Abstract
The final decade of the 20th century was marked by an alarming resurgence in infectious diseases caused by tropical parasites belonging to the kinetoplastid protozoan order. Among the pathogenic trypanosomatids, some species are of particular interest due to their medical importance. These species include the agent responsible for Chagas’ disease, Trypanosoma cruzi. Approximately 8 to 10 million people are infected in the Americas, and approximately 40 million are at risk. In the present review, we discuss in detail the immune mechanisms elicited during infection by T. cruzi and the effects of chemotherapy in controlling parasite proliferation and on the host immune system.
Keywords: Chagas’ disease, Trypanosoma cruzi, immunity, chemotherapy, immunoparasitology
Introduction
Diseases caused by trypanosomatids constitute a substantial health and socioeconomic problem in several countries, mainly in the Americas, sub-Saharan Africa and tropical and subtropical belt regions. In particular, Chagas’ disease, (caused by Trypanosoma cruzi) affects 8 to 10 million people in the Americas, with an additional 40 million people at risk (http://www.who.int/tdr). T. cruzi has a complex life cycle involving a reduviid insect vector and a mammalian host (Fig. 1). Insect vectors become infected when they bite an infected mammal that has trypomastigote forms of the parasite circulating in its bloodstream. Trypomastigotes, infective non-dividing forms, are ingested with the blood; in the insect’s digestive tube, they differentiate into dividing and non-infective epimastigote forms. In the terminal portion of the digestive tube, epimastigotes differentiate into metacyclic trypomastigotes, which are eliminated in faeces and deposited on mammals’ skin while the triatomine bug bites and feeds. Trypomastigotes enter the body and invade host cells; they differentiate into dividing amastigote forms and after proliferating, differentiate into trypomastigotes, passing through a transient epimastigote-like stage. Finally, the trypomastigotes lyse host cells and are released into the extracellular medium, where they can invade other cells or the bloodstream, becoming capable of invading other tissues or a non-infected reduviid insect, thus completing the cycle [1].
Fig 1.
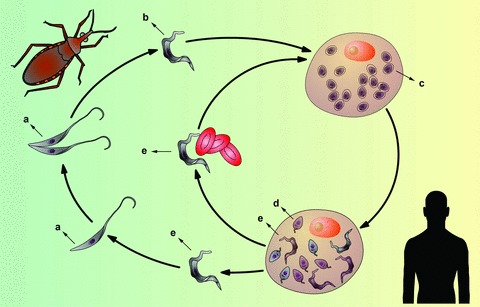
Schematic representation of the Trypanosoma cruzi life cycle. Replicative, non-infective epimastigote forms (A), predominantly present in the insect vector, give rise to non-replicative, infective metacyclic trypomastigotes (B). Metacyclic forms must invade the host cells and differentiate into replicative amastigote forms (C) to establish the infection. These forms give rise to a transient stage called intracellular epimastigotes (D), which subsequently differentiate into trypomastigotes (E). Trypomastigotes can disseminate in the mammalian host through the bloodstream. The insect vector eventually can take these forms during its bloodmeal. The cycle ends when the ingested trypomastigotes differentiate again into epimastigotes (A), which colonize the digestive tube of a new insect.
Chagas’ disease
Chagas’ disease presents mainly as two clinical phases in human beings: acute and chronic. The acute phase happens shortly after infection, beginning when the parasite enters the mammalian host. It is either largely asymptomatic or accompanied by non-specific symptoms such as fever and headache. It is characterized by an absence of antibodies and, in most patients, a conspicuous para sitemia starting 1 or 2 weeks after parasite entry. In some cases, specific symptoms such as lymphadenopathy and splenomegaly, myalgia, malaise, muscle pains, sweating, hepatosplenomegaly or heart failure from myocarditis or pericardial effusion may be present. Less often, meningoencephalitis can occur, which can lead to death [2]. The chronic phase, in principle, can last for the patient’s entire lifetime [3], beginning with the decline of parasitemia. It is defined by an initial absence of symptoms. The main chronic forms are indeterminate, cardiac (chronic chagasic cardiomyopathy, or CCC) and digestive. At lower frequencies, the chronic phase can consist of alterations in the peripheral nervous system. The indeterminate form is characterized by the absence of evident tissue damage and organ dysfunction and can last from several months to the patient’s entire life, which is the case for approximately 70% of chronically infected people. The remaining 30% develop one of the symptomatic forms, most frequently CCC. This form presents different degrees of severity, ranging from mild symptoms to heart failure (caused by inflammation and fibrosis), frequently followed by sudden death. The main clinical manifestation of CCC is cardiomegaly caused by inflammatory infiltrations, arrhythmias and thromboembolism. The lesions can affect the right ventricle, causing oedema and congestive hepatomegaly [3]. The digestive form consists of two syndromes: megaesophagus, leading to dysphagia and regurgitation, and megacolon, leading to severe constipation and faecal retention [4]. In immunocompromised patients, severe compromise of the central nervous system has been also reported [5]. In conclusion, although the majority of T. cruzi infected individuals remain asymptomatic for their entire lives, a percentage of the infected population will develop serious symptoms.
Chemotherapy
Despite the fact that Chagas’ disease was first described a century ago, only two therapeutic compounds presently in use have been shown to be useful against human infections by T. cruzi: benznidazole (BZL) and nifurtimox (NF) (Fig. 2). BZL, a nitroimidazole, was launched in the 1970s; in most Latin American countries, it is the only drug used to treat Chagas’ disease. This treatment is effective for acute phase infections, congenital infections, reactivated infections and early chronic disease. However, its efficacy during the chronic phase is controversial [6]. The key mode of action of BZL seems to be based on interference with the synthesis of macromolecules via covalent binding between nitroreduction intermediates and various cellular components such as DNA, lipids and proteins of the parasite. BZL has also been shown to improve phagocytosis, increase trypanosomal death through interferon (IFN)- production and inhibit T. cruzi NADH-fumarate reductase [7]. The mechanism of action of NF involves the generation of nitroanion radicals by nitroreductases that, in the presence of oxygen, produce reactive intermediates to which T. cruzi is susceptible. Considerable efforts are being made to identify promising targets for new drugs. A detailed discussion of new drugs with chemotherapeutic perspectives is outside the scope of this review. However, some T. cruzi specific pathways contain proteins/enzymes that are being validated as targets; several drugs that interfere with these targets are particularly promising [6]. The cysteine proteinases of T. cruzi, which participate in cellular processes such as energy metabolism, differentiation, host cell invasion and evasion of the immune system, have been validated as drug targets [8]. In particular, the use of synthetic inhibitors such as vinyl sulfone-derivatized dipeptides has shown promising results in vivo[9, 10]. In addition, sterol and polyisoprenoid biosynthesis pathways provide promising targets, because ergosterol (rather than cholesterol) is the main sterol in T. cruzi membranes [11]. Specific inhibitors such as azole derivatives [12] and bisphosphonates [13, 14] have been tested in vitro or in vivo. Inhibitors of T. cruzi specific enzymes such as trypanothione reductase [15], arginine kinase [16] and proline racemase [17] have shown promising trypanocidal activities. It was recently shown that proline transporters could also be relevant targets [18]. Allopurinol, an inhibitor of purine (hypoxanthine/guanine)-phosphoribosyl-transferase, has been proposed for treating T. cruzi reactivation infection in patients after heart transplantation [19]. Inhibitors of topoisomerases I and II [20, 21], which are involved in nuclear and kinetoplastid DNA replication, were efficient in blocking T. cruzi growth. Parasite DNA has also been proposed as a target for intercalators and binders with trypanocidal activity [22–24].
Fig 2.
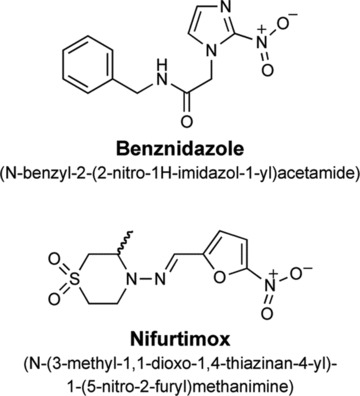
Molecular formula of BZL and NF.
