Abstract
A blockade of CD44 can interfere with haematopoietic and leukemic stem cell homing, the latter being considered as a therapeutic option in haematological malignancies. We here aimed to explore the molecular mechanism underlying the therapeutic efficacy of anti-CD44. We noted that in irradiated mice reconstituted with a bone marrow cell transplant, anti-CD44 exerts a stronger effect on haematopoietic reconstitution than on T lymphoma (EL4) growth. Nonetheless, in the non-reconstituted mouse anti-CD44 suffices for a prolonged survival of EL4-bearing mice, where anti-CD44-prohibited homing actively drives EL4 cells into apoptosis. In vitro, a CD44 occupancy results in a 2–4-fold increase in apoptotic EL4 cells. Death receptor expression (CD95, TRAIL, TNFRI) remains unaltered and CD95 cross-linking-mediated apoptosis is not affected. Instead, CD44 ligation promotes mitochondrial depolarization that is accompanied by caspase-9 cleavage and is inhibited in the presence of a caspase-9 inhibitor. Apoptosis becomes initiated by activation of CD44-associated phosphatase 2A (PP2A) and proceeds via ERK1/2 dephosphorylation without ERK1/2 degradation. Accordingly, CD44-induced apoptosis could be mimicked by ERK1/2 inhibition, that also promotes EL4 cell apoptosis through the mitochondrial pathway. Thus, during haematopoietic stem cell reconstitution care should be taken not to interfere by a blockade of CD44 with haematopoiesis, which could be circumvented by selectively targeting leukemic CD44 isoforms. Beyond homing/settlement in the bone marrow niche, anti-CD44 drives leukemic T cells into apoptosis via the mitochondrial death pathway by CD44 associating with PP2A. Uncovering this new pathway of CD44-induced leukemic cell death provides new options of therapeutic interference.
Keywords: rodent, apoptosis, CD44, signal transduction, PP2A
Introduction
CD44 comprises a family of glycoproteins encoded by a single gene [1], that vary in size due to N- and O-glycosylation and insertion of alternatively spliced variable exon products in the extracellular domains of the molecule [2]. CD44, besides many other activities [3, 4], plays an important role in haematopoiesis [5]. More recently, CD44 has been described as a cancer initiating marker in several types of carcinoma [6], but also in leukaemia [7–10].
The importance of CD44 in haematopoiesis has first been described in 1990 by Miyake et al.[11], noting that anti-CD44 prohibited the development of cobblestone areas and of non-adherent progenitors in murine long-term bone marrow cultures [11]. At least in vitro, bone marrow stroma formation also requires CD44, which supports the process by induction of IL-6 secretion [12, 13]. Notably, the most primitive human and mouse HSCs synthesize and express hyaluronan (HA) [14] and HA expression correlates with selective migration of HSCs to the endosteal niche [14]. HSC homing can be blocked by anti-CD44 or soluble HA or hyaluronidase treatment [15]. In addition, CXCL12 stimulates adhesion of progenitor cells via CD44 demonstrating a cross-talk between CD44 and CXCR4 signalling, which suggests a key role of HA and CD44 in CXCL12-dependent transendothelial migration of HSCs and their anchorage within specific niches [15]. Thus, CD44 contributes to homing and settlement of HSCs in the bone marrow niche [16]. In addition, depending on associations with integrins, CD44 has been suggested to promote quiescence versus differentiation [17].
Two recent reports describe that CD44 also accounts for leukaemia cell homing, some haematological malignancies revealing high CD44 expression particularly on the subpopulation of cancer initiating cells [8]. The acute myeloid leukaemia stem cell requires CD44 for the transport to the stem cell-supportive microenvironmental niche and anti-CD44 alters the fate of acute myeloid leukaemia stem cells by inducing differentiation [18]. In a mouse model of chronic myeloid leukaemia, BCR-ABL1-transduced progenitors from CD44-mutant donors were defective in bone marrow homing, which resulted in decreased engraftment and impaired CML-like disease induction. These studies provided additional evidence for a higher degree of CD44 dependence of leukemic than of haematopoietic stem cells [19]. The findings in both studies suggest that anti-CD44 promoted death of leukaemia-initiating cells, which possibly could be translated into a therapeutic strategy to eliminate quiescent leukemic stem cells (LSCs) by a blockade of CD44. However, because HSCs and LSCs are CD44 dependent, it would be desirable to know the pathway of anti-CD44-induced apoptosis and whether differences between HSCs and LSCs can be elaborated, where death could be a consequence of a deficit in survival signals from the haematopoietic niche or CD44 occupancy could actively initiate signals that promote apoptosis, where both the receptor-mediated and mitochondrial pathway have been assigned to CD44 [20, 21].
During haematopoiesis as well as lymphocyte activation, CD44 has become assigned with promoting proliferation [22, 23], cytokine secretion [24], apoptosis protection as well as induction [8, 9]. CD44-induced apoptosis protection proceeds via activation of anti-apoptotic molecules [25, 26]. CD44-induced apoptosis obviously can proceed via different pathways [27]. CD44-initiated apoptosis has been described to be accompanied by up-regulation of apoptosis receptors [28, 29] or by HA binding in activated T cells, which has been CD95 and caspase independent [30] or by CD40 and CD44v7 cross-linking, which also is CD95 independent, but proceeds through caspase-3 and caspase-9 activation [31] suggestive of the involvement of the mitochondrial apoptosis pathway. In haematological malignancies, too, CD44 ligation can induce cell cycle arrest [32, 33], differentiation and apoptosis [34], which in erythroid leukaemia cells has been observed to be caspase independent, but accompanied by mitochondrial membrane destabilization and release of apoptosis-inducing factor, but not of cytochrome c [35].
We have been particularly interested in the role of CD44 in progenitor T-cell homing and maturation. CD44 is known as a thymus homing receptor for T progenitor cells [36], which we recently could confirm [37] for the subpopulation of common lymphoid progenitor 2 cells (CLP2) [38]. Thus we asked, whether leukemic T-cell growth in vivo also requires CD44 and whether antibody occupancy of CD44 hampers settlement or actively promotes apoptosis. Using the EL4 thymoma line we demonstrate that CD44 contributes to T lymphoma homing and that a blockade of CD44 drives thymoma cells into apoptosis. Unravelling the molecular pathway of CD44-induced apoptosis in T lymphoma revealed a new pathway, initiated via activation of CD44-associated phosphatase 2A (PP2A), which inhibits ERK1/2 phosphorylation leading to mitochondrial membrane depolarization and caspase-9 activation.
Material and methods
Mice and tumours lines
C57BL6 (H-2b) mice were bred at the central animal facilities of the German Cancer Research Center; 8–10-week-old mice were used for experiments. The C57BL6 EL4 thymoma line and CD44v6 cDNA transfected EL4 cells (EL4-v6) were maintained in RPMI1640, 10% FCS, L-glutamine and antibiotics. Cells were regularly screened for mycoplasma infection and were mycoplasma free. Cells were split when reaching a density of 106/ml.
Antibodies
Hybridoma supernatant of anti-CD4, -CD8, -panCD44 (IM7), (European Association of Animal Cell Cultures, Salisbury, England), anti-H-2Db (K7–65) [39] and anti-CD49d (PS/2) [40] were purified by affinity chromatography. JO2 (hamster antimouse CD95) [41] was a kind gift from S. Nagata (Kyoto University, Japan). Unlabelled, biotinylated or dye-labelled anti-CD44v6, -CD95, -CD95L, -(p)c-jun, -NFκB, -TNFRI, -TNFRII, -TRAIL, -(p)ERK1,2, -pTyr, -pThr, -caspase-3, -caspase-9, -PI3K, -(p)Akt, -Ras, -Bcl-xL, -(p)BAD, -BAX, -(p)PP1, -PP2A, -CK2 (casein kinase 2), -actin, biotinylated, dye- or HRPO-labelled secondary antibodies and streptavidin were obtained commercially (BD/Pharmingen, Heidelberg, Germany; Dianova, Hamburg, Germany; Biotrend, Köln, Germany; Bender Medsystems, Vienna, Austria; Santa Cruz, CA, USA).
Haematopoietic cell preparation
Bone marrow, thymus and lymph nodes were teased through fine gauze. Bone marrow cells (BMC) were T-cell depleted by panning on anti-CD4- and anti-CD8-coated Petri dishes (purity: ∼98%). Where indicated, cells were CFSE (Invitrogen, Karlsruhe, Germany) labelled.
Flow cytometry
Cells (5 × 105) were stained according to routine procedures. For intracellular staining (cytokines, chemokines, signalling molecules) cells were fixed and permeabilized in advance. Apoptosis was determined by annexinV-FITC or -APC/PI staining. Mitochondrial integrity was determined by DilC1 staining, measuring fluorescence in the FL4 channel. The cell cycle was controlled by PI staining (1 μg/ml, 0.1% TritonX-100, RNAase A) after fixation in ethanol. Samples were processed in a FACS-Calibur using the Cell Quest program for analysis (BD, Heidelberg, Germany).
