Abstract
Cholinergic neurotransmission is essential for many important functions in the brain, including cognitive mechanisms. Here we demonstrate that human embryonic stem (hES) cells differentiate into a population of neuronal cells that express the cholinergic enzyme choline acetyltransferase and homeobox proteins specifying neuronal progenitors of ventral telencephalic lineage. These differentiated cells express transcripts for cholinergic α3, α4 and α7 nicotinic acetylcholine (ACh) receptor subunits and for M1, M2 and M3 muscarinic acetylcholine receptor (mAChR) subtypes. Stimulation with brain-derived neurotrophic factor, neurotrophin-3, ciliary neurotrophic factor and nerve growth factor increases the proportion of cholinergic neurons. These cholinergic receptors also mediate ACh-evoked increase in cytosolic calcium levels, and this response was unaffected by extracellular calcium removal and was abolished by the mAChR antagonist scopolamine. Our findings demonstrate expression of functional cholinergic receptors on hES cell-derived neurons, which may provide a source of expandable cells to facilitate screening of novel cholinergic drugs and useful for evaluating cell transplantation in animal models of cholinergic dysfunction.
Keywords: acetylcholine receptor, cholinergic, human embryonic stem cells, neuronal differentiation, neurotrophic factors
Introduction
Recent studies have demonstrated that multiple cell types can be derived in vitro from human embryonic stem (hES) cells. Transplanted neurons developed from hES cells have also been reported to integrate successfully in vivo[1]. Together, these important findings indicate the great advantages and opportunities that these cells may offer as a source of specialized human cells for biotechnological and future clinical therapeutic applications. Crucial to the success of generating specialized cell populations is an understanding of the mechanisms influencing the control of cell growth and differentiation by extrinsic and intrinsic factors.
During embryonic development, morphogens such as bone morphogenic protein, sonic hedgehog, fibroblast growth factors (FGFs) and retinoic acid, act as inductive signals for neuronal specification by interacting in unique temporal orders [2–4]. The neurotrophins are a family of growth factors that include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and NT-4, which have central roles in the development of the nervous system and act by stimulating receptor tyrosine kinases (Trk) and the p75 neurotrophin receptor (p75NTR). These factors mediate proliferation and differentiation of neuronal precursor cells and can also regulate neurotransmitter release, long-term potentiation, axonal and dendritic growth and guidance, and synaptic plasticity [5]. Earlier studies on hES cells have shown that neurotrophic factors can mediate hES cell survival and also modulate their fate [6, 7].
Both neuronal nicotinic (nAChRs) and muscarinic AChRs (mAChRs) have important roles in cognitive functions [8, 9]. The majority of high affinity nAChRs in the brain are of the α4β2 nAChR subtype, whereas α7 nAChRs are expressed early in development and have been shown to be important in the modulation of synaptic plasticity and the transduction of neuroprotective effects against β-amyloid induced neurotoxicity [9–11]. Cognitive impairment in patients with Alzheimer’s disease (AD) has been correlated with losses of nAChRs detected by measurements carried out by positron emission tomography (PET) [12]. We have previously observed an increase in the number of 11C-nicotine binding sites by PET in AD patients following ventricular administration of NGF [13]. Another recent clinical study revealed a reduced rate of cognitive decline in AD patients who received implants of genetically modified fibroblasts expressing NGF [14]. However, up to now, there have been no reported studies exploring the possibility of using hES cell-derived neurons for enhancing cholinergic functions in AD.
Here we provide evidence that hES cell-derived neuronal cells express both nAChR and mAChR subtypes. The functionality of cholinergic receptors was demonstrated by measuring acetylcholine (ACh)-evoked calcium responses. Furthermore, exposure of hES cells to neurotrophic factors during differentiation differentially modulated the expression of various subunits of nAChRs and mAChRs, and the ACh-synthesizing enzyme choline acetyltransferase (ChAT).