Immune response in experimental T. cruzi infection
T. cruzi’s success in maintaining its life cycle is dependent on its capacity to cause chronic infection in the host, thus favouring encounters with insect vectors. To maintain latent infection, a balance between parasite and host immune response is necessary.
Host resistance to Chagas’ disease depends on both innate and adaptive immunity (Fig. 3) [25–27]. Different cell types and molecules are involved in the response against experimental T. cruzi infection, as summarized in Table 1. Generally, the absence of some component(s) of the immune response leads to greater susceptibility to T. cruzi infection, resulting in higher parasitemia and mortality rates.
Fig 3.
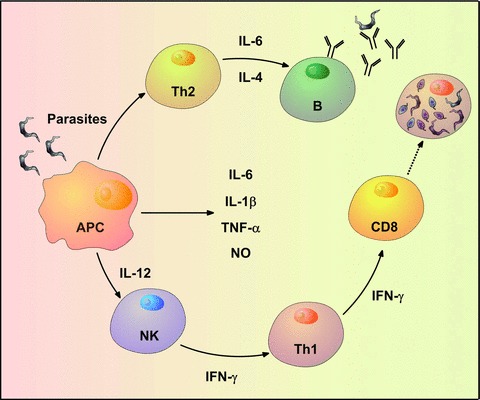
Schematic representation of the protective immune response during T. cruzi acute infection. Antigen-presenting cells are among the first cells that become infected by the trypomastigotes when they enter the mammalian host. Normally, the cells react by up-regulating IL-6, IL-1β, TNF-α, IL-12 and nitric oxide in an attempt to control the infection. NK cells are among the first line of responders and usually produce high levels of IFN-γ when stimulated by IL-12. The stimulus of the Th1 profile and the CD8+ T cells contributes to eliminate the intracellular amastigotes in infected tissues. On the other hand, the parasite antigens stimulate a Th2 profile which contributes to the production of specific antibodies.
Table 1.
Effect of the absence of different molecules of the immune system in the experimental acute infection by Trypanosoma cruzi
| KO | Phenotype/absence of: | Effect on parasitemia | Effect on mortality | Reference |
|---|---|---|---|---|
| Immunoglobulin heavy chain | B lymphocytes | Increase (end of acute phase) | Increase | [35] |
| μ Chain | Mature B lymphocytes | Increase | Unaltered | [75] |
| CD4 | CD4+ T lymphocytes | Increase | Increase | [26] |
| CD8 | CD8+ T lymphocytes | Increase | Increase | [26] |
| MHC class I and II | CD4+ and CD8+, T lymphocytes | Increase | Increase | [27] |
| β2 Microglobulin | CD8+ T lymphocytes and NK cells | Increase | Increase | [27] |
| γ chain of the T lymphocyte receptor | γδ T lymphocytes | Unaltered | Decrease | [48] |
| IFN-γ | IFN-γ production | Increase | Increase | [57] |
| IFN-γ receptor | Activation by IFNγ | Increase | Increase | [55] |
| NOS2 | Nitric oxide production | Increase | Increase | [55, 58] |
| IL-10 | IL-10 production | Decrease | Increase | [67] |
| IL-12 | IL-12 production | Increase | Increase | [57, 64] |
| TNF-α receptor | Activation by TNF-α, reduction of Ig production | Increase | Increase | [65] |
| Perforin | Perforin | Unaltered | Unaltered | [35] |
| Granzime B | Granzime B | Unaltered | Unaltered | [35] |
| Stat4 | CD4+ Th1 response | Increase | Increase | [37] |
| Stat6 | CD4+ Th2 response | Unaltered | Unaltered | [37] |
| MyD88 | Absence of signalling through some TLRs | Increase | Increase | [30] |
| TLR-2 | Absence of signalling through TLR 2 | Unaltered | Unaltered | [30] |
| TLR-4 | Absence of signalling through TLR 4 | Increase | Increase | [28] |
| TLR-9 | Absence of signalling through TLR 9 | Increase | Increase | [33] |
| CD1d | NK T cells | Decrease | Not assessed | [52] |
With respect to the innate immune mechanisms triggered by the parasite, it has been found that some pathogen-associated molecular patterns derived from T. cruzi are recognized by specific receptors known as pattern recognition receptors (PRRs) [28]. The toll-like receptor (TLR) family is the best characterized class of mammalian PRRs [29]. Mice that are unable to signal through most TLRs (MyD88 knockout [KO] or MyD88/TRIF double KO) are highly susceptible to T. cruzi infection, suggesting that resistance to acute T. cruzi infection is dependent on TLR signalling [30, 31]. T. cruzi derived glycosylphosphatidylinositol (GPI) anchors and glycoinositolphospholipids (GIPLs) were shown to have immunoregulatory properties and were present in significant quantities on the parasitic surface. GPIs are recognized by the transmembrane receptor TLR-2, which is associated with CD14. Interestingly, T. cruzi infected TLR-2 KO mice are able to produce pro-inflammatory cytokines, yet parasitemia and mortality rates are not different from wild-type animals [30]. It has also been shown that GIPLs are recognized via TLR-4, and TLR-4 KO mice are more susceptible to T. cruzi infection than wild-type mice [28]. However, the role of these molecules in natural infection has not been assessed. T. cruzi DNA binds TLR-9 and has been reported to stimulate macrophages and DCs to express interleukin (IL)-12, tumour necrosis factor (TNF)-α and nitric oxide [32]. In fact, TLR-9 KO mice are highly susceptible to T. cruzi infection, and some results suggest that TLR-2 and TLR-9 cooperate to control T. cruzi replication during acute infection [33]. In summary, these data suggest an important role for TLR signalling pathways in the innate immune response to T. cruzi infection.
To unravel the role of cellular immune responses, initial experiments have been performed in which T cells were adoptively transferred from chronically infected to naïve mice. Significant protection was observed after experimental challenge with T. cruzi[34]. Both CD4+ and CD8+ (αβ) T-cell subsets appear to be important for the generation of protective immunity against acute experimental T. cruzi infection [26, 27, 35, 36].
Tarleton and collaborators showed that Th1 CD4+ T cells are important for controlling T. cruzi infection, while Th2 cells contribute to parasite persistence and increased disease severity [37]. Attempts to obtain protective vaccines against defined T. cruzi antigens have also provided valuable information about the role of Th1-type responses during infection. For example, cruzipain, when used as an antigen in immunization protocols, significantly improved immune responses in animals challenged with a lethal dose of the parasite [38] and precluded tissue damage [39].
CD8+ T cells also seem to play a role in T. cruzi infection by killing infected cells through the production of perforin and granzyme B or through the Fas/Fas ligand pathway. Acute infection with the Brazil strain of T. cruzi did not enhance the susceptibility of either perforin or granzyme B KO mice [35]. By contrast, previously reported data have shown that mice lacking perforin and granzymes A and B are more susceptible to infection with the Tulahuen strain [40]. Increased susceptibility to infection with the Tulahuen strain was also observed in mice deficient in the Fas/Fas ligand pathway [40]. T. cruzi antigens that are CD8+ T-cell targets have also been studied using chronically infected mice. These studies showed that epitopes from the trans-sialidase family of proteins are immunodominant, and the CD8+ T-cell response is focused mainly on very specific epitopes [41, 42]. When surface markers of some of these cell populations were analysed, it was found that they were central memory T cells that are maintained even during persistent T. cruzi infection [43]. Immunization protocols using plasmid DNA coding for the proteins trans-sialidase and amastigote surface protein-2 have also shown that both cellular and humuoral immune responses were induced, and the presence of CD4+ Th1 and CD8+ specific T cells was shown. Furthermore, parasitemia and/or mortality reduction was seen in mice infected with the Y [44–46] or Brazil strains of T. cruzi[47].
The effective participation of γδ T cells in the immune response against T. cruzi is still controversial. These cells were found to be deleterious for the host in a study in which γδ-KO mice had lower mortality rates and fewer areas with skeletal and cardiac inflammatory lesions compared to wild-type mice [48]. On the other hand, an increase in susceptibility was observed in γδ T-cell depleted animals after experimental infection associated with a reduction in IFN-γ production [49].