Apoptosis induction
Apoptosis was induced by death receptor cross-linking, or a CD44 blockade by anti-panCD44 (IM7) or anti-CD44v6 (10 μg/ml) or HA (15 μg/ml). Campothectin (1 μM/ml) served as a positive control. The percentage of respiratory active cells was evaluated by DilC1 staining. Cells were treated with SL327 (MEK1,2 inhibitor, 5 μM), tetra-bromo-benzotriazole (CK2 inhibitor, 2 μM), LEDH-CHO, (caspase-9 inhibitor, 2 μM), DEVD-CHO, (caspase-3 inhibitor, 2 μM) or okadaic acid (OA) (1–20 μM).
Raf-GST fusion protein and pull-down assay
Escherichia coli were transformed with a GST-Raf (AA1–149 of cRaf1) cDNA [42]. The purified fusion protein was incubated with cell lysate and 1/10 volume of glutathione beads. After washing, the beads were resuspended in 6× Laemmli buffer and run on SDS-PAGE.
Phosphatase assay
Cell lysates, centrifuged at 12,000 ×g for 10 min., were incubated with anti-PP2A and ProteinG-agarose (Roche, Mannheim, Germany) (2 hrs, 4°C). The immunoprecipitate was washed and incubated with 900 μg/ml p-NPP (30 min., 37°C). The amount of para-nitrophenol produced by dephosphorylation was determined by measuring the absorbance at 405 nm.
Sucrose gradient centrifugation
Cells were lysed in 1% Lubrol, lysates were centrifuged (10 min., 20,000 ×g), adjusted to 40% sucrose (4.5 ml) and were layered on 1.3 ml of 50% sucrose and overlaid with 2.3 ml of 30%, 2.3 ml of 20% and 1.3 ml of 5% sucrose. After centrifugation (200,000 ×g, 16 hrs), 12 fractions (1 ml) were collected from the top of the tubes. Fractions 1–4, 5–8 and 9–12 were pooled and precipitated with anti-CD44.
Cytosol, nuclei and mitochondria preparation
Cells were incubated in hypotonic buffer, homogenized and centrifuged at 800 rpm for pelleting the nuclei. For separating the cytosolic from the mitochondrial fraction, 2.5 × 106 cells were lysed in 0.5 ml lysis buffer. After adding Nonidet-P40 (0.5%), vortexing and centrifugation (1600 rpm, 5 min.), cytosolic proteins are recovered from the supernatant. The pellet (mitochondria) and the pelleted nuclei were washed, resuspended in lysis buffer (1% TritonX-100, 1% SDS) and sonicated (7 sec., nine cycles).
Immunoprecipitation
Cells (2 × 107) were washed twice in TNE-buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 1 mM Na2VO4, 10 mM NaF) and lysed (TNE-buffer, 1% Lubrol, 1 mM PMSF, a protease inhibitor mix) (Boehringer, Mannheim, Germany) (30 min., 4°C). Centrifuged (13,000 ×g, 10 min., 4°C) lysates were pre-cleared, incubated with the indicated antibodies (1 hr, 4°C) followed by incubation with ProteinG Sepharose (1 hr) and washing.
Western blot (WB)
EL4 cells were lysed in Laemmli buffer, sonicated and boiled (5 min.). Lysates (30 μl) were resolved on 10% SDS-PAGE. Proteins were transferred to Nitrocellulose membranes (30 V, 16 hrs, 4°C) and detected by WB with indicated antibodies using ECL (GE Healthcare, Munich, Germany) detection system.
Reconstitution and tumour cell injection
C57BL6 mice received an i.v. or s.c. injection of 104 EL4 cells or were lethally irradiated (9.5 Gy) and reconstituted with 1 × 107 T-cell-depleted BMC and received EL4 cells 2 days after reconstitution. Both groups of mice received twice per week an i.v. injection of 150 μg control IgG or anti-panCD44 (IM7). Survival time and reconstitution were controlled. In a second setting, C57BL6 mice were treated as described above, but received 2 × 106 CFSE-labelled EL4 cells. The% of CFSE-labelled EL4 cells and of apoptotic (annexinV-APC stained) CFSE-labelled EL4 cells in bone marrow, thymus and spleen was evaluated during 96 hrs. Animal experimentations were approved by the governmental authorities of Baden-Wuerttemberg, Germany.
Statistical analysis
Significance of differences was calculated according to the Wilcoxon rank sum test (in vivo assays) or the Student’s t-test (in vitro studies). Mean values ± S.D. of in vivo experiments are derived from 3–5 experiments with 2–3 mice/group in each experiment corresponding to 6–15 mice/group. Mean ± S.D. of in vitro studies are derived from three experiments and/or are based on three to four replicates. P-values <0.05 were considered as statistically significant.
Results
Anti-panCD44 interferes with haematopoiesis and thymoma growth
C57BL6 mice received 104 EL4 cells, i.v. or s.c., with/without anti-panCD44 (IM7, 150 μg/ml, i.v.). The antibody application was repeated two times per week. After s.c. application of the lymphoma cells the mean tumour diameter was measured twice per week and mice were killed, when the mean diameter of the s.c. growing tumour reached 2.5 cm. After i.v. lymphoma cell application, mice were killed upon weight loss and fatigue (survival time). The development of metastasis was evaluated microscopically and by surveying lymphoma cell growth in cultures of dispersed BMC, thymocytes (TC), SC and liver. Subcutaneous lymphoma growth was retarded by IM7 and the survival time significantly exceeded the survival time of mice receiving control IgG. After i.v. application of lymphoma cells, the survival time of IM7 treated mice was prolonged, however, not at a statistically significant level (Fig. 1A). Furthermore, after i.v. lymphoma application, thymic metastasis developed in 3/10 anti-panCD44-treated, but only in 1/10 mice not receiving IM7. After s.c. EL4 application, the number of mice that developed metastases was also increased in IM7-tretaed as compared to control mice (Fig. 1A–C, Table 1). Distinct to immunocompetent mice, application of IM7 accelerated tumour growth in irradiated and reconstituted mice (Fig. 1D). In addition, bone marrow and thymus reconstitution was severely impaired by IM7 treatment in reconstituted, leukaemia-bearing mice (Fig. 1E).
Fig 1.
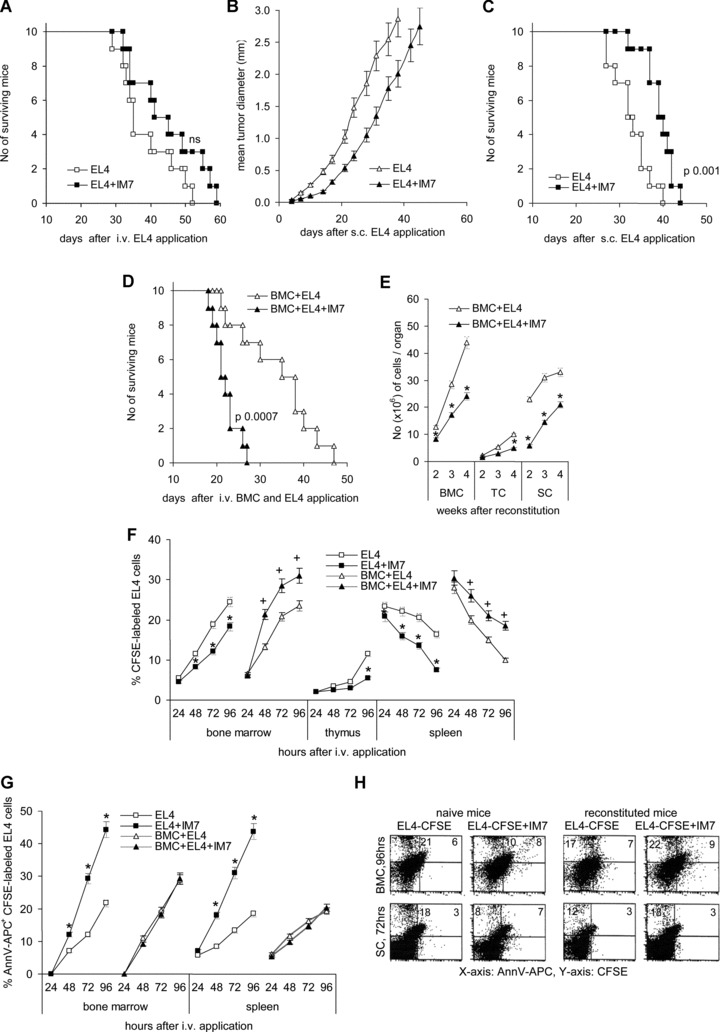
The impact of anti-CD44 on thymoma growth in vivo: (A–E) C57BL6 mice received an i.v. (A) or s.c. (B, C) injection of 104 EL4 cells or (C, D) were lethally irradiated and reconstituted with 1 × 107 T-cell-depleted BMC and received EL4 cells, i.v. 2 days after reconstitution. Mice received twice per week an i.v. injection of 150 μg control IgG or IM7. (A) The survival time of mice receiving EL4 cells i.v. is shown. Survival was slightly, but not to a significant level prolonged by IM7 application. (B, C) After s.c. application of EL4 cells, IM7 retarded the start of the tumour growth and the survival time became significantly prolonged. (D) The survival time of IM7-treated, EL4 tumour-bearing reconstituted mice was significantly shortened as compared to mice receiving control IgG and (E) the recovery of BMC, TC and SC (mean number ± S.D. of three mice/group) was significantly delayed (*). (F) C57BL6 mice were treated as described above, but received 2 × 106 CFSE-labelled EL4 cells. The% of CFSE-labelled EL4 cells in bone marrow, thymus and spleen was evaluated during 96 hrs. Mean values ± S.D. of three mice are shown. The recovery of tumour cells is significantly reduced in IM7-treated, non-reconstituted mice (*), but unaltered or increased in IM7-treated reconstituted mice (+). (G) The percentage (mean ± S.D.) of apoptotic CFSE-labelled EL4 cells during the starting 96 hrs after i.v. application was evaluated by annexinV-APC staining. The percentage of apoptotic EL4 cells is significantly increased in non-reconstituted IM7-treated mice (*), but largely unaltered in reconstituted IM7-treated mice. (H) Examples of apoptotic EL4 cells in the bone marrow 96 hrs and in the spleen 72 hrs after application of CFSE-labelled EL4 cells in naive and reconstituted C57BL6 mice.
