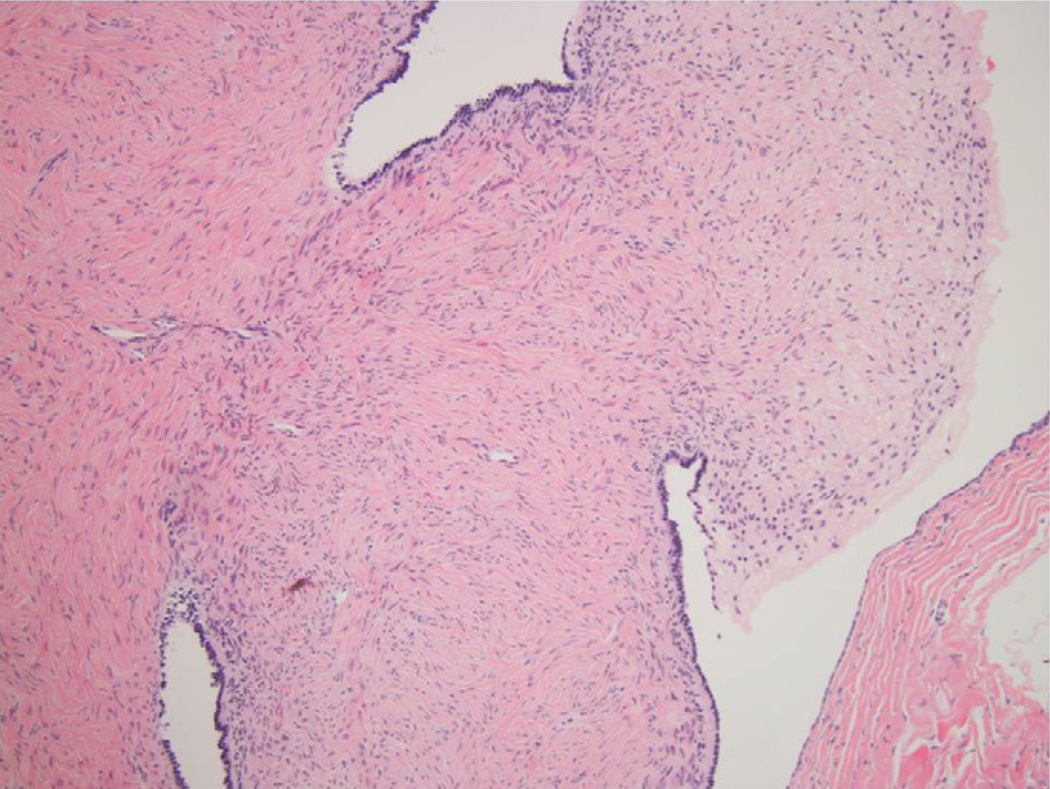
By the third week, the symptoms progressed, and the patient had difficulty swallowing. She became mute with hypersomnia and showed signs of autonomic instability, including hypertension. Because of the patient’s rapid deterioration in clinical status, a left anterior temporal brain biopsy was performed to exclude primary CNS vasculitis. It showed a nonspecific, lymphocytic infiltration of the leptomeninges and neocortex with a minimal increase in microglia (Fig 2). There was no evidence of cerebral vasculitis. Pathology stains and polymerase chain reaction were negative for bacteria, fungus, tuberculosis, cytomegalovirus, herpes simplex virus, and parvovirus.
Figure 2.
Brain biopsy showed chronic meningitis with extension into the Virchow-Robin space and minimal activity in the brain parenchyma. The inflammatory infiltrate extended into the perivascular space (Hematoxylin & eosin staining; original magnification × 100).
Serum NMDAR antibody was positive by the indirect fluorescent antibody technique. The patient’s serum was not sent for confirmatory titer by enzyme-linked immunosorbent assay because, at the time of the indirect fluorescent antibody–positive result, she had already been treated with immunotherapy. CSFNMDAR antibody was not sent after the initial lumbar puncture. Other paraneoplastic markers including anti-YO, anti-HU, anti-Ri, anti-voltage-gated calcium channel, anti-voltage-gated potassium channel, and glutamic acid decarboxylase antibody were negative.
The patient was treated with a 5-day course of intravenous immunoglobulin (IVIG) with a total dose of 2 g/kg. There was an initial improvement in verbal skills, but then the patient became increasingly lethargic and difficult to arouse. She was started on plasmapheresis. She became more alert but was noted to have persistent fluctuations in mental status, as well as abnormal facial and limb movements consisting of more severe choreiform movements, dystonic posturing, and orofacial dyskinesia. Evaluation for occult neoplasm including abdominal ultrasound scanning, an MRI of the chest and abdomen was negative. However, MRI of the pelvis showed a 2.5-cm cyst in the left ovary, which contained a septation or calcification, most consistent with an ovarian cyst or atypical dermoid cyst. Transvaginal ultrasonography confirmed the presence of left-sided hemorrhagic, mobile ovarian cyst, not consistent with tumor or malignancy. The patient’s β human chorionic gonadotropin and cancer antigen 125 test results were negative.