The immune response against T. cruzi is also influenced by NK, NK T and regulatory T cells (T reg). The relevance of NK cells in acute T. cruzi infection was demonstrated when normal, NK-depleted mice showed a significant increase in parasitemia and mortality rates [50, 51]. NK T cells are activated by glycolipids presented via CD1d molecules and act by limiting parasitemia. These cells also seem to influence antibody responses during chronic Chagas’ disease [52]. The role of regulatory T cells (CD4+ CD25+) during T. cruzi infection was also shown through depletion. Controversial results were obtained with mice infected with different T. cruzi strains: no effect was seen when Brazil and Tulahuen strains were used [53], while limited effects were seen with the Colombian strain [54]. No role was observed for these cells during chronic infection of mice with the Colombian strain [54].
The role of IFN-γ during T. cruzi infection was demonstrated when IFN-γ and IFN-γ receptor KO mice showed higher rates of parasitemia and mortality [55]. Infected IFN-γ KO mice showed increases in cellular infiltrates in the heart and skeletal muscles and reduced survival [36, 56, 57]. The role of the inducible nitric oxide synthase (iNOS) was also studied. iNOS KO mice showed greater parasitemia in the acute phase and rapid mortality compared to control animals [55, 57, 58]. Additionally, nitric oxide seems to have an effect on the generation of the inflammatory heart infiltrate seen during T. cruzi infection by modulating chemokine expression. Cardiomyocytes from iNOS KO mice that were stimulated with IFN-γ and TNF produced significantly higher levels of the chemokines CCL2, CCL4, CCL5 and CXCL2 [59]. Other chemokines and chemokine receptors have also been analysed during acute or chronic T. cruzi infections. CXCL9, CXCL10 and CCL5 are expressed in the heart during both phases of T. cruzi infection [60, 61]. Their presence correlates with the expression of IFN-γ and TNF-α and the presence of inflammatory cells. However, their ablation did not modulate the severity of heart inflammation [61]. During the acute phase, CCR5 seems to be critical to controlling the migration of CD4+ and CD8+ T cells to the heart [62], but it does not seem to play an important role in maintaining an inflammatory response in the heart during chronic infection [63].
IL-12 is also extremely important for controlling the infection, as IL-12 KO mice show higher rates of parasitemia and mortality compared to controls [57, 64]. The role of TNF-α is controversial: TNF receptor p55 KO mice [25, 65] showed increased parasitemia, while parasitemia and mortality rates in TNFR1 KO mice did not differ from wild-type [66]. IL-10 deficiency leads to parasitemia reduction; however, mortality is accelerated due to a dramatic increase in acute pathology [67]. IL-4 seems to have different effects according to the parasite strain. IL-4 KO mice infected with the Y strain did not differ from wild-type in terms of parasitemia and mortality [68]. However, when the Colombian strain was used, IL-4 KO mice showed reduced parasitemia and mortality rates [57].
The importance of antibodies for controlling chronic infection was demonstrated when sera from chronically infected chagasic patients or mice were transferred to naïve mice, significantly reducing parasitemia and prolonging survival after challenge with T. cruzi[69, 70]. Protective antibodies are also able to agglutinate trypomastigotes in vitro[70], lyse them in a complement-mediated fashion [71], facilitate phagocytosis/opsonization [72] and mediate antibody-dependent cellular cytotoxicity [73, 74]. During acute T. cruzi infection, B cells also play fundamental roles in both the recruitment of CD4+ T cells and CD8+ T cells to the spleen and in the generation and maintenance of central memory and effector T cells. KO mice lacking mature B cells (μMT KO) produced decreased amounts of inflammatory cytokines and fewer central and memory CD4+ and CD8+ T cells compared to wild-type T. cruzi infected mice. An increase in parasitemia was observed in muMT KO mice, but no difference was seen in terms of survival [75]. B cells are also able to participate in the cross-priming of specific CD8+ T cells, inducing systemic and mucosal protective immunity against experimental infection [76]. Taken together, these results suggest the important participation of both innate and adaptive immune responses during experimental T. cruzi infection.
Immune response in human beings infected with T. cruzi
During the chronic phase of Chagas’ disease, the majority of individuals show potent cellular and humuoral immune responses [77, 78]. The relevance of a strong immune response for parasite control has been shown by the fact that chemically immunosuppressed individuals [79] and AIDS patients can develop symptomatic forms of the disease [80]. The mechanisms underlying the transition from asymptomatic to clinically symptomatic are still unclear. Several factors may be involved, such as differences in parasite strain, parasite load, infection time, host genetic background and immune response. In animal models, different parasite strains, mouse backgrounds and re-infections can play a role in the development of heart pathology and/or protection [81–83].
Regarding human infections, a study with patients acutely infected with T. cruzi has shown that CD4+ and CD8+ T cells are present in endomyocardial biopsies where myocarditis was also detected in 100% of the cases, reinforcing the role of the immune response in acute pathology [84]. With respect to the chronic phase, a predominance of activated CD8+ T cells was found in myocardial biopsy fragments from patients with CCC [85, 86]. It is also of interest that peripheral blood mononuclear cells (PBMCs) from chronically infected chagasic patients were able to produce IFN-γ upon stimulation with recombinant T. cruzi derived proteins [87, 88]. Furthermore, PBMCs from CCC patients produced more IFN-γ and less IL-10 [87, 89]. IL-10 expression in monocytes from patients with the indeterminate form was higher than in cardiac patients [89, 90]. By contrast, analysis of IFN-γ-producing CD8+ T cells present in infected patients (with undetectable, mild or more severe forms of clinical disease) showed that there was a negative correlation between the capacity of their cells to respond to T. cruzi amastigote antigens and disease severity [91]. A careful analysis of these cells showed that in responding individuals (individuals with milder heart disease), there were early differentiated (CD27+CD28+) and effector memory (CD45RA−CCR7−) CD8+ T cells. On the other hand, individuals with more severe forms of the disease presented fully differentiated (CD27−CD28−) CD8+ T cells [77]. The authors suggest that this profile is compatible with the hypothesis that as the disease progresses, there is a gradual clonal exhaustion of the CD8+ T-cell population, probably due to continuous antigenic stimulation. Another study showed that patients with cardiac forms of Chagas disease display a high frequency of circulating CD4+ and CD8+ T cells lacking the CD28 surface molecule [78]. Two cytokines (IL-7 and IL-15) were suggested as being important for the survival of CD8+ T cells in the cardiac infiltrate [92].
On the other hand, there is also evidence that activated T cells are involved in Chagas disease pathology. Cells from patients with chronic infections (either symptomatic or asymptomatic) express both inflammatory and anti-inflammatory cytokines [93, 94], suggesting that there is probably immune regulation during the chronic phase. However, preferential expression of TNF-α and IFN-γ (both inflammatory cytokines) was observed in cardiac lesions [33, 86] and has been associated with progressively severe cardiac disease [89, 95]. Few reports have focused on the identification the CD8+ T-cell targets in chronic chagasic patients [41, 91, 96–98], probably due to the fact that these responses are not very strong. CD8+ T-cell responses against T. cruzi peptides derived from the proteins cruzipain, FL-160, KMP-11 and the trans-sialidase family were detected in infected individuals [41, 97, 98]. Peptides derived from the trans-sialidase protein family were able to bind to six of the most common class I HLA supertypes, and the stimulated cells showed a lack of polyfunctional cytokine responses, producing only IFN-γ[96].
Evidence has also led to the proposal of an autoimmune hypothesis for the disease, suggesting that the symptoms presented by infected individuals are a consequence of the triggered immune response rather than parasite persistence [99]. However, autoimmunity is not sufficient to explain the multifocal nature of myocarditis and the preferential location of fibrosis in certain regions, such as the apical or posterior left ventricular wall in the cardiac form of Chagas’ disease [100]. Moreover, as mentioned above, frequent positive xenodiagnosis during the chronic phase of the disease and episodes of reactivation in immunocompromised patients has provided evidence that the parasite is present even under active control of the host immunological system [79]. In fact, recent studies have described a positive correlation between myocardial parasite persistence and high-grade myocarditis [101, 102]. These studies reinforce the notion that a combination of immune response and parasite persistence determines the development of Chagas’ disease pathology. However, the specific mechanisms that trigger the symptoms seen during the chronic phase are still elusive.