Table 1.
Impact of anti-CD44 on EL4 tumour growth after subcutaneous and intravenous application
| Tumour cells (104)* | No. of mice | Application | Treatment | Survival time | P-values | Metastasis |
|---|---|---|---|---|---|---|
| EL4 | 10 | s.c. | rIgG | 32.7 + 4.2 | Spleen (1) | |
| EL4 | 10 | s.c. | IM7 | 39.9 + 4.0 | 0.001 | Spleen (4), liver (1) |
| EL4 | 10 | i.v. | rIgG | 38.6 + 8.0 | Thymus (1) | |
| EL4 | 10 | i.v. | IM7 | 44.6 + 10.0 | 0.16 | Thymus (3) |
Tumour cells were injected into immunocompetent, 8-week-old C57BL6 mice.
Evaluating short term recovery of CFSE-labelled EL4 cells in spleen, bone marrow and thymus in dependence on concomitant IM7 application revealed that IM7 interfered with the settlement of EL4 cells in spleen, bone marrow and thymus in immunocompetent mice. Instead, in line with the survival study, this effect was not observed in irradiated and reconstituted mice (Fig. 1F). When analysing the percentage of annexinV stained EL4 cells, it became obvious that hampering EL4 settlement by anti-CD44 was accompanied by a significant increase in the percentage of apoptotic cells between 24 and 48 hrs after intravenous application. Anti-CD44-induced apoptosis of lymphoma cells was not seen in concomitantly reconstituted mice (Fig. 1G and H).
The data are in line with several reports demonstrating that anti-CD44 interferes with thymoma growth, but concomitantly severely hampers the reconstitution process [21, 24], such that during reconstitution the benefit of retarding lymphoma growth becomes overridden. In addition, our data provided evidence that anti-CD44-mediated retardation of thymoma growth under steady state conditions was accompanied by an increase in apoptotic tumour cells. Thus, it became important to know, whether death was a consequence solely of a failure to embed or whether IM7 may have actively triggered apoptosis.
Anti-CD44 promotes thymoma cell apoptosis
To control for this hypothesis and to evaluate the underlying mechanism(s), EL4 and EL4v6 cells were cultured in the presence of 10 μg/ml anti-CD44. After 16 hrs of culture in the presence of IM7 the percentage of apoptotic EL4/EL4v6 cells was measured by annexinV-FITC/PI staining. In the presence of soluble anti-panCD44 the apoptosis rate was increased after 6 hrs from ∼2% to ∼25% and after o/n culture from ∼10% to ∼30% (Fig. 2A). Similar effects were observed with YC18 cells, a BALB/c-derived lymphoma (data not shown). In order to check for the specificity of apoptosis induction through CD44, we used HA and for EL4v6 cells anti-CD44v6. HA and anti-CD44v6 induced apoptosis in EL4 and EL4v6 cells comparably to anti-panCD44. Instead, the percentage of apoptotic cells was not increased in cultures containing an anti-MHC antibody or anti-CD49d, CD49d being expressed at a high level on EL4 cells (Fig. 2B). The increase in apoptotic cells obviously is not a consequence of activation induced cell death, as the distribution of cells in G1, S and G2 or M-phase did not vary significantly depending on the culture condition (Fig. 2C).
Fig 2.
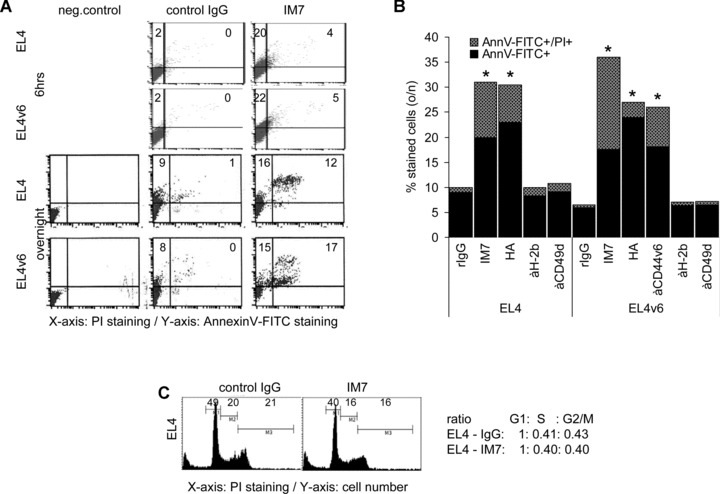
CD44 ligation induces apoptosis in EL4 cells: (A and B) EL4 and EL4v6 cells were cultured for 6 hrs or o/n in the presence of 10 μg/ml IM7, anti-CD44v6 or rIgG or 15 μg/ml HA and, as controls, 10 μg/ml anti-H-2b or anti-CD49d. Cells were stained with annexinV-FITC/PI. A representative example (A) and mean values ± S.D. of apoptotic cells are shown (B). Significant differences in comparison to cells cultured in the presence of rIgG are indicated by *. (C) Cells were stained with PI and cultured o/n in the presence of rIgG or IM7. The percentage of cells in G1, S and G2 or M phase was evaluated by flow cytometry. Cell cycle progression did not vary significantly in dependence on the presence of IM7.
Thus, occupancy of CD44, independently of the particular epitope, can induce apoptosis in T lymphoma cells.
Apoptosis induction, loss of mitochondrial membrane polarization and caspase-9 activation
Asking for the molecular mechanism accounting for CD44-induced apoptosis, expression levels of Fas, FasL, Trail, TNFRI and TNFRII were evaluated. EL4 and EL4v6 cells express Fas at about 55–60%, FasL at less than 10%, TRAIL at >70%, TNFRI and TNFRII at ∼10% to ∼20%. Expression and intensity of expression of these death receptors was not changed after overnight culture in the presence of anti-CD44 (Fig. 3A and B). Moreover, EL4 and EL4v6 cells were resistant to apoptosis by CD95 cross-linking with the JO2 anti-CD95 mAb as well as to TNFRI and TNFRII cross-linking. Death receptor cross-linking also did not strengthen anti-CD44-induced apoptosis (Fig. 3C). In view of these findings the possibility of death receptor-induced apoptosis by a cross-talk between CD44 and the Fas machinery becomes unlikely.
Fig 3.