Materials and methods
Cell culture and proliferation assays
Human ES cells, derived at Karolinska Institutet, Karolinska University Hospital Huddinge (lines HS293 and HS346) were grown on human foreskin fibroblasts as earlier described [15]. Colonies of cells were then removed from the feeder layer and the culture was expanded in DMEM:F12 medium supplemented with B27, heparin and epidermal growth factor (EGF) + bFGF (20 ng/ml) as free-floating aggregates (neurospheres) and routinely passaged mechanically every 2–3 weeks. Neurospheres were passaged two to five times prior to analysis.
For proliferative assays, neurospheres derived from hES cells were dissociated with 0.1 mg/ml dispase for 10 min. at 37°C. Cells were adjusted to 50,000 cells/ml and plated on culture dishes 24 hrs prior to the administration of BDNF, CNTF, NT-4 or NGF (all at a concentration of 50 ng/ml; Invitrogen, Bleiswijk, Holland). Measurement of cell proliferation using a BrdU incorporation assay was carried out according to the manufacturer’s instructions (Roche, Mannheim, Germany).
Reverse transcription-PCR
Extraction of RNA was carried out with Trizol reagent (Invitrogen). RT-PCR analyses were performed as previously described [16]. Amplification of cyclophilin or omission of reverse transcriptase served as positive and negative controls, respectively. The cDNA was amplified by the following primers; Cyclophilin (sense) 5′-TTGTTTTAGAATTGTTTGCAGATATT-3′, (anti-sense) 5′- TTTTTAATAGCCATCTCCCAGTTCT-3′; TrkB (sense) 5′-CCCACTCACATGAACAATGG-3′, (anti-sense) 5′-TCAGTGACGTCTGTGGAAGG-3′; TrkC (sense) 5′-AAGCGAGAACTGGGTGAGG-3′, (anti-sense) 5′-ATGTGGAGCATTTGGGAGAG-3′; p75NTR (sense) 5′-CCTACGGCTACTACCAGGATG-3′, (anti-sense) 5′-TGGCCTCGTCGGAATACG-3; Dlx1 (sense) 5′-CCG AGT TGA CGT AGG GGT AGC-3′, (anti-sense) 5′- GAT GAC CAT GAC CAC CAT GCC-3′; Dlx2 (sense) 5′- CTCTGCCTGCCTCATAAGG-3′, (anti-sense) 5′-ATCGTAAGAACAGCGCAACC-3′; Gbx2 (sense) 5′-GACTTTTCGCCTCTCGCTGGCCTCTA-3′, (anti-sense) 5′- GTTGCTTCAAACACAGTGGAGTCCAC-3′; Mash1 (sense) 5′-CTCGTCTTCGCCCGAACTGATG-3′, (anti-sense) 5′-CGACAGGACGCCTGCCTGGAAG-3′; MAP2 (sense) 5′-AATAGACCTAAGCCATGTGACATCC-3′, (anti-sense) 5′-AGAACCAACTTTAGCTTGGGCC -3′; Gsh2 (sense) 5′-GGCGGACCCGCGGAGATTCC-3′, (anti-sense) 5′-CGCGTAGTGCACCTGGCTCCC-3′; Lhx6 (sense) 5′-TCAACAACCTCATCTGGCAC-3′, (anti-sense) 5′-CATGGTGTCGTAGTGGATGC-3′; Lhx8 (sense) 5′-TGCTCTCGATGTGGGAGACACAT-3′, (anti-sense) 5′-CAGAGGACTTTCTCTTCCACCAA-3′; α3 nAChR (sense) 5′-TGAAGCAAATCTGGAATGACTACAA-3′, (anti-sense) 5′-AGTCAAACGGGAAGTAGGTCACAT-3′; α4 nAChR (sense) 5′-TGG GTA CGC AGG GTC TTC C-3′, (anti-sense) 5′-GCT CAG CCG GCA CAT CCA-3′; α7 nAChR (sense) 5′-CGCCACATTCCACACTAAC-3′, (anti-sense) 5′-ACCTTTCACTCCTCTTGCC-3′; ChAT (sense) 5′-TGCTGCAATCAGTTCTTTGT-3′, (anti-sense) 5′-AGGCAGATGCAGCGCTCAATCATGTC-3′; M1 (sense) 5′-TGGAAGGAAGAAGAGGAAGA-3′, (anti-sense) 5′-AGGAGAGGGGACTATCAGCATT-3′; M2 (sense) 5′-GGGTCCTCTCTTTCATCCTCT-3′, (anti-sense) 5′-TCCTGGGTTATTTCATCATCT-3′; M3 (sense) 5′-GTCTGGCTTGGGTCATCTCCT-3′, (anti-sense) 5′-ACTTGCTGCTGTGGTCTTGGTC-3′; NMDAR1 (sense) 5′- GGAAGGCGCCCCCAGAAG-3′, (anti-sense) 5′- AGCCCGAGCGGAAAAACAGC-3′.