Immune response in the treatment of chagasic infection
As previously mentioned, the resistance developed during experimental Chagas’ disease depends on innate immune responses as well as on a prevalent Th1 response at the beginning of infection.
In addition, the antibodies produced by a Th2 response can contribute to the control of in vivo parasite replication (schematized in Fig. 3). Several works have shown that chemotherapy acts by unbalancing the equilibrium between the host immune response and the parasite in favour of the host [66, 103]. However, it is important to stress that the results obtained with both drugs used for treatment, BZL and NF, differ strongly according to the disease phase, the time extension and the drug dose, as well as the patient’s age and geographical origin [104]. In vitro phagocytosis and intracellular destruction of trypomastigotes by mouse peritoneal macrophages collected from animals treated with NF and BZL are remarkably increased compared to untreated controls [105]. The effects of in vivo treatment with BZL on parasite-macrophage interaction have been studied using the Y strain of T. cruzi. Drug-resistant and drug-susceptible parasites from this strain were used, and it was observed that BZL enhanced phagocytosis, parasite destruction and cytokine release by macrophages when a drug-sensitive strain was used. Splenocytes from these animals produced very high levels of TNF-α and reactive nitrogen intermediates [106]. BZL treatment was also evaluated in IFN-γ, IL-12, TNF receptor and iNOS KO mice. Although BZL treatment was able to cure 100% of wild-type mice, it was not as effective when various KO mice were used [107]. The induction of a stable parasite-specific CD8+ T-cell population with the characteristics of central memory was observed in chronically infected mice treated with BZL. These cells also expand more rapidly and provide greater protection after challenge compared to cells from non-treated chronically infected mice [108]. It was also observed that splenomegaly persisted in spite of amelioration of clinical and parasitological signs in infected and BZL-treated mice. This was due to a preferential expansion of effector and memory CD8+ T cells but not of recently activated CD4+ and CD8+ T cells, suggesting that BZL directly affects immunoregulation in T. cruzi infected mice [109]. The effect of BZL treatment was also studied in two CCC mouse models, showing contradictory results. One model showed that treatment was able to reduce the severity of cardiac autoimmune disease [82], while in the other, early treatment with BZL did not alter the intensity of CCC [110].
PBMCs from cured patients treated with BZL during the acute phase showed stronger proliferative responses and IFN-γ production compared to non-cured patients. IFN-γ could be acting synergistically with chemotherapy to eliminate parasites [111], as previously suggested [112]. It was also demonstrated that most individuals with an indeterminate clinical form show a dominant regulatory cytokine profile, whereas individuals with CCC display a dominant inflammatory cytokine pattern. Interestingly, an inversion of the cytokine profile (from an inflammatory to a regulatory profile or vice versa) was found after in vivo treatment of indeterminate and cardiac individuals with BZL [113]. Cytokine expression in T. cruzi infected children treated with BZL shifted toward a type 1 modulated immune profile, and IFN-γ was mainly produced by NK cells and CD8+ T lymphocytes [103]. Although a pro-inflammatory immune response is commonly related to Chagas’ disease pathogenesis, it is also important for treatment effectiveness. A longitudinal study performed to evaluate immunological status following BZL treatment during early indeterminate Chagas’ disease demonstrated that BZL treatment induced substantial T- and B-cell activation [114].
As discussed above, several lines of evidence show that BZL, in addition to having trypanocidal activity, acts as an immunomodulator. More specifically, the fact that BZL is known to affect mammalian host cells raised the hypothesis that it might be affecting macrophage metabolism. Revelli and collaborators showed that BZL down-regulates nitric oxide and pro-inflammatory cytokine synthesis (IL-6, IL-1β) by lipopolysaccharide (LPS)-stimulated RAW 264.7 murine macrophages and leads to an inhibition of iNOS gene expression [115] through the inhibition of NF-κB activation [116]. Moreover, in a rat model of acute T. cruzi infection, systemic treatment with BZL led to a marked reduction of nitric oxide derived metabolites, suggesting that the beneficial properties of BZL may depend on both trypanocidal action and immunomodulating effects [117]. These properties of BZL have been further demonstrated by its ability to increase survival and decrease serum levels of IL-6 and TNF-α in C57BL/6 mice challenged systemically with LPS [118]. In an infectious-based situation of systemic inflammatory response (cecal ligation and puncture, CLP), mice treated with BZL had an increased survival rate and a significant reduction of TNF-α levels and bacteremia 24 hrs after CLP [119]. Such beneficial properties may broaden the potential use of BZL in hyperacute cases where inflammatory responses become harmful.
There are limited data concerning the effects of NF on immune responses, mainly because treatment with this drug was discontinued in the 1980s. At the experimental level, no gross changes in cell-mediated immunity were recorded in NF-treated mice [120]. However, an impaired PPD skin reaction was seen in guinea pigs when this compound was administered [121]. From a clinical standpoint, chronically infected human beings who were treated for two months with NF had a detectable peripheral leukocyte migration inhibition test to T. cruzi antigens. Migration was lower in untreated patients, and further treatment improved such responses [122]. The effect of NF treatment on re-infection has also been evaluated. Animals that were parasitologically sterilized by NF treatment and re-infected with a small number of parasites showed parasitemia and mortality rates similar to control animals (infected, non-treated). However, when re-infection was performed with a large number of parasites, parasitemia and mortality were increased compared to controls. Immunological studies have shown that NF treatment reduces the levels of antibodies engaged in parasite destruction, reducing either complement-dependent lysis or antibody-dependent cytotoxicity. No difference was observed when treated and non-treated mice were compared in terms of T-cell mediated immunity. Therefore, it seems that NF treatment leads to a loss of resistance to re-infection with a high number of parasites [120].
The need for new therapeutic alternatives for Chagas’ disease
During the last 40 years, therapy for Chagas’ disease has been based solely on two drugs: NF and BZL. The search for new trypanocidal drugs as well as other strategies to improve immune responses during infection (i.e. therapeutic vaccines) are currently under way [123, 124]. Treatment of the symptomatic complications of Chagas’ disease, mainly related to heart failure, is also effective in improving quality of life. Heart transplantation [125] and cellular therapy with stem cells [126] are therapeutic options for patients with advanced CCC, but both require optimization of the etiological treatment. As more data become available regarding the relevance of parasite persistence in the development of chronic infection, the search for new treatments becomes extremely critical. Future challenges for developing new drugs for Chagas’ disease reside in finding compounds that show both trypanocidal and immunomodulatory activities. Despite the vast literature regarding the anti-T. cruzi activity of myriad compounds, only a small fraction of studies explore the characteristics mentioned above. Furthermore, drugs with modest anti-T. cruzi activity could be evaluated as co-adjuvant drugs, interacting synergistically with NF or BZL. This strategy would increase the spectrum of available combinations and possibly contribute to reducing the dose and thus avoiding the toxic effects of the drugs currently in use.
Conclusions
In summary, the drugs that are available to treat diseases caused by trypanosomatids are somewhat effective. However, for the reasons discussed above, new drugs that are able to act alone or in concert with current treatments as well as with strategies such as immunotherapy and vaccines are urgently needed. These are relevant goals because they could aid in reducing undesired secondary effects, thereby optimizing the quality of life of patients and diminishing treatment evasion. In this way, funding of all steps related to the development of new therapies, from validation of new targets to evaluation of new drugs (from the bench top to clinical trials), is a necessity.
Acknowledgments
This work was supported by grants from the Fundação de Amparo ‡ Pesquisa do Estado de São Paulo (FAPESP grants #08/57596-4 to A.M.S. and #07/08648-9 to S.B.B.), Conselho Nacional de desenvolvimento Cientìfico e Tecnológico (CNPq Grant #473906/2008-2), and Instituto Nacional de Biologia Estrutural e Quìmica Medicinal em Doenças Infecciosas (INBEQMeDI). We are deeply acknowledged to Dr. Oscar Bottasso for his critical review of this paper.