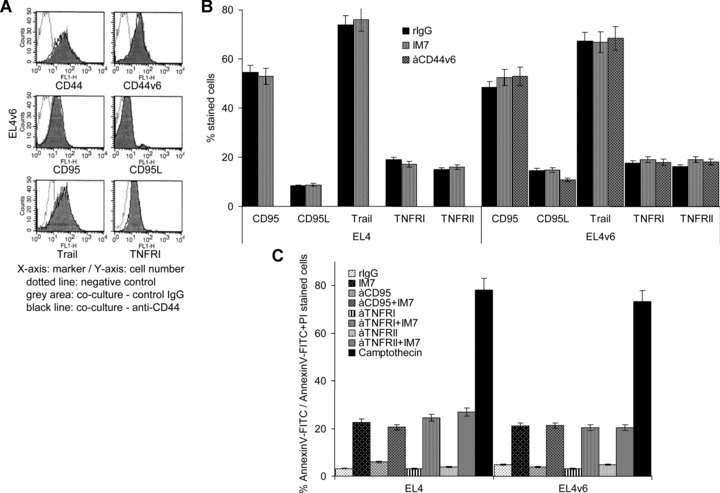
EL4 and EL4v6 cells are resistant towards Fas-induced apoptosis: (A and B) CD44, CD44v6, CD95, CD95L, Trail, TNFRI and TNFRII expression on EL4 cells and EL4v6 cells was evaluated after o/n incubation in the presence of rIgG, IM7 or anti-CD44v6 (10 μg/ml). (A) A representative example and (B) mean values ± S.D. are shown. IM7 had no impact on CD44 and apoptosis receptor expression. (C) EL4 and EL4v6 cells were cultured o/n on uncoated or anti-CD95-, anti-TNFRI- or anti-TNFRII-coated plates. Cultures contained 10 μg/ml rIgG or IM7 or 1 μM/ml camptothecin (positive control). The percentage (mean ± S.D. of triplicates) of annexinV-FITC/PI stained cells is shown. EL4 and EL4v6 cells were resistant towards receptor-induced apoptosis and IM7-induced apoptosis was independent of death receptor cross-linking.
To strengthen this interpretation, the involvement of caspase-3 and caspase-9 in anti-CD44-initiated apoptosis was evaluated. EL4 and EL4v6 cells were cultured overnight in the presence of IM7 and caspase-3 or caspase-9 inhibitors. Apoptosis was measured by annexinV-FITC/PI staining. Caspase-3 and caspase-9 inhibitors reduced IM7-induced apoptosis in EL4 and EL4v6 cells (Fig. 4A and B). As revealed by WB, cleavage of caspase-9 and caspase-3 in EL4 and EL4v6 cells was more pronounced when cultured in the presence of IM7 and caspase-9 cleavage was partly inhibited in the presence of a caspase-9 inhibitor (Fig. 4C). Caspase-9 becoming activated within the apoptosome after cytochrome c release from the mitochondria, this result points towards anti-CD44-initiated activation of the intrinsic apoptosis pathway, which is characterized by loss of mitochondrial membrane polarization. To confirm the involvement of the mitochondrial pathway, we measured mitochondrial membrane depolarization using DilC1, which accumulates in intact mitochondria [43]. Untreated cells show high level of dye intensity. The intensity of fluorescence decreased already after 2 hrs incubation with anti-CD44 (data not shown), and more pronounced after 12 hrs (Fig. 4D). Also, cytochrome c was recovered in the cytosol only in IM7-treated EL4 and EL4v6 cells, albeit at a low level (Fig. 4E).
Fig 4.
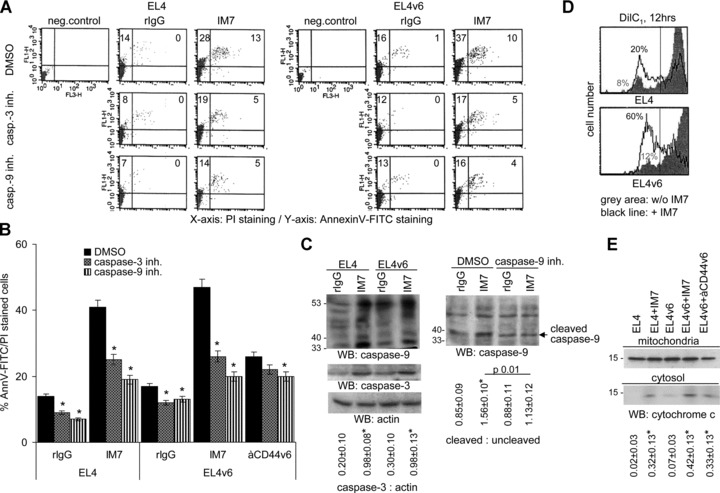
CD44 ligation induces apoptosis via the mitochondrial pathway: (A and B) EL4 and EL4v6 cells were cultured o/n in the presence of rIgG, IM7 or anti-CD44v6. Cultures contained in addition DMSO (control) or 2 μM caspase-3 or caspase-9 inhibitor. Apoptosis was measured by annexinV-FITC/PI staining. (A) A representative example and (B) mean values ± S.D. of triplicates are shown. Significant differences in the percentage of apoptotic cells in the presence of caspase inhibitors are indicated by *. (C) WB of caspase-3 and caspase-9 cleavage in lysates of EL4 cells cultured o/n in the presence of rIgG or IM7 or in the presence of a caspase-9 inhibitor. The mean values ± S.D. of the ratio of caspase-3: actin and cleaved: uncleaved caspase-9 are shown. Significant differences by IM7 or a caspase-9 inhibitor are indicated by an asterisk. Caspase-3 and capase-9 cleavage is enhanced in IM7 treated cells and caspase-9 cleavage is reduced in the presence of the caspase-9 inhibitor. (D) EL4 cells were stained with the mitochondrial dye DilC1 and incubated with 10 μg/ml IM7 for 12 hrs. DilC1 staining was evaluated by flow cytometry. (E) EL4 cells were incubated with 10 μg/ml IM7 for 12 hrs. Mitochondria were separated from the cytosol. Lysates were separated by SDS-PAGE, proteins were transferred to a nitrocellulose membrane and blotted with anti-cytochrome c. The mean values ± S.D. of the ratio of cytochrome c in the cytosol: mitochondria is shown. Significant differences by IM7 or anti-CD44v6 are indicated by an asterisk. (D and E) Mitochondria integrity is strongly affected after 12 hrs of culture in the presence of IM7.
These results indicate that CD44-induced apoptosis proceeds via caspase-9 and caspase-3 activation through the mitochondrial pathway.
Anti-CD44 induces ERK inhibition through PP2A
To unravel the molecular pathway initiated downstream of CD44 engagement, we checked for early activation signalling events which might be involved. EL4 cells stimulated with IM7 for up to 2 hrs did not reveal any change in the phosphotyrosine profile (Fig. 5A), PI3K activation (data not shown) and Akt phosphorylation (Fig. 5B). Instead, ERK phosphorylation became strongly inhibited. Inhibition of ERK phosphorylation started 15 min. after IM7 addition and lasted for at least 1 hr in EL4 and EL4v6 cells (Fig. 5C). Because the total amount of ERK remained stable, this result rules out an effect of CD44 on ERK degradation. To differentiate between the possibilities that a blockade of CD44 interferes with ERK1/2 activation directly or via the Ras pathway, we used a GST-raf fusion protein to pull down the active form of Ras. The GST-raf pull-down experiment revealed no effect of IM7 treatment on Ras activity in EL4 cells (data not shown). This finding suggests that CD44 affects ERK phosphorylation independently of the Ras pathway. Next we evaluated, whether a blockade of CD44 leads to ERK1/2 dephosphorylation via activation of a phosphatase. Addition of OA, an inhibitor of the protein phosphatases 1 (PP1) and 2A (PP2A), to the culture stabilized ERK1/2 phosphorylation and reversed the effect of anti-CD44 (Fig. 5D).
Fig 5.