Analysis of mitogen-activated protein kinase
Cells cultured as neurospheres were stimulated for 15 min. using 50 ng/ml of each factor and then lysed in tris-buffered saline containing 1% triton x-100 and phosphatase inhibitors. For inhibition of Trk signalling, cells were exposed to the K252a inhibitor (200 nM; Biosource, Invitrogen, Carlsbad, CA, USA) 4 hrs prior to growth factor stimulation. To remove cell debris, lysates were centrifuged at 13,000 ×g for 5 min. and equal amount of proteins were separated by 10% SDS-PAGE followed by western blotting. The membranes were probed with phospho-extracellular signal-regulated kinase (ERK)42/44 (1:1000, Cell Signaling Technology, Beverly, MA, USA) and the phosphorylated bands were visualized with anti-rabbit IgG conjugated to horseradish peroxidase.
Immunocytochemistry
Cells cultured on poly-D-lysine and laminin-coated culture dishes were fixed in methanol for 15 min. at −20°C. Immunocytochemistry was carried out using standard protocols. Primary antibodies used were: Bf-1 polyclonal (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), brain lipid-binding protein (BLBP) polyclonal (1:200; Chemicon, Temecula, CA, USA), β-tubulin type III monoclonal (1:500; Sigma, St. Louis, MO, USA), ChAT polyclonal (1:200; Chemicon), glial fibrillary acidic protein (GFAP) polyclonal (1:200; Dako, Glostrup, Denmark), Islet-1/2 polyclonal (1:100; Santa Cruz), Ki67 monoclonal (1:200; Chemicon), MAP2 monoclonal (1:200; Sigma), nestin polyclonal (1:200; Santa Cruz), Nkx2.1 polyclonal (1:100; Santa Cruz), Pax6 polyclonal (1:200; Chemicon), p75 polyclonal (1:100; Santa Cruz), Trk polyclonal (1:200; Santa Cruz). Cells were then exposed to appropriate secondary antibodies conjugated to either Texas Red or fluorescein isothiocyanate (FITC) for 1 hr at room temperature. ACh receptors were labelled by FITC-conjugated α-bungarotoxin (Molecular Probes, Eugene, OR, USA). Hoechst nuclear stain (5 μg/ml) was performed for 15 min. Images were observed on a Nikon (Japan) E800 microscope equipped with appropriate filters.