References
- 1.Alves MJ, Colli W. Trypanosoma cruzi: adhesion to the host cell and intracellular survival. IUBMB Life. 2007;59:274–9. doi: 10.1080/15216540701200084. [DOI] [PubMed] [Google Scholar]
- 2.Punukollu G, Gowda RM, Khan IA, et al. Clinical aspects of the Chagas’ heart disease. Int J Cardiol. 2007;115:279–83. doi: 10.1016/j.ijcard.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Moncayo A, Ortiz Yanine MI. An update on Chagas disease (human American trypanosomiasis) Ann Trop Med Parasitol. 2006;100:663–77. doi: 10.1179/136485906X112248. [DOI] [PubMed] [Google Scholar]
- 4.Gattuso JM, Kamm MA. The management of constipation in adults. Aliment Pharmacol Ther. 1993;7:487–500. doi: 10.1111/j.1365-2036.1993.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 5.Walker M, Zunt JR. Parasitic central nervous system infections in immunocompromised hosts. Clin Infect Dis. 2005;40:1005–15. doi: 10.1086/428621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duschak VG, Couto AS. An insight on targets and patented drugs for chemotherapy of Chagas disease. Recent Pat Antiinfect Drug Discov. 2007;2:19–51. doi: 10.2174/157489107779561625. [DOI] [PubMed] [Google Scholar]
- 7.Maya JD, Cassels BK, Iturriaga-Vasquez P, et al. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp Biochem Physiol. 2007;146:601–20. doi: 10.1016/j.cbpa.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Cazzulo JJ. Proteinases of Trypanosoma cruzi: patential targets for the chemotherapy of Changas desease. Curr Top Med Chem. 2002;2:1261–71. doi: 10.2174/1568026023392995. [DOI] [PubMed] [Google Scholar]
- 9.Barr SC, Warner KL, Kornreic BG, et al. A cysteine protease inhibitor protects dogs from cardiac damage during infection by Trypanosoma cruzi. Antimicrob Agents Chemother. 2005;49:5160–1. doi: 10.1128/AAC.49.12.5160-5161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel JC, Doyle PS, Hsieh I, et al. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J Exp Med. 1998;188:725–34. doi: 10.1084/jem.188.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbina JA. Chemotherapy of Chagas’ disease: the how and the why. J Mol Med. 1999;77:332–8. doi: 10.1007/s001090050359. [DOI] [PubMed] [Google Scholar]
- 12.Urbina JA. Ergosterol biosynthesis and drug development for Chagas disease. Mem Inst Oswaldo Cruz. 2009;104:311–8. doi: 10.1590/s0074-02762009000900041. [DOI] [PubMed] [Google Scholar]
- 13.Docampo R, Moreno SN. Bisphosphonates as chemotherapeutic agents against trypanosomatid and apicomplexan parasites. Curr Drug Targets Infect Disord. 2001;1:51–61. doi: 10.2174/1568005013343191. [DOI] [PubMed] [Google Scholar]
- 14.Esteva MI, Kettler K, Maidana C, et al. Benzophenone-based farnesyltransferase inhibitors with high activity against Trypanosoma cruzi. J Med Chem. 2005;48:7186–91. doi: 10.1021/jm050456x. [DOI] [PubMed] [Google Scholar]
- 15.Rivarola HW, Paglini-Oliva PA. Trypanosoma cruzi trypanothione reductase inhibitors: phenothiazines and related compounds modify experimental Chagas’ disease evolution. Curr Drug Targets Cardiovasc Haematol Disord. 2002;2:43–52. doi: 10.2174/1568006023337745. [DOI] [PubMed] [Google Scholar]
- 16.Paveto C, Guida MC, Esteva MI, et al. Anti-Trypanosoma cruzi activity of green tea (Camellia sinensis) catechins. Antimicrob Agents Chemother. 2004;48:69–74. doi: 10.1128/AAC.48.1.69-74.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamond N, Goytia M, Coatnoan N, et al. Trypanosoma cruzi proline racemases are involved in parasite differentiation and infectivity. Mol Microbiol. 2005;58:46–60. doi: 10.1111/j.1365-2958.2005.04808.x. [DOI] [PubMed] [Google Scholar]
- 18.Magdaleno A, Ahn IY, Paes LS, et al. Actions of a proline analogue, L-thiazolidine-4-carboxylic acid (T4C), on Trypanosoma cruzi. PLoS ONE. 2009;4:e4534. doi: 10.1371/journal.pone.0004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida DR, Carvalho AC, Branco JN, et al. Chagas’ disease reactivation after heart transplantation: efficacy of allopurinol treatment. J Heart Lung Transplant. 1996;15:988–92. [PubMed] [Google Scholar]
- 20.Bodley AL, Shapiro TA. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc Natl Acad Sci USA. 1995;92:3726–30. doi: 10.1073/pnas.92.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzales-Perdomo M, de Castro SL, Meirelles MN, et al. Trypanosoma cruzi proliferation and differentiation are blocked by topoisomerase II inhibitors. Antimicrob Agents Chemother. 1990;34:1707–14. doi: 10.1128/aac.34.9.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowland EC, Moore-Lai D, Seed JR, et al. Inhibition of in vitro intracellular growth of Trypanosoma cruzi by dicationic compounds. J Parasitol. 2003;89:1078–80. doi: 10.1645/GE-53R. [DOI] [PubMed] [Google Scholar]
- 23.Stephens CE, Brun R, Salem MM, et al. The activity of diguanidino and ‘reversed’ diamidino 2,5-diarylfurans versus Trypanosoma cruzi and Leishmania donovani. Bioorg Med Chem Lett. 2003;13:2065–9. doi: 10.1016/s0960-894x(03)00319-6. [DOI] [PubMed] [Google Scholar]
- 24.Stolić I, Miškovic K, Magdaleno A, et al. Effect of 3,4-ethylenedioxy-extension of thiophene core on the DNA/RNA binding properties and biological activity of bisbenzimidazole amidines. Bioorg Med Chem. 2009;17:2544–54. doi: 10.1016/j.bmc.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 25.Abrahamsohn IA, Coffman RL. Trypanosoma cruzi: IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol. 1996;84:231–44. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 26.Rottenberg ME, Bakhiet M, Olsson T, et al. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect Immun. 1993;61:5129–33. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarleton RL, Koller BH, Latour A, et al. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992;356:338–40. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 28.Campos MA, Gazzinelli RT. Trypanosoma cruzi and its components as exogenous mediators of inflammation recognized through Toll-like receptors. Mediators Inflamm. 2004;13:139–43. doi: 10.1080/09511920410001713565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im1412s77. ; Chapter 14:Unit 14 2. [DOI] [PubMed] [Google Scholar]
- 30.Campos MA, Closel M, Valente EP, et al. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J Immunol. 2004;172:1711–8. doi: 10.4049/jimmunol.172.3.1711. [DOI] [PubMed] [Google Scholar]
- 31.Koga R, Hamano S, Kuwata H, et al. TLR-dependent induction of IFN-beta mediates host defense against Trypanosoma cruzi. J Immunol. 2006;177:7059–66. doi: 10.4049/jimmunol.177.10.7059. [DOI] [PubMed] [Google Scholar]
- 32.Shoda LK, Kegerreis KA, Suarez CE, et al. DNA from protozoan parasites Babesia bovis, Trypanosoma cruzi, and T. brucei is mitogenic for B lymphocytes and stimulates macrophage expression of interleukin-12, tumor necrosis factor alpha, and nitric oxide. Infect Immun. 2001;69:2162–71. doi: 10.1128/IAI.69.4.2162-2171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bafica A, Santiago HC, Goldszmid R, et al. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–9. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 34.Rottenberg ME, Rodriguez DA, Orn A. Control of Trypanosoma cruzi infection in mice deprived of T-cell help. Scand J Immunol. 1992;36:261–8. doi: 10.1111/j.1365-3083.1992.tb03098.x. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, Tarleton RL. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol. 1998;20:207–16. doi: 10.1046/j.1365-3024.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 36.Tzelepis F, de Alencar BC, Penido ML, et al. Distinct kinetics of effector CD8+ cytotoxic T cells after infection with Trypanosoma cruzi in naive or vaccinated mice. Infect Immun. 2006;74:2477–81. doi: 10.1128/IAI.74.4.2477-2481.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarleton RL, Grusby MJ, Zhang L. Increased susceptibility of Stat4-deficient and enhanced resistance in Stat6-deficient mice to infection with Trypanosoma cruzi. J Immunol. 2000;165:1520–5. doi: 10.4049/jimmunol.165.3.1520. [DOI] [PubMed] [Google Scholar]