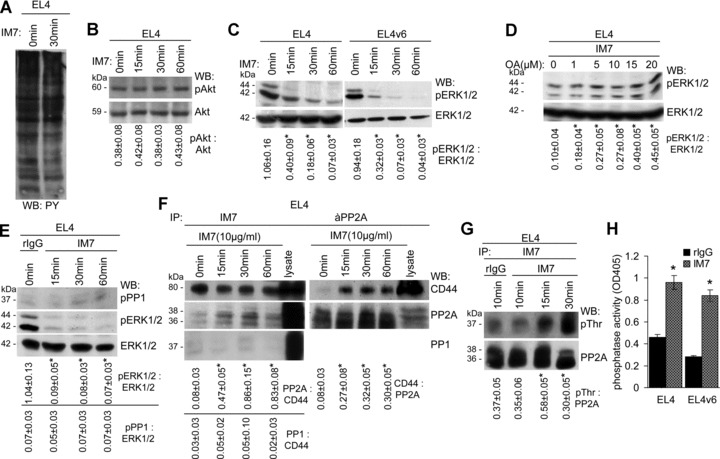
CD44 ligation induces PP2A activation and ERK1/2 dephosphorylation: (A) EL4 cells were cultured for 30 min. in the presence of IM7. Cells were lysed and proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane and probed with anti-phosphotyrosine. (B and C) EL4 and EL4v6 cells were cultured for 15–60 min. in the presence of IM7. After lysis, SDS-PAGE and transfer, membranes were incubated with (B) anti-Akt and anti-pAkt and (C) anti-ERK1/2 and anti-pERK1/2. The mean values ± S.D. of the ratio of pAkt: Akt and pERK1/2: ERK1/2 are shown. Significant differences by IM7 are indicated by an asterisk. Tyrosine and Akt phosphorylation remained unaltered. ERK1/2 phosphorylation of EL4 and EL4v6 cells cultured in the presence of IM7 becomes strikingly reduced. (D) EL4 cells were cultured in the presence of rIgG or IM7 and increasing amounts of OA. Cells were lysed, lysates were separated by SDS-PAGE, transferred and blotted with anti-ERK1/2 and anti-pERK1/2. The mean values ± S.D. of the ratio of pERK1/2: ERK1/2 are shown. Significant differences by OA are indicated by an asterisk. In the presence of the phosphatase inhibitor, the IM7-induced reduction of ERK1/2 phosphorylation was prevented. (E) EL4 cells were cultured in the presence of rIgG or IM7. Cells were lysed, lysates were separated by SDS-PAGE, transferred and blotted with anti-pPP1, anti-ERK1/2 and anti-pERK1/2. The mean values ± S.D. of the ratio of pPP1: ERK1/2 and for comparison of pERK1/2: ERK1/2 are shown. Significant differences by IM7 are indicated by an asterisk. PP1 did not become phosphorylated concomitantly with ERK1/2 dephosphorylation. (F) EL4 cells were cultured in the presence of IM7. Cells were lysed and immunoprecipitated with IM7 or anti-PP2A. Precipitates were separated by SDS-PAGE, transferred and blotted with IM7, anti-PP2A and anti-PP1. The mean values ± S.D. of the ratio of PP2A: CD44, PP1: CD44, respectively, of CD44: PP2A are shown. Significant differences by IM7 are indicated by an asterisk. PP2A, but not PP1 co-immunoprecipitated with CD44 and vice versa. (G) EL4 cells were cultured in the presence of rIgG or IM7. Cells were lysed and lysates were immunoprecipitated with IM7. Lysates were separated by SDS-PAGE, transferred and blotted with anti-PP2A and anti-pThr. The mean values ± S.D. of the ratio of pThr: PP2A are shown. Significant differences by IM7 are indicated by an asterisk. Threonine phosphorylation of PP2A became strengthened in the presence of IM7. (H) Phosphatase activity of the anti-CD44 immunoprecipitate was tested by ELISA. Phosphatase activity was strongly increased after culture of EL4 and EL4v6 cells in the presence of IM7.
These results suggesting that CD44 engagement leads to ERK inhibition through the activation of PP1 or PP2A, we controlled for the potential contribution of PP1 or PP2A. We did not observe PP1 phosphorylation concomitantly with ERK1/2 dephosphorylation (Fig. 5E). In fact, PP2A, but not PP1 associates with CD44 upon CD44 binding. EL4 cells, cultured in the presence of IM7 for different time-points, were lysed and immunoprecipitated with anti-CD44 and the immunoprecipitate was blotted with anti-PP1 and -PP2A. PP1 was not detected in the immunoprecipitates. PP2A was absent from the IM7 precipitates at time 0, but was detected after 15 min. of activation. Immunoprecipitating PP2A and blotting with anti-CD44 confirmed the association between CD44 and PP2A upon CD44 ligation, the association remaining stable for at least 1 hr (Fig. 5F). The association of PP2A is accompanied by PP2A activation as revealed by blotting the PP2A immunoprecipitates with anti-pThr (Fig. 5G) and evaluating phosphatase activity of PP2A immunoprecipitates after CD44 ligation (Fig. 5H).
Having demonstrated PP2A activation upon association with CD44, we wanted to identify the mechanism responsible for PP2A activation, expecting due to CD44 association-dependent activation of PP2A that the upstream activator possibly may also be associated with CD44. MALDI-TOF analysis of proteins co-immunoprecipitating with CD44 revealed the presence of CK2, a constitutively active ser/thr kinase, which has a wide range of substrates including PP2A, which becomes threonine phosphorylated [44]. We confirmed a constitutive, though weak association between CK2 and CD44 by WB, which was increased after CD44 engagement (Fig. 6A). To strengthen the assumption that CK2 and PP2A concomitantly associate with CD44, EL4 cells, cultured in the presence of IM7, were lysed and light and dense membrane fractions were separated by sucrose gradient centrifugation. Light, medium dense and heavy fractions were pooled and immunoprecipitated with IM7. After SDS-PAGE and transfer, membranes were blotted with IM7, anti-PP2A and anti-CK2. CD44 was recovered in all three fractions. Instead, both PP2A and CK2 co-immunoprecipitated with CD44 exclusively in the medium dense fractions of 1.15–1.21 (Fig. 6B). To see, whether CK2 accounts for PP2A activation, EL4 cells, cultured in the presence of anti-CD44, were pre-treated with a CK2 inhibitor to block its catalytic activity. This treatment resulted in a mild reduction in PP2A phosphorylation (Fig. 6C) and in a reduction of the basal level of pERK1/2 (Fig. 6D). Instead, the CK2 inhibitor strongly interfered with spontaneous and IM7-promoted apoptosis (Fig. 6E). These findings suggest that CK2 does not exclusively account for PP2A activation, despite that both molecules can become associated with CD44.
Fig 6.
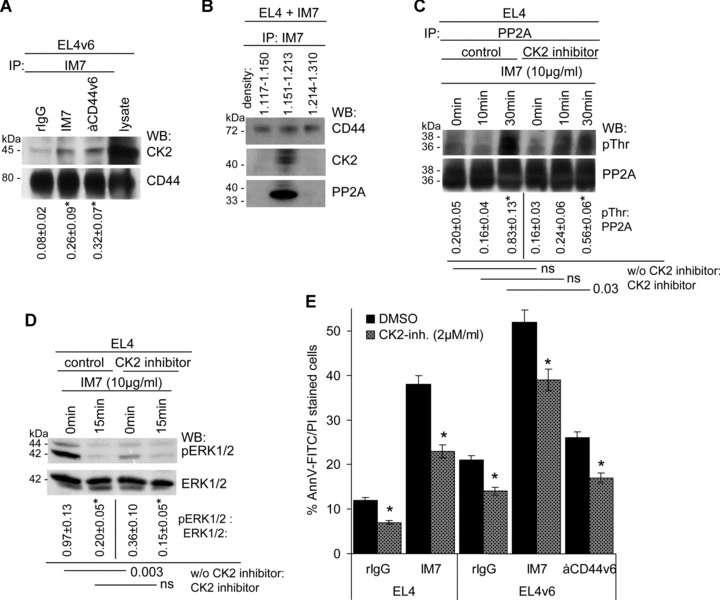
CK2 is associated with CD44 and promotes partial PP2A activation: (A) EL4 cells were cultured in the presence of rIgG or IM7. Cells were lysed and lysates were immunoprecipitated with IM7. After SDS-PAGE of the precipitate, proteins were transferred and blotted with anti-CD44 and anti-CK2. The mean values ± S.D. of the ratio of CK2: CD44 are shown. Significant differences by IM7 and anti-CD44v6 are indicated by an asterisk. The CD44 precipitate contained low amounts of CK2. (B) EL4 cells, cultured in the presence of IM7, were lysed and light and dense membrane fractions were separated by sucrose gradient centrifugation. Light, medium dense and heavy fractions were pooled and immunoprecipitated with IM7. After SDS-PAGE and transfer, membranes were blotted with IM7, anti-PP2A and anti-CK2. PP2A and CK2 co-immunoprecipitated with CD44 in the pooled fractions of density 1.15–1.21. (C) EL4 cells were cultured in the presence of IM7 and, where indicated a CK2 inhibitor (2 μM/ml). Cells were lysed and the lysates were precipitated with anti-PP2A, separated by SDS-PAGE, transferred and blotted with anti-PP2A and anti-pThr. The mean values ± S.D. of the ratio of pThr: PP2A are shown. Significant differences by IM7 and the CK2 inhibitor are indicated. The CK2 inhibitor partly inhibited PP2A phosphorylation. (D) EL4 cells were cultured in the presence of IM7 and, where indicated the CK2 inhibitor, lysates were precipitated by SDS-PAGE, transferred and blotted with anti-ERK1/2 and anti-pERK1/2. The mean values ± S.D. of the ratio of pERK1/2: ERK1/2 are shown. Significant differences by IM7 and the CK2 inhibitor are indicated. ERK1/2 phosphorylation was reduced in the presence of the CK2 inhibitor. (E) EL4 and EL4v6 cells were cultured in the presence of rIgG, IM7 or anti-CD44v6. Where indicated the cultures contained a CK2 inhibitor. Apoptosis was evaluated after 24 hrs by annexinV-FITC/PI staining. The CK2 inhibitor interfered with apoptosis induction independent of the presence of IM7 or anti-CD44v6.
Taken together, CD44 ligation is accompanied by apoptosis induction which becomes initiated by its association with PP2A. PP2A activation proceeds towards ERK1/2 dephosphorylation. CK2, also associated with CD44, promotes PP2A activation. However, in view of the low impact of a CK2 inhibitor on PP2A activation, an involvement of additional molecules in PP2A activation becomes likely.