After gynecologic oncology consultation, the patient underwent a left salpingo-oophorectomy and pelvic washing. The ovary and cyst were evaluated in their entirety; pathology reported a serous cystadenofibroma comprising the entire tumor. There was no teratoma element in the ovary or cyst (Fig 3). After surgery, within the first week she had marked clinical improvement. She became more alert, oriented, and able to communicate with minimally slurred speech. No abnormal movements were observed. She regained the ability to swallow and to ambulate with assistance. Ten days after the oopherectomy, serum and CSF NMDAR antibodies were negative.
Figure 3.
Ovarian cyst consisting of polypoid excrescences with columnar ciliated epithelium lining and dense fibrous tissue (Hematoxylin & eosin staining; original magnification × 10).
In the second and third postoperative week, the patient’s persistent symptoms were psychiatric with episodic agitation and mood swings throughout the day with bouts of inattention. She began to have interrupted sleep patterns. Video EEG showed continuous polymorphic delta slowing over the left temporal region, as well as intermittent polymorphic delta slowing over the right temporal region and generalized background slowing. No seizures were captured. Over a period of several weeks, the psychiatric symptoms and sleep disturbances steadily improved until the time of discharge. Her mini-mental status examination at the time of discharge was 23/30.
Three weeks after discharge from the hospital, the patient showed continued improvement. Her mini-mental status examination was 29/30. Within 6 months of discharge, the patient was weaned off antiepileptic drugs. Twelve months later she was employed in her original position, which included management of other employees, with plans to return to college. She will be monitored for tumor surveillance of her remaining ovary with surveillance visits every 6 months for 2 years and then yearly thereafter.
Discussion
We report the clinical course of an adolescent female with anti-NMDAR encephalitis with ovarian cystadenofibroma. The first presenting symptom was focal seizures, followed within 1 week by hemichorea. As her disease progressed, she had development of agitation, speech disturbance (mutism), hypersomnia, and autonomic dysfunction (hypertension), all consistent with her final diagnosis of anti-NMDAR encephalitis.
This case illustrates the challenges associated with making a diagnosis of anti-NMDAR encephalitis early in the course of the disease. Anti-NMDAR encephalitis is a syndrome with multistage symptom development comprised of various psychiatric symptoms, movement disorders, speech disturbances, seizures, autonomic dysfunction, hypoventilation, and insomnia [3]. These can occur in varying degrees of severity over variable periods of time. Most adult patients first present with psychiatric symptoms or memory problems [4]. Unlike adults, nonpsychiatric symptoms such as seizures and abnormal movements are the most common presenting symptoms in children [2,5].
NMDAR antibodies from the serum or CSF are required to make the diagnosis of anti-NMDAR encephalitis. The CSF in patients with anti-NMDAR encephalitis can show a lymphocytic pleocytosis or be normal; oligoclonal bands in CSF were present in more than 80% of children and adolescents with anti-NMDAR encephalitis [2]. EEG findings are nonspecific and often show generalized background slowing or focal slowing—typically in the frontotemporal regions—, and occasionally associated with epileptiform discharges [4]. Dalmau et al. [6] show that MRI of the brain is unremarkable in half of patients with anti-NMDAR encephalitis. In the other 50%, the brain MRI shows T2 or fluid-attenuated inversion recovery signal hyperintensity in the temporal, parietal, frontobasal, and insular regions [6].
Brain biopsy was performed to exclude primary CNS vasculitis. The result was consistent with previously published cases and showed minimal inflammatory cells in the brain parenchyma, and microglial activation [4,7,8]. Currently the pathogenesis of anti-NMDAR encephalitis is believed to be a humoral-mediated process. NMDAR antibodies are directed at surface antigens, causing decreased NMDA receptor density, which is believed to alter NMDA-mediated synaptic currents [9]. Another explanation is that the immune response is primarily mediated by cytotoxic T cells. This is based in part on a detailed immuno-histopathologic study of encephalitis in a patient with NMDA and glycine autoantibodies, which demonstrated prominent lymphocytic inflammation apposed to neurons and only patchy immunoglobulin staining of neurons and astrocytes [5,7].