Calcium measurements
Cultures plated on poly-D-lysine-coated cover slips were imaged using an inverted Meta-Zeiss (Carl Zeiss AG, Göttingen, Germany) 510 LSM confocal microscope with a ×40 (numerical aperture [NA], 1.3) objective. The pinhole was set to produce optical sections thinner than 4 μm. Neuronal cells derived from hES cells were maintained in culture medium with pH adjusted to 7.4. The cells were loaded with the calcium-sensitive dyes Fluo-3 AM and Fura-Red AM (5 μM; Molecular Probes) for 30 min. in a buffer containing pluronic acid (0.02%). When both probes were loaded together at a ratio of 3:1 (Fura-Red: Fluo-3) this allowed semi-quantitative tracking of intracellular calcium. Experiments were carried out at 25–27°C. The excitation wavelength was 488 nm. Fluo-3 was imaged at 505–550 nm emission and Fura-Red was imaged simultaneously at >615 nm emission. Calcium measurements were conducted in DMEM/F12 (1:1, pH 7.4) or in Krebs–Ringer–Hepes (KRH) buffer supplemented with glucose (20 mM) (KRH mM concentrations: NaCl 136, KCl 4.7, CaCl2 1.25, MgSO4 1.25, HEPES 20; pH 7.4). Calcium-free buffer was prepared without CaCl2, and EGTA (500 μM) was added. Ionomycin (2 μM; Sigma) was used to verify the responsiveness of the dyes to calcium at the end of each experiment.
Statistical analysis
Results are expressed as mean ± S.E.M. Experimental groups that were significantly different from control groups in ANOVA with Dunnett’s test (GraphPad Prism 3.0) are identified by asterisks.
Results
Radial glial differentiation from hES cells in free-floating cultures
We have previously reported that cells from six hES cell lines differentiate into neuroepithelial cells that subsequently gave rise to neurons in serum- and feeder-free culture conditions [17]. Here we used hES cells from two of these lines (HS293 and HS346), that were cultured as free-floating neurospheres (Fig. 1A) in serum- and feeder-free medium and routinely passaged every 2–3 weeks. One day after plating on poly-D-lysine/laminin-coated culture dishes, proliferating cells migrated from the neurospheres (Fig. 1B). Immunocytochemical staining showed that these cells expressed the radial glial markers Pax6, BLBP and GFAP (Fig. 1C–E). A few cells expressed the neuronal marker βIII-tubulin. Neither the primitive endodermal marker α-fetoprotein nor the mesodermal marker brachyury were expressed in these cells (data not shown), suggesting that the generation of neurospheres occurred without induction of endodermal or mesodermal contamination. Neural precursors derived from these neurospheres expressed Trk receptors (Fig. 1G) and these precursors gave subsequently rise to post-mitotic neurons (Fig. 1F). To activate the neurotrophin receptors, hES cell-derived neuronal cells were treated with NGF, BDNF and NT-3 for 6 days (early differentiation) and 18 days (late differentiation). CNTF was also used as a candidate factor to induce cholinergic differentiation. Measurement of cell proliferation following neurotrophic factor exposure revealed increased proliferation with NT-3 (14.1 ± 2.4%), CNTF (19.4 ± 4.0%) and NGF (18.3 ± 3.0%) but not following BDNF treatment for 18 days (Fig. 1H). RT-PCR analysis was performed on cells differentiated for 18 days and similar mRNA levels of TrkB, TrkC and p75NTR were observed following stimulation with growth factors (Fig. S1). These results suggest that activation of these receptors does not affect their gene expression.
Fig 1.