ERK1/2 inhibition promotes apoptosis and caspase-9 cleavage
Our data so far suggest that PP2A activation-induced apoptosis proceeds through ERK1/2 inhibition. PP2A also is known to affect the PI3K/Akt pathway, that has already been excluded (Fig. 5B), to inhibit JNK or to regulate NFκB via IKKB/IKB [45]. However, we did not obtain evidence for impaired c-Jun phosphorylation in EL4 cells cultured in the presence of IM7 (data not shown). NFκB did not become regulated by CD44 occupancy and the low level of nuclear NFκB in EL4 cells remained unaltered. In line with this finding IKKB (data not shown) and IKB phosphorylation was not affected (Fig. 7A).
Fig 7.
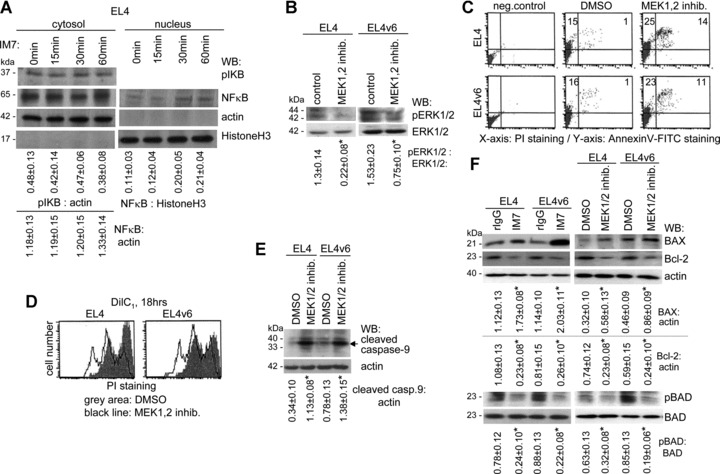
ERK1/2 dephosphorylation promotes mitochondrial membrane disintegration and caspase-9 cleavage: (A) EL4 cells were cultured for 15–60 min. in the presence of IM7. After separation of the cytosolic from the nuclear fraction, lysates were separated by SDS-PAGE, transferred and blotted with anti-pIKB and anti-NFκB. Actin and HistoneH3 served as controls. The mean values ± S.D. of the ratio of pIKB and NFκB: actin (cytosol) and of NF-kB: HistoneH3 (nucleus) are shown. Significant differences by IM7 are indicated by an asterisk. IM7 treatment did not influence IKB phosphorylation and did not significantly alter NFκB liberation. (B–F) EL4 and EL4v6 cells were cultured o/n in the presence of a MEK1,2 inhibitor. (B) The mean values ± S.D. of the ratio of pERK1/2: ERK1/2 are shown. Significant differences by the MEK1,2 inhibitor are indicated by an asterisk. ERK1/2 phosphorylation in EL4 and EL4v6 cells was inhibited in the presence of MEK1,2 inhibitor. (C) Apoptosis (annexinV-FITC/PI staining) was significantly increased in the presence of the MEK1,2 inhibitor. (D) Cells were stained with DilC1 and mitochondrial membrane integrity was determined after 18 hrs incubation by flow cytometry. In the presence of the MEK1,2 inhibitor, mitochondrial membrane integrity was decreased. (E) Cells were lysed, proteins were separated by SDS-PAGE, transferred and blotted with anti-caspase-9. The mean values ± S.D. of the ratio of cleaved caspase-9: actin are shown. Significant differences by IM7 and the MEK1,2 inhibitor are indicated by an asterisk. Caspase-9 cleavage was pronounced in the presence of the MEK1,2 inhibitor. (F) Cells were cultured o/n in the presence of the MEK1,2 inhibitor or in the presence of IM7. Cells were lysed and proteins were separated by SDS-PAGE, transferred and blotted with anti-BAX, anti-Bcl-2, anti-BAD and anti-pBAD. The mean values ± S.D. of the ratio of BAX and Bcl-2: actin and of pBAD: BAD are shown. Significant differences by IM7 and the MEK1,2 inhibitor are indicated by an asterisk. The MEK1,2 inhibitor and IM7 promoted BAX activation and prohibited Bcl-2 expression and BAD phosphorylation.
IM7-mediated PP2A activation, at least predominantly, inducing apoptosis through ERK1/2 inhibition, we evaluated whether an ERK1/2 inhibitor would induce apoptosis and whether apoptosis induction would proceed via caspase-9. EL4/EL4v6 cells show a high basic level of ERK activation, which was significantly inhibited in the presence of a MEK1,2 inhibitor (Fig. 7B). In fact, when EL4 and EL4v6 cells were cultured in the presence of a MEK1,2 inhibitor, apoptosis became increased (Fig. 7C), mitochondrial membrane depolarization was strengthened, as shown by the higher percentage of cells displaying reduced DilC1 staining (Fig. 7D), and caspase-9 cleavage was pronounced (Fig. 7E). Concomitantly, Bcl-2 expression and BAD phosphorylated was reduced and BAX expression was up-regulated. The same findings accounted for IM7-treated EL4 and EL4v6 cells (Fig. 7F).
Taken together, occupancy of CD44 drives leukemic T cells into apoptosis. This is accompanied by PP2A associating with CD44 and PP2A activation, which strongly promotes ERK1/2 dephosphorylation, mitochondrial membrane depolarization and caspase-9 cleavage.
Discussion
CD44 plays an important role in HSC homing and embedding in the bone marrow niche, which is supposed to support HSC survival [5]. CD44 is also important for progenitor T-cell homing into the thymus as well as for thymocyte differentiation via a crosstalk with the thymic stroma [36–38, 46]. There is strong evidence that leukaemia initiating cells may also require CD44 for niche embedding and that an antibody blockade of CD44 can hamper leukaemia growth such that LSC survival becomes impaired and/or LSCs are driven into differentiation [16, 18, 19, 33–35]. Thus, anti-CD44 treatment could be considered as a therapeutic option in leukaemia. However, because CD44 is also required during haematopoiesis, it becomes important to elaborate possible differences in CD44 activities in haematopoiesis versus leukaemia growth.
T lymphoma cells are less CD44-dependent than haematopoietic progenitors
Anti-CD44 treatment retards T lymphoma growth, particularly at a subcutaneous site. This corresponds, although far less pronounced, to the effect of anti-CD44 treatment in myeloid leukaemia [18, 19]. However, IM7-treatment obviously affects homing and settlement in the bone marrow more efficiently than homing in the thymus and the liver, with the consequence that metastatic growth in the thymus and the liver is more frequently observed in anti-CD44- than control IgG-treated mice. The reasons for this phenomenon have not yet been explored. However, several reports [reviewed in 3] demonstrate convincingly that there is a balance between CD44 isoform expression, HA binding and settlement by lymphoma cells in lymphoid organs. Accordingly, anti-CD44 inhibits metastasis formation in selective organs, but may be ineffective in other organs. We would suggest, in addition, that preventing settlement of lymphoma cells in one organ may actively support lymphoma cell settlement in others, which stroma provides ligands, including additional adhesion molecules, that allow lymphoma cell adhesion.
An even more serious drawback of anti-CD44 treatment has been seen in irradiated and syngeneically reconstituted mice. In anti-CD44-treated syngeneically reconstituted mice the recovery of BMC, TC and SC was severely impaired and mice succumbed with the tumour before regaining immunocompetence (data not shown). Concomitantly, T lymphoma growth became accelerated.
Though differing from studies on the impact of anti-CD44 on myeloid leukaemia homing, the finding that a CD44 blockade can more efficiently interfere with progenitor than T lymphoma cell homing is of clinical relevance, taking into account that BMC/HSC reconstitution is frequently considered as a therapeutic option in leukaemia. In clinical settings also the possibility of anti-CD44-promoted metastasis formation should be taken into account. Nonetheless, both these serious drawbacks should not hamper considering anti-CD44 as a therapeutic option in leukaemia, as these side effects possibly can be circumvented by the use of either CD44 variant isoform (CD44v) specific or bispecific antibodies [3]. As far as the leukaemia strongly expresses a CD44v, the therapeutic application of CD44v-specific antibodies may not attack HSC. A bispecific antibody that recognizes CD44 and an additional leukaemia marker, will also spare HSC and progenitor cells [47]. We plan to control this hypothesis in the murine leukaemia model.
A CD44 antibody blockade promotes death receptor-independent leukemic T cell apoptosis
Independently of these side effects, in the non-reconstituted mouse anti-CD44 treatment retarded lymphoma growth. As a first step towards a therapeutic exploitation, we aimed to clarify the underlying mechanism. In line with several reports [16, 33–35], our in vivo findings provided evidence that a blockade of CD44 can drive leukemic T cells into apoptosis.