- 38.Frank FM, Petray PB, Cazorla SI, et al. Use of a purified Trypanosoma cruzi antigen and CpG oligodeoxynucleotides for immunoprotection against a lethal challenge with trypomastigotes. Vaccine. 2003;22:77–86. doi: 10.1016/s0264-410x(03)00541-3. [DOI] [PubMed] [Google Scholar]
- 39.Frank FM, Cazorla SI, Sartori MJ, et al. Elicitation of specific, Th1-biased immune response precludes skeletal muscle damage in cruzipain-vaccinated mice. Exp Mol Pathol. 2008;84:64–70. doi: 10.1016/j.yexmp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Muller U, Sobek V, Balkow S, et al. Concerted action of perforin and granzymes is critical for the elimination of Trypanosoma cruzi from mouse tissues, but prevention of early host death is in addition dependent on the FasL/Fas pathway. Eur J Immunol. 2003;33:70–8. doi: 10.1002/immu.200390009. [DOI] [PubMed] [Google Scholar]
- 41.Martin DL, Weatherly DB, Laucella SA, et al. CD8+ T-Cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2006;2:e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzelepis F, de Alencar BC, Penido ML, et al. Infection with Trypanosoma cruzi restricts the repertoire of parasite-specific CD8+ T cells leading to immunodominance. J Immunol. 2008;180:1737–48. doi: 10.4049/jimmunol.180.3.1737. [DOI] [PubMed] [Google Scholar]
- 43.Bixby LM, Tarleton RL. Stable CD8+ T cell memory during persistent Trypanosoma cruzi infection. J Immunol. 2008;181:2644–50. doi: 10.4049/jimmunol.181.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araujo AF, de Alencar BC, Vasconcelos JR, et al. CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2. Infect Immun. 2005;73:6017–25. doi: 10.1128/IAI.73.9.6017-6025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boscardin SB, Kinoshita SS, Fujimura AE, et al. Immunization with cDNA expressed by amastigotes of Trypanosoma cruzi elicits protective immune response against experimental infection. Infect Immun. 2003;71:2744–57. doi: 10.1128/IAI.71.5.2744-2757.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasconcelos JR, Hiyane MI, Marinho CR, et al. Protective immunity against Trypanosoma cruzi infection in a highly susceptible mouse strain after vaccination with genes encoding the amastigote surface protein-2 and trans-sialidase. Hum Gene Ther. 2004;15:878–86. doi: 10.1089/hum.2004.15.878. [DOI] [PubMed] [Google Scholar]
- 47.Garg N, Tarleton RL. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect Immun. 2002;70:5547–55. doi: 10.1128/IAI.70.10.5547-5555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos Lima EC, Minoprio P. Chagas’ disease is attenuated in mice lacking gamma delta T cells. Infect Immun. 1996;64:215–21. doi: 10.1128/iai.64.1.215-221.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomizo A, Cardillo F, Postol E, et al. V gamma 1 gammadelta T cells regulate type-1/type-2 immune responses and participate in the resistance to infection and development of heart inflammation in Trypanosoma cruzi-infected BALB/c mice. Microbes Infect. 2006;8:880–8. doi: 10.1016/j.micinf.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Lieke T, Graefe SE, Klauenberg U, et al. NK cells contribute to the control of Trypanosoma cruzi infection by killing free parasites by perforin-independent mechanisms. Infect Immun. 2004;72:6817–25. doi: 10.1128/IAI.72.12.6817-6825.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rottenberg M, Cardoni RL, Andersson R, et al. Role of T helper/inducer cells as well as natural killer cells in resistance to Trypanosoma cruzi infection. Scand J Immunol. 1988;28:573–82. doi: 10.1111/j.1365-3083.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 52.Duthie MS, Wleklinski-Lee M, Smith S, et al. During Trypanosoma cruzi infection CD1d-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect Immun. 2002;70:36–48. doi: 10.1128/IAI.70.1.36-48.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotner J, Tarleton R. Endogenous CD4+ CD25+ regulatory T cells have a limited role in the control of Trypanosoma cruzi infection in mice. Infect Immun. 2007;75:861–9. doi: 10.1128/IAI.01500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sales PA, Jr, Golgher D, Oliveira RV, et al. The regulatory CD4+CD25+ T cells have a limited role on pathogenesis of infection with Trypanosoma cruzi. Microbes Infect. 2008;10:680–8. doi: 10.1016/j.micinf.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Holscher C, Kohler G, Muller U, et al. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–15. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marinho CR, Nunez-Apaza LN, Martins-Santos R, et al. IFN-gamma, but not nitric oxide or specific IgG, is essential for the in vivo control of low-virulence Sylvio X10/4 Trypanosoma cruzi parasites. Scand J Immunol. 2007;66:297–308. doi: 10.1111/j.1365-3083.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- 57.Michailowsky V, Silva NM, Rocha CD, et al. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am J Pathol. 2001;159:1723–33. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodrigues MM, Ribeirao M, Boscardin SB. CD4 Th1 but not Th2 clones efficiently activate macrophages to eliminate Trypanosoma cruzi through a nitric oxide dependent mechanism. Immunol Lett. 2000;73:43–50. doi: 10.1016/s0165-2478(00)00205-4. [DOI] [PubMed] [Google Scholar]
- 59.Machado FS, Souto JT, Rossi MA, et al. Nitric oxide synthase-2 modulates chemokine production by Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect. 2008;10:1558–66. doi: 10.1016/j.micinf.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talvani A, Ribeiro CS, Aliberti JC, et al. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect. 2000;2:851–66. doi: 10.1016/s1286-4579(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 61.Hardison JL, Wrightsman RA, Carpenter PM, et al. The chemokines CXCL9 and CXCL10 promote a protective immune response but do not contribute to cardiac inflammation following infection with Trypanosoma cruzi. Infect Immun. 2006;74:125–34. doi: 10.1128/IAI.74.1.125-134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Machado FS, Koyama NS, Carregaro V, et al. CCR5 plays a critical role in the development of myocarditis and host protection in mice infected with Trypanosoma cruzi. J Infect Dis. 2005;191:627–36. doi: 10.1086/427515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardison JL, Kuziel WA, Manning JE, et al. Chemokine CC receptor 2 is important for acute control of cardiac parasitism but does not contribute to cardiac inflammation after infection with Trypanosoma cruzi. J Infect Dis. 2006;193:1584–8. doi: 10.1086/503812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galvao Da Silva AP, Jacysyn JF, De Almeida Abrahamsohn I. Resistant mice lacking interleukin-12 become susceptible to Trypanosoma cruzi infection but fail to mount a T helper type 2 response. Immunology. 2003;108:230–7. doi: 10.1046/j.1365-2567.2003.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castanos-Velez E, Maerlan S, Osorio LM, et al. Trypanosoma cruzi infection in tumor necrosis factor receptor p55-deficient mice. Infect Immun. 1998;66:2960–8. doi: 10.1128/iai.66.6.2960-2968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kroll-Palhares K, Silverio JC, Silva AA, et al. TNF/TNFR1 signaling up-regulates CCR5 expression by CD8+ T lymphocytes and promotes heart tissue damage during Trypanosoma cruzi infection: beneficial effects of TNF-alpha blockade. Mem Inst Oswaldo Cruz. 2008;103:375–85. doi: 10.1590/s0074-02762008000400011. [DOI] [PubMed] [Google Scholar]