Demonstration of the presence of antibodies to the NR1 subunit of the NMDAR in serum or CSF establishes the diagnosis. Florance et al. [2] reported that although one third of the children diagnosed with anti-NMDAR encephalitis had negative serum antibody results, all patients had significant levels of NMDAR antibodies in the CSF. Antibodies might be detected only in CSF if the diagnosis is delayed or patients have received immunotherapy [2,10]. In a series of 412 patients in whom serum and CSF were tested for antibodies reported by Dalmau [11], there were no patients in whom antibodies were only present in the serum. The CSF antibody titer is higher in patients with tumors or in those with worse clinical outcomes, than in patients without tumors or with milder symptoms [12]. Our patient had positive serum NMDAR antibodies sent 10 days after symptom onset and before treatment. We did not check for CSF NMDAR antibodies in the first LP.
A significant clinical challenge posed by this case was the significance of the pelvic imaging findings and determining the necessity of an oophorectomy. Anti-NMDAR encephalitis has a strong association with ovarian teratoma, present in more than 50% of young adult women and 30% in girls [2,11]. Teratoma is the only ovarian disease reported to be associated with anti-NMDAR encephalitis. The decision to proceed with unilateral oopherectomy was strongly influenced by the patient’s progressive worsening despite maximal immunotherapy with IVIG and plasmapheresis and low risk of affecting the patient’s fertility. The final pathologic report was cystoadenofibroma without evidence of a teratoma. Ovarian cystadenofibroma is a benign tumor that consists of an epithelial and fibrous stromal component. Ovarian cystadenofibromas account for 1.7% of benign ovarian cysts [13].
It is uncertain whether the ovarian cystadenofibroma could have caused anti-NMDAR encephalitis or was a coincidental finding, and the patient’s clinical improvement was a consequence of delayed response to immune therapy. The patient will receive regular tumor surveillance in the form of pelvic examination and sonography every 6 months for 2 years and then yearly thereafter.
The overall prognosis of anti-NMDAR encephalitis is favorable. About 75% had complete recovery or mild sequelae, significantly better than other paraneoplastic encephalitides [12]. Dalmau et al. [11] reported that in 105 cases of anti-NMDAR encephalitis, most (80%) patients with tumors had improvement after receiving first-line immunotherapy (IVIG, plasmapheresis, steroids) and tumor removal, as compared with only half (50%) of patients in whom no tumor was identified and treated with immune therapy alone (P = 0.001). Those without tumor and no response to first-line immunotherapy may require secondline immunotherapy (rituximab, cyclophosphamide). However, the final long-term outcomes were similar in those with and without tumor [11].
Acknowledgments
The authors thank Dr. Seema Rani, and Dr. Karen Weidenheim, neuropathologist, Department of Pathology, Albert Einstein College of Medicine and Montefiore Medical Center for the brain biopsy review.
References
- 1.Iizuka T, Sakai F, Ide T, et al. Anti-NMDA receptor encephalitis in Japan: Long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Florance-Ryan N, Dalmau J. Update on anti-N-methyl-D-aspartate receptor encephalitis in children and adolescents. Curr Opin Pediatr. 2010;22:739–744. doi: 10.1097/MOP.0b013e3283402d2f. [DOI] [PubMed] [Google Scholar]
- 4.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner MR, Irani SR, Leite MI, Nithi K, Vincent A, Ansorge O. Progressive encephalomyelitis with rigidity and myoclonus: Glycine and NMDA receptor antibodies. Neurology. 2011;77:439–443. doi: 10.1212/WNL.0b013e318227b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bien CG, Bauer J. Which pathomechanism damages the brain in antibody-associated CNS disease? Neurology. 2011;77:414–415. doi: 10.1212/WNL.0b013e318227b262. [DOI] [PubMed] [Google Scholar]
- 8.Camdessanche JP, Streichenberger N, Cavillon G, et al. Brain immunohistopathological study in a patient with anti-NMDAR encephalitis. Eur J Neurol. 2011;18:929–931. doi: 10.1111/j.1468-1331.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- 9.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruss H, Dalmau J, Harms L, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology. 2010;75:1735–1739. doi: 10.1212/WNL.0b013e3181fc2a06. [DOI] [PubMed] [Google Scholar]
- 11.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent A. Bien CG Anti-NMDA-receptor encephalitis: A cause of psychiatric, seizure, and movement disorders in young adults. Lancet Neurol. 2008;7:1074–1075. doi: 10.1016/S1474-4422(08)70225-4. [DOI] [PubMed] [Google Scholar]
- 13.Cho SM, Byun JY, Rha SE, et al. CT and MRI findings of cys-tadenofibromas of the ovary. Eur Radiol. 2004;14:798–804. doi: 10.1007/s00330-003-2060-z. [DOI] [PubMed] [Google Scholar]