Though CD44 occupancy has repeatedly been described to drive activated T cells into apoptosis [29–31, 48], possible pathways have not been elucidated for T-cell leukaemia. This knowledge will be important when considering a blockade in signal transduction as a therapeutic option.
In the presence of anti-panCD44, anti-CD44v6 or HA the rate of apoptotic EL4 or EL4v6 cells increased 2–3 fold within 48 hrs of culture. We interpret the observation that a CD44-specific antibody, that does not recognize the HA binding site as well as an antibody specific for a variant isoform and HA supported apoptosis induction as an indication that apoptotic signalling likely proceeds directly via CD44 and not via associating transmembrane molecules or a feedback via ligand binding. The finding does not exclude apoptosis by neglect due to the absence of survival supporting signals. Nonetheless, co-operative activity of CD44 with receptor-mediated apoptosis was excluded by unaltered apoptosis induction upon death receptor cross-linking, the failure to observe cooperative activity in apoptosis induction by anti-CD44 plus death receptor cross-linking and the unaltered death receptor expression in EL4 cells cultured in the presence of anti-CD44.
Anti-CD44-promoted apoptosis proceeds via CD44 redistribution and PP2A association
PP2A, that mostly resides in the cytosol, but can attach to the plasma membrane depending on the activation state [49], activates pro-apoptotic and inhibits anti-apoptotic proteins of the Bcl-2 family [50–52]. PP2A associated with CD44 only in cultures containing anti-CD44, and became activated upon association. PP2A regulation is a complex mechanism and the impact of PP2A phosphorylation on its activation is debated [53–55]. However, several publicatiobs showed that PP2A is a substrate for CK2 and can become activated through CK2 [44, 53, 54]. Because we observed that CD44 antibody occupancy was accompanied by both PP2A and CK2 associating with CD44, we suggest that by the induced proximity between CK2 and PP2A, CK2 may account for PP2A activation. In fact, PP2A phosphorylation was slightly reduced in the presence of a CK2 inhibitor. On the other hand, spontaneous and CD44-induced apoptosis was strongly inhibited in the presence of a CK2 inhibitor. Therefore we suggest that PP2A activation does not exclusively rely on CK2. In line with this hypothesis, the CK2α association with PP2A can be disrupted by activated raf [55], which could provide an explanation for the low impact of CK2 on PP2A activation. However, we did not yet succeed in defining an additional upstream activator of PP2A.
CD44-associated PP2A promotes mitochondrial membrane destabilization via ERK1/2 dephosphorylation
EL4 cells show a high level of activated ERK1/2 in the absence of an external stimulus. Upon IM7 treatment, ERK1/2 phosphorylation becomes strikingly diminished without evidence for ERK1/2 degradation. ERK activation mainly proceeding via Ras activation, we first evaluated whether anti-CD44 treatment may alter Ras activation. This has not been the case. Ras is highly activated in EL4 cells and a pull down of activated Ras by a raf-GST fusion protein provided no evidence for altered Ras activity in IM7-treated EL4 cells (data not shown). Alternatively, PP2A may account for ERK1/2 dephosphorylation [56, 57]. Indeed, ERK1/2 remained phosphorylated in the presence of OA. Furthermore, albeit less pronounced, apoptosis could be induced by culturing EL4 cells in the presence of MEK1/2 inhibitor. Both the antibody blockade of CD44 and the blockade of ERK1/2 phosphorylation was accompanied by low level of BAD phosphorylation and up-regulation of pro-apoptotic BAX, of mitochondria depolarization and caspase-9 cleavage. Both inhibition of ERK1/2 phosphorylation and PP2A activation promoting mitochondrial depolarization, caspase activation and apoptosis induction, it becomes most likely that in EL4 cells pERK1/2 is the preferential target of PP2A. Apoptosis induction by ERK1/2 dephosphorylation could proceed via preventing dissociation of BAD from Bcl-2 [58]. Also, pERK1/2 is directly involved in Bcl-2 activation [59]. The missing support for Bcl-2 activation from pERK1/2 may become aggravated by a direct interaction between PP2A and Bcl-2 [51, 60]. Thus, IL-2 deprivation-induced apoptosis operates by BAD dephosphorylation and proceeds directly via caspase-9 dephosphorylation/activation by PP1 [61]. Whether this pathway accounts for anti-CD44 initiated activation of caspase-9 via PP2A activation and ERK1/2 dephosphorylation remains to be answered. PP2A can also interfere with activation of Akt, JNK and NFκB inhibitors [45]. However, anti-CD44-initiated PP2A activation did not display significant effects. Thus, in leukemic T cells PPA2-mediated ERK1/2 dephosphorylation appears to be the dominating theme in anti-CD44-induced apoptosis.
Taken together, the finding that anti-CD44 can interfere more efficiently with HSC than leukemic T-cell homing into the bone marrow is clinically important for patients with a T-cell leukaemia that receive a HSC transplant. In addition, anti-CD44 may more effectively block leukemic cell settlement in the bone marrow than in the liver and the thymus, which could promote metastatic growth. As both these drawbacks likely can be circumvented by the use of selected CD44 antibodies, it became important to evaluate the consequences of a CD44 occupancy on leukemic T cells.
Anti-CD44 actively induces apoptosis in leukemic T cells. By PP2A relocation in the proximity of CD44 and PP2A activation, the basic level of ERK1/2 phosphorylation cannot be maintained, which drives leukemic T cells into apoptosis by up-regulation of pro- and down-regulation of anti-apoptotic proteins, mitochondrial membrane destabilization and caspase activation. Unravelling this new pathway of anti-CD44-initiated apoptosis in leukemic T cells strengthens the clinical relevance of anti-CD44 in T leukaemia and should allow for well-targeted therapeutic interference.
Acknowledgments
This investigation was supported by the Tumorzentrum HD/MA (M.Z.).
Conflict of interests
None of the authors has any conflict of interest.
References
- 1.Screaton GR, Bell MV, Jackson DG, et al. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA. 1992;89:12160–4. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch KW. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev Immunol. 2004;4:931–40. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- 3.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–7. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Orian-Rousseau V, Ponta H. Adhesion proteins meet receptors: a common theme. Adv Cancer Res. 2008;101:63–92. doi: 10.1016/S0065-230X(08)00404-1. [DOI] [PubMed] [Google Scholar]
- 5.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 6.Marhaba R, Klingbeil P, Nübel T, et al. CD44 and EpCAM: cancer-initiating cell markers. Curr Mol Med. 2008;8:784–804. doi: 10.2174/156652408786733667. [DOI] [PubMed] [Google Scholar]
- 7.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 8.Zou GM. Cancer stem cells in leukemia, recent advances. J Cell Physiol. 2007;213:440–4. doi: 10.1002/jcp.21140. [DOI] [PubMed] [Google Scholar]
- 9.Misaghian N, Ligresti G, Steelman LS, et al. Targeting the leukemic stem cell: the holy grail of leukemia therapy. Leukemia. 2009;23:25–42. doi: 10.1038/leu.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu LC, Foltz G, Lin E, et al. Targeting stem cells-clinical implications for cancer therapy. Curr Stem Cell Res Ther. 2009;4:147–53. doi: 10.2174/157488809788167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake K, Medina KL, Hayashi S, et al. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990;171:477–88. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khaldoyanidi S, Denzel A, Zöller M. Requirement for CD44 in proliferation and homing of hematopoietic precursor cells. J Leukoc Biol. 1996;60:579–92. doi: 10.1002/jlb.60.5.579. [DOI] [PubMed] [Google Scholar]
- 13.Khaldoyanidi S, Karakhanova S, Sleeman J, et al. CD44 variant-specific antibodies trigger hemopoiesis by selective release of cytokines from bone marrow macrophages. Blood. 2002;99:3955–61. doi: 10.1182/blood.v99.11.3955. [DOI] [PubMed] [Google Scholar]
- 14.Ratajczak MZ, Reca R, Wysoczynski M, et al. Heterogeneous populations of bone marrow stem cells – are we spotting on the same cells from the different angles? Folia Histochem Cytobiol. 2004;42:139–46. [PubMed] [Google Scholar]
- 15.Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–9. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Jiang G. CD44 and hematologic malignancies. Cell Mol Immunol. 2006;3:359–65. [PubMed] [Google Scholar]