- 67.Hunter CA, Ellis-Neyes LA, Slifer T, et al. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–6. [PubMed] [Google Scholar]
- 68.Abrahamsohn IA, da Silva AP, Coffman RL. Effects of interleukin-4 deprivation and treatment on resistance to Trypanosoma cruzi. Infect Immun. 2000;68:1975–9. doi: 10.1128/iai.68.4.1975-1979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kierszenbaum F. Protection of congenitally athymic mice against Trypanosoma cruzi infection by passive antibody transfer. J Parasitol. 1980;66:673–5. [PubMed] [Google Scholar]
- 70.Krettli AU, Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976;116:755–60. [PubMed] [Google Scholar]
- 71.Krettli AU, Weisz-Carrington P, Nussenzweig RS. Membrane-bound antibodies to bloodstream Trypanosoma cruzi in mice: strain differences in susceptibility to complement-mediated lysis. Clin Exp Immunol. 1979;37:416–23. [PMC free article] [PubMed] [Google Scholar]
- 72.Lages-Silva E, Ramirez LE, Krettli AU, et al. Effect of protective and non-protective antibodies in the phagocytosis rate of Trypanosoma cruzi blood forms by mouse peritoneal macrophages. Parasite Immunol. 1987;9:21–30. doi: 10.1111/j.1365-3024.1987.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 73.Lima-Martins MV, Sanchez GA, Krettli AU, et al. Antibody-dependent cell cytotoxicity against Trypanosoma cruzi is only mediated by protective antibodies. Parasite Immunol. 1985;7:367–76. doi: 10.1111/j.1365-3024.1985.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 74.Okabe K, Kipnis TL, Calich VL, et al. Cell-mediated cytotoxicity to Trypanosoma cruzi. I. Antibody-dependent cell mediated cytotoxicity to trypomastigote bloodstream forms. Clin Immunol Immunopathol. 1980;16:344–53. doi: 10.1016/0090-1229(80)90140-3. [DOI] [PubMed] [Google Scholar]
- 75.Cardillo F, Postol E, Nihei J, et al. B cells modulate T cells so as to favour T helper type 1 and CD8+ T-cell responses in the acute phase of Trypanosoma cruzi infection. Immunology. 2007;122:584–95. doi: 10.1111/j.1365-2567.2007.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoft DF, Eickhoff CS, Giddings OK, et al. Trans-sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic Trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming. J Immunol. 2007;179:6889–900. doi: 10.4049/jimmunol.179.10.6889. [DOI] [PubMed] [Google Scholar]
- 77.Albareda MC, Laucella SA, Alvarez MG, et al. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas’ disease patients. Int Immunol. 2006;18:465–71. doi: 10.1093/intimm/dxh387. [DOI] [PubMed] [Google Scholar]
- 78.Dutra WO, Martins-Filho OA, Cancado JR, et al. Chagasic patients lack CD28 expression on many of their circulating T lymphocytes. Scand J Immunol. 1996;43:88–93. doi: 10.1046/j.1365-3083.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 79.Stolf NA, Higushi L, Bocchi E, et al. Heart transplantation in patients with Chagas’ disease cardiomyopathy. J Heart Transplant. 1987;6:307–12. [PubMed] [Google Scholar]
- 80.Vaidian AK, Weiss LM, Tanowitz HB. Chagas’ disease and AIDS. Kinetoplastid Biol Dis. 2004;3:2. doi: 10.1186/1475-9292-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bustamante JM, Novarese M, Rivarola HW, et al. Reinfections and Trypanosoma cruzi strains can determine the prognosis of the chronic chagasic cardiopathy in mice. Parasitol Res. 2007;100:1407–10. doi: 10.1007/s00436-006-0425-3. [DOI] [PubMed] [Google Scholar]
- 82.Hyland KV, Leon JS, Daniels MD, et al. Modulation of autoimmunity by treatment of an infectious disease. Infect Immun. 2007;75:3641–50. doi: 10.1128/IAI.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tzelepis F, Persechini PM, Rodrigues MM. Modulation of CD4+ T cell-dependent specific cytotoxic CD8+ T cells differentiation and proliferation by the timing of increase in the pathogen load. PLoS ONE. 2007;2:e393. doi: 10.1371/journal.pone.0000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuenmayor C, Higuchi ML, Carrasco H, et al. Acute Chagas’ disease: immunohistochemical characteristics of T cell infiltrate and its relationship with T. cruzi parasitic antigens. Acta Cardiol. 2005;60:33–7. doi: 10.2143/AC.60.1.2005046. [DOI] [PubMed] [Google Scholar]
- 85.Higuchi MD, Ries MM, Aiello VD, et al. Association of an increase in CD8+ T cells with the presence of Trypanosoma cruzi antigens in chronic, human, chagasic myocarditis. Am J Trop Med Hyg. 1997;56:485–9. doi: 10.4269/ajtmh.1997.56.485. [DOI] [PubMed] [Google Scholar]
- 86.Reis DD, Jones EM, Tostes S, Jr, et al. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am J Trop Med Hyg. 1993;48:637–44. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 87.Abel LC, Rizzo LV, Ianni B, et al. Chronic Chagas’ disease cardiomyopathy patients display an increased IFN-gamma response to Trypanosoma cruzi infection. J Autoimmun. 2001;17:99–107. doi: 10.1006/jaut.2001.0523. [DOI] [PubMed] [Google Scholar]
- 88.Ribeirao M, Pereira-Chioccola VL, Renia L, et al. Chagasic patients develop a type 1 immune response to Trypanosoma cruzi trans-sialidase. Parasite Immunol. 2000;22:49–53. doi: 10.1046/j.1365-3024.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 89.Gomes JA, Bahia-Oliveira LM, Rocha MO, et al. Evidence that development of severe cardiomyopathy in human Chagas’ disease is due to a Th1-specific immune response. Infect Immun. 2003;71:1185–93. doi: 10.1128/IAI.71.3.1185-1193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Souza PE, Rocha MO, Rocha-Vieira E, et al. Monocytes from patients with indeterminate and cardiac forms of Chagas’ disease display distinct phenotypic and functional characteristics associated with morbidity. Infect Immun. 2004;72:5283–91. doi: 10.1128/IAI.72.9.5283-5291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laucella SA, Postan M, Martin D, et al. Frequency of interferon- gamma -producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J Infect Dis. 2004;189:909–18. doi: 10.1086/381682. [DOI] [PubMed] [Google Scholar]
- 92.Fonseca SG, Reis MM, Coelho V, et al. Locally produced survival cytokines IL-15 and IL-7 may be associated to the predominance of CD8+ T cells at heart lesions of human chronic Chagas disease cardiomyopathy. Scand J Immunol. 2007;66:362–71. doi: 10.1111/j.1365-3083.2007.01987.x. [DOI] [PubMed] [Google Scholar]
- 93.Cunha-Neto E, Dzau VJ, Allen PD, et al. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas’ disease cardiomyopathy. Am J Pathol. 2005;167:305–13. doi: 10.1016/S0002-9440(10)62976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dutra WO, Gollob KJ, Pinto-Dias JC, et al. Cytokine mRNA profile of peripheral blood mononuclear cells isolated from individuals with Trypanosoma cruzi chronic infection. Scand J Immunol. 1997;45:74–80. doi: 10.1046/j.1365-3083.1997.d01-362.x. [DOI] [PubMed] [Google Scholar]
- 95.Talvani A, Rocha MO, Barcelos LS, et al. Elevated concentrations of CCL2 and tumor necrosis factor-alpha in chagasic cardiomyopathy. Clin Infect Dis. 2004;38:943–50. doi: 10.1086/381892. [DOI] [PubMed] [Google Scholar]