- 17.Hidalgo A, Robledo MM, Teixidó J. CD44-mediated hematopoietic progenitor cell adhesion and its complex role in myelopoiesis. J Hematother Stem Cell Res. 2002;11:539–47. doi: 10.1089/15258160260091004. [DOI] [PubMed] [Google Scholar]
- 18.Jin L, Hope KJ, Zhai Q, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–74. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 19.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–80. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 20.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 21.Marhaba R, Zöller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–31. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 22.Hegde VL, Singh NP, Nagarkatti PS, et al. CD44 mobilization in allogeneic dendritic cell-T cell immunological synapse plays a key role in T cell activation. J Leukoc Biol. 2008;84:134–42. doi: 10.1189/jlb.1107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu B, Symonds AL, Martin JE, et al. Early growth response gene 2 (Egr-2) controls the self-tolerance of T cells and prevents the development of lupuslike autoimmune disease. J Exp Med. 2008;205:2295–307. doi: 10.1084/jem.20080187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 25.Föger N, Marhaba R, Zöller M. CD44 supports T cell proliferation and apoptosis by apposition of protein kinases. Eur J Immunol. 2000;30:2888–99. doi: 10.1002/1521-4141(200010)30:10<2888::AID-IMMU2888>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Marhaba R, Freyschmidt-Paul P, Zöller M. In vivo CD44-CD49d complex formation in autoimmune disease has consequences on T cell activation and apoptosis resistance. Eur J Immunol. 2006;36:3017–32. doi: 10.1002/eji.200636158. [DOI] [PubMed] [Google Scholar]
- 27.Gadhoum Z, Delaunay J, Maquarre E, et al. The effect of anti-CD44 monoclonal antibodies on differentiation and proliferation of human acute myeloid leukemia cells. Leuk Lymphoma. 2004;45:1501–10. doi: 10.1080/1042819042000206687. [DOI] [PubMed] [Google Scholar]
- 28.Mielgo A, Brondani V, Landmann L, et al. The CD44 standard/ezrin complex regulates Fas-mediated apoptosis in Jurkat cells. Apoptosis. 2007;12:2051–61. doi: 10.1007/s10495-007-0115-3. [DOI] [PubMed] [Google Scholar]
- 29.Nakano K, Saito K, Mine S, et al. Engagement of CD44 up-regulates Fas ligand expression on T cells leading to activation-induced cell death. Apoptosis. 2007;12:45–54. doi: 10.1007/s10495-006-0488-8. [DOI] [PubMed] [Google Scholar]
- 30.Ruffell B, Johnson P. Hyaluronan induces cell death in activated T cells through CD44. J Immunol. 2008;181:7044–54. doi: 10.4049/jimmunol.181.10.7044. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann U, Heilmann K, Hayford C, et al. CD44v7 ligation downregulates the inflammatory immune response in Crohn’s disease patients by apoptosis induction in mononuclear cells from the lamina propria. Cell Death Differ. 2007;14:1542–51. doi: 10.1038/sj.cdd.4402153. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Reiman T, Li W, et al. Targeting aurora kinases as therapy in multiple myeloma. Blood. 2007;109:3915–21. doi: 10.1182/blood-2006-07-037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zada AA, Singh SM, Reddy VA, et al. Downregulation of c-Jun expression and cell cycle regulatory molecules in acute myeloid leukemia cells upon CD44 ligation. Oncogene. 2003;22:2296–308. doi: 10.1038/sj.onc.1206393. [DOI] [PubMed] [Google Scholar]
- 34.Artus C, Maquarre E, Moubarak RS, et al. CD44 ligation induces caspase-independent cell death via a novel calpain/AIF pathway in human erythroleukemia cells. Oncogene. 2006;25:5741–51. doi: 10.1038/sj.onc.1209581. [DOI] [PubMed] [Google Scholar]
- 35.Song G, Liao X, Zhou L, et al. HI44a, an anti-CD44 monoclonal antibody, induces differentiation and apoptosis of human acute myeloid leukemia cells. Leuk Res. 2004;28:1089–96. doi: 10.1016/j.leukres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Wu L, Kincade PW, Shortman K. The CD44 expressed on the earliest intrathymic precursor population functions as a thymus homing molecule but does not bind to hyaluronate. Immunol Lett. 1993;38:69–75. doi: 10.1016/0165-2478(93)90121-h. [DOI] [PubMed] [Google Scholar]
- 37.Rajasagi M, Vitacolonna M, Benjak B, et al. CD44 promotes progenitor homing into the thymus and T cell maturation. J Leukoc Biol. 2009;85:251–61. doi: 10.1189/jlb.0608389. [DOI] [PubMed] [Google Scholar]
- 38.Scimone ML, Aifantis I, Apostolou I, et al. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci USA. 2006;103:7006–11. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch N, Hämmerling GJ, Tada N, et al. Cross-blocking studies with monoclonal antibodies against I-A molecules of haplotypes b, d and k. Eur J Immunol. 1982;12:909–14. doi: 10.1002/eji.1830121103. [DOI] [PubMed] [Google Scholar]
- 40.Hession C, Moy P, Tizard R, et al. Cloning of murine and rat vascular cell adhesion molecule-1. Biochem Biophys Res Commun. 1992;183:163–9. doi: 10.1016/0006-291x(92)91623-x. [DOI] [PubMed] [Google Scholar]
- 41.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–9. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 42.Taylor SJ, Resnick RJ, Shalloway D. Nonradioactive determination of Ras-GTP levels using activated ras interaction assay. Methods Enzymol. 2001;333:333–42. doi: 10.1016/s0076-6879(01)33067-7. [DOI] [PubMed] [Google Scholar]
- 43.Cossarizza A, Salviolit S. Analysis of mitochondria during cell death. Methods Cell Biol. 2001;63:467–86. doi: 10.1016/s0091-679x(01)63025-5. [DOI] [PubMed] [Google Scholar]
- 44.Hériché JK, Lebrin F, Rabilloud T, et al. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2 alpha. Science. 1997;276:952–5. doi: 10.1126/science.276.5314.952. [DOI] [PubMed] [Google Scholar]
- 45.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Schwärzler C, Oliferenko S, Günthert U. Variant isoforms of CD44 are required in early thymocyte development. Eur J Immunol. 2001;31:2997–3005. doi: 10.1002/1521-4141(2001010)31:10<2997::aid-immu2997>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 47.Avin E, Haimovich J, Hollander N. Anti-idiotype x anti-CD44 bispecific antibodies inhibit invasion of lymphoid organs by B cell lymphoma. J Immunol. 2004;173:4736–43. doi: 10.4049/jimmunol.173.7.4736. [DOI] [PubMed] [Google Scholar]
- 48.Alpdogan SO, Lu SX, Patel N, et al. Rapidly proliferating CD44hi peripheral T cells undergo apoptosis and delay posttransplantation T-cell reconstitution after allogeneic bone marrow transplantation. Blood. 2008;112:4755–64. doi: 10.1182/blood-2008-02-142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ludowyke RI, Holst J, Mudge LM, et al. Transient translocation and activation of protein phosphatase 2A during mast cell secretion. J Biol Chem. 2000;275:6144–52. doi: 10.1074/jbc.275.9.6144. [DOI] [PubMed] [Google Scholar]
- 50.Van Hoof C, Goris J. Phosphatases in apoptosis: to be or not to be, PP2A is in the heart of the question. Biochim Biophys Acta. 2003;1640:97–104. doi: 10.1016/s0167-4889(03)00029-6. [DOI] [PubMed] [Google Scholar]
- 51.Ruvolo PP, Clark W, Mumby M, et al. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem. 2002;277:22847–52. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- 52.Chiang CW, Kanies C, Kim KW, et al. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol. 2003;23:6350–62. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol. 2000;60:1225–35. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]
- 54.Pérez M, Avila J. The expression of casein kinase 2alpha’ and phosphatase 2A activity. Biochim Biophys Acta. 1999;1449:150–6. doi: 10.1016/s0167-4889(99)00008-7. [DOI] [PubMed] [Google Scholar]
- 55.Lebrin F, Bianchini L, Rabilloud T, et al. CK2alpha-protein phosphatase 2A molecular complex: possible interaction with the MAP kinase pathway. Mol Cell Biochem. 1999;191:207–12. [PubMed] [Google Scholar]
- 56.Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–19. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Letourneux C, Rocher G, Porteu F. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J. 2006;25:727–38. doi: 10.1038/sj.emboj.7600980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ewings KE, Wiggins CM, Cook SJ. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle. 2007;6:2236–40. doi: 10.4161/cc.6.18.4728. [DOI] [PubMed] [Google Scholar]
- 59.Deng X, Ruvolo P, Carr B, et al. Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci USA. 2000;97:1578–83. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvarado-Kristensson M, Andersson T. Protein phosphatase 2A regulates apoptosis in neutrophils by dephosphorylating both p38 MAPK and its substrate caspase 3. J Biol Chem. 2005;280:6238–44. doi: 10.1074/jbc.M409718200. [DOI] [PubMed] [Google Scholar]
- 61.Dessauge F, Cayla X, Albar JP, et al. Identification of PP1alpha as a caspase-9 regulator in IL-2 deprivation-induced apoptosis. J Immunol. 2006;177:2441–51. doi: 10.4049/jimmunol.177.4.2441. [DOI] [PubMed] [Google Scholar]