- 96.Alvarez MG, Postan M, Weatherly DB, et al. HLA Class I-T Cell Epitopes from trans-Sialidase Proteins Reveal Functionally Distinct Subsets of CD8 T Cells in Chronic Chagas Disease. PLoS Negl Trop Dis. 2008;2:e288. doi: 10.1371/journal.pntd.0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diez H, Lopez MC, Del Carmen Thomas M, et al. Evaluation of IFN-gamma production by CD8 T lymphocytes in response to the K1 peptide from KMP-11 protein in patients infected with Trypanosoma cruzi. Parasite Immunol. 2006;28:101–5. doi: 10.1111/j.1365-3024.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 98.Fonseca SG, Moins-Teisserenc H, Clave E, et al. Identification of multiple HLA-A*0201-restricted cruzipain and FL-160 CD8+ epitopes recognized by T cells from chronically Trypanosoma cruzi-infected patients. Microbes Infect. 2005;7:688–97. doi: 10.1016/j.micinf.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 99.Marin-Neto JA, Cunha-Neto E, Maciel BC, et al. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–23. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 100.Bonney KM, Engman DM. Chagas heart disease pathogenesis: one mechanism or many. Curr Mol Med. 2008;8:510–8. doi: 10.2174/156652408785748004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benvenuti LA, Roggerio A, Freitas HF, et al. Chronic American trypanosomiasis: parasite persistence in endomyocardial biopsies is associated with high-grade myocarditis. Ann Trop Med Parasitol. 2008;102:481–7. doi: 10.1179/136485908X311740. [DOI] [PubMed] [Google Scholar]
- 102.Bilate AM, Teixeira PC, Ribeiro SP, et al. Distinct outcomes of Trypanosoma cruzi infection in hamsters are related to myocardial parasitism, cytokine/chemokine gene expression, and protein expression profile. J Infect Dis. 2008;198:614–23. doi: 10.1086/590347. [DOI] [PubMed] [Google Scholar]
- 103.Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, et al. Benznidazole treatment during early-indeterminate Chagas’ disease shifted the cytokine expression by innate and adaptive immunity cells toward a type 1-modulated immune profile. Scand J Immunol. 2006;64:554–63. doi: 10.1111/j.1365-3083.2006.01843.x. [DOI] [PubMed] [Google Scholar]
- 104.Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 105.Lages-Silva E, Filardi LS, Brener Z. Effect of the host specific treatment in the phagocytosis of Trypanosoma cruzi blood forms by mouse peritoneal macrophages. Mem Inst Oswaldo Cruz. 1990;85:401–5. doi: 10.1590/s0074-02761990000400003. [DOI] [PubMed] [Google Scholar]
- 106.Murta SM, Ropert C, Alves RO, et al. In-vivo treatment with benznidazole enhances phagocytosis, parasite destruction and cytokine release by macrophages during infection with a drug-susceptible but not with a derived drug-resistant Trypansoma cruzi population. Parasite Immunol. 1999;21:535–44. doi: 10.1046/j.1365-3024.1999.00251.x. [DOI] [PubMed] [Google Scholar]
- 107.Romanha AJ, Alves RO, Murta SM, et al. Experimental chemotherapy against Trypanosoma cruzi infection: essential role of endogenous interferon-gamma in mediating parasitologic cure. J Infect Dis. 2002;186:823–8. doi: 10.1086/342415. [DOI] [PubMed] [Google Scholar]
- 108.Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–50. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olivieri BP, Cotta-De-Almeida V, Araujo-Jorge T. Benznidazole treatment following acute Trypanosoma cruzi infection triggers CD8+ T-cell expansion and promotes resistance to reinfection. Antimicrob Agents Chemother. 2002;46:3790–6. doi: 10.1128/AAC.46.12.3790-3796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caldas IS, Talvani A, Caldas S, et al. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol Res. 2008;103:413–21. doi: 10.1007/s00436-008-0992-6. [DOI] [PubMed] [Google Scholar]
- 111.Bahia-Oliveira LM, Gomes JA, Cancado JR, et al. Immunological and clinical evaluation of chagasic patients subjected to chemotherapy during the acute phase of Trypanosoma cruzi infection 14–30 years ago. J Infect Dis. 2000;182:634–8. doi: 10.1086/315743. [DOI] [PubMed] [Google Scholar]
- 112.Michailowsky V, Murta SM, Carvalho-Oliveira L, et al. Interleukin-12 enhances in vivo parasiticidal effect of benznidazole during acute experimental infection with a naturally drug-resistant strain of Trypanosoma cruzi. Antimicrob Agents Chemother. 1998;42:2549–56. doi: 10.1128/aac.42.10.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vitelli-Avelar DM, Sathler-Avelar R, Teixeira-Carvalho A, et al. Strategy to assess the overall cytokine profile of circulating leukocytes and its association with distinct clinical forms of human Chagas disease. Scand J Immunol. 2008;68:516–25. doi: 10.1111/j.1365-3083.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 114.Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, et al. Etiological treatment during early chronic indeterminate Chagas disease incites an activated status on innate and adaptive immunity associated with a type 1-modulated cytokine pattern. Microbes Infect. 2008;10:103–13. doi: 10.1016/j.micinf.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 115.Revelli S, Le Page C, Piaggio E, et al. Benznidazole, a drug employed in the treatment of Chagas’ disease, down-regulates the synthesis of nitrite and cytokines by murine stimulated macrophages. Clin Exp Immunol. 1999;118:271–7. doi: 10.1046/j.1365-2249.1999.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Piaggio E, Sanceau J, Revelli S, et al. Trypanocidal drug benznidazole impairs lipopolysaccharide induction of macrophage nitric oxide synthase gene transcription through inhibition of NF-kb activation. J Immunol. 2001;167:3422–6. doi: 10.4049/jimmunol.167.6.3422. [DOI] [PubMed] [Google Scholar]
- 117.Piaggio E, Roggero E, Pitashny M, et al. Treatment with benznidazole and its immunomodulating effects on Trypanosoma cruzi-infected rats. Parasitol Res. 2001;87:539–47. doi: 10.1007/s004360000357. [DOI] [PubMed] [Google Scholar]
- 118.Pascutti MF, Pitashny M, Nocito AL, et al. Benznidazole, a drug used in Chagas’ disease, ameliorates LPS-induced inflammatory response in mice. Life Sci. 2004;76:685–97. doi: 10.1016/j.lfs.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 119.Manarin R, Bottasso E, Bottasso O, et al. Beneficial effects of benznidazole during an infectious-based situation of systemic inflammatory response: cecal ligation and puncture. Am J Trop Med Hyg. 2008;79:793–6. [PubMed] [Google Scholar]
- 120.Cabeza Meckert P, Chambo JG, Laguens RP. Differences in resistance to reinfection with low and high inocula of Trypanosoma cruzi in chagasic mice treated with nifurtimox and relation to immune response. Antimicrob Agents Chemother. 1988;32:241–5. doi: 10.1128/aac.32.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lelchuk R, Cardoni RL, Levis S. Nifurtimox-induced alterations in the cell-mediated immune response to PPD tin guinea-pigs. Clin Exp Immunol. 1977;30:469–73. [PMC free article] [PubMed] [Google Scholar]
- 122.Lelchuk R, Cardoni RL, Fuks AS. Cell-mediated immunity in Chagas’ disease: alterations induced by treatment with a trypanocidal drug (nifurtimox) Clin Exp Immunol. 1977;30:434–8. [PMC free article] [PubMed] [Google Scholar]
- 123.Sanchez-Burgos G, Mezquita-Vega RG, Escobedo-Ortegon J, et al. Comparative evaluation of therapeutic DNA vaccines against Trypanosoma cruzi in mice. FEMS Immunol Med Microbiol. 2007;50:333–41. doi: 10.1111/j.1574-695X.2007.00251.x. [DOI] [PubMed] [Google Scholar]
- 124.Tarleton RL. Chagas disease: a role for autoimmunity? Trends Parasitol. 2003;19:447–51. doi: 10.1016/j.pt.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 125.da Silva AL. Chagas disease surgery. Mem Inst Oswaldo Cruz. 1999;94:343–7. doi: 10.1590/S0074-02761999000700067. [DOI] [PubMed] [Google Scholar]
- 126.Soares MB, Garcia S, Campos de Carvalho AC, et al. Cellular therapy in Chagas’ disease: potential applications in patients with chronic cardiomyopathy. Regen Med. 2007;2:257–64. doi: 10.2217/17460751.2.3.257. [DOI] [PubMed] [Google Scholar]