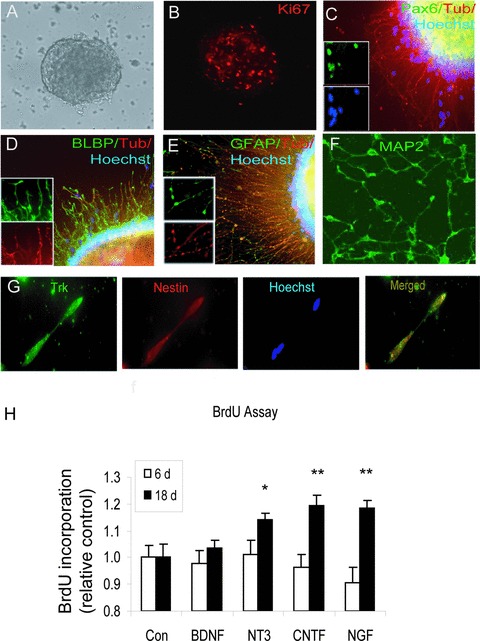
Efficient generation of neurons from hES cells that were cultured as free-floating aggregates (neurospheres). Cells in these aggregates first differentiated to radial glial cells and then further to neurons. (A, B) Proliferative neurospheres expressed Ki67 (at 10×). (C–E) Cells migrated from neurospheres, expressing the radial glial markers Pax6, BLBP and GFAP 1 day after plating on poly-D-lysine and laminin. A few cells expressed the neuronal marker βIII-tubulin (red) (cells are shown at 20×). (F) Post-mitotic (MAP2-expressing) neurons were developed under these culture conditions. (G) Tyrosine kinase receptors were expressed on neural precursors. (H) Effects of neurotrophic factors (BDNF, NT-3 and NGF) and CNTF on cell proliferation. After 18 days, proliferation was increased following NT-3, CNTF and NGF (50 ng/ml) at *P < 0.05; **P < 0.01.
Forebrain identity of hES cell-derived cholinergic neurons
To define the sub-regional identity of neurons generated in the presence of neurotrophic factors, we analysed transcription factors which play key roles in brain development (Fig. 2). The pro-neural transcription factor Mash1 was expressed, which is involved in the regulation of telencephalon dorsal/ventral fates in the developing forebrain. In addition, we detected several genes expressed in the ventral telencephalon [18, 19] including Gsh2, Dlx1, Dlx2 and Gbx2 (Fig. 2A). A total of 76 ± 1.8% of the cells also expressed the forebrain restricted homeodomain protein Nkx2.1, indicating hES cell-derived progenitors with a telencephalic identity. In addition, the brain factor-1 (Bf-1) with a restricted pattern of expression in neural progenitors of the telencephalic neuroepithelium and in structures in the adult brain derived from the telencephalon, was expressed (Figs S2 and 3). To characterize medial ganglionic eminence (MGE) areas further, the LIM-homeobox genes Lhx6 and Lhx8 were analysed. The expression of Lhx6, a marker for neurons in the subventricular and submantle zones of MGE [20], was induced by exposure to CNTF. In contrast, BDNF and NGF up-regulated Lhx8 (Fig. 2A), a neuronal marker for the submantle and mantle zone of MGE, which is also involved in the specification of many cholinergic neurons [21].
Fig 2.
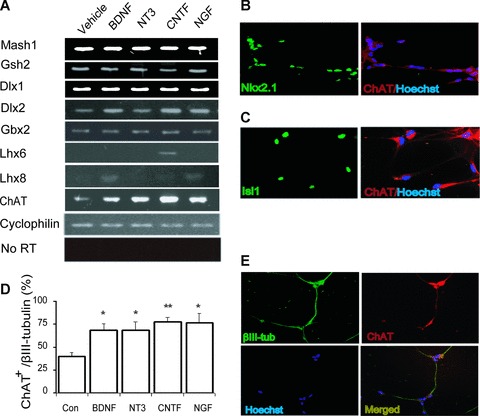
Expression of markers for sub-regional identity and for cholinergic neurotransmission in differentiated hES cells. (A) After 18 days of differentiation with BDNF, NT-3, CNTF and NGF, the cells expressed transcripts of several genes expressed the ventral telencephalon, including Mash1, Dlx1, Dlx2, Gbx2 and Gsh2. The LIM-homeobox genes Lhx6 and Lhx8, both confined to the MGE, were up-regulated with CNTF, and with BDNF and NGF treatment, respectively. (B, C) Immunostaining of hES cell-derived cholinergic cells with anti-Nkx2.1 and anti-Islet-1 antibodies 18 days after plating (at 40×). (D) The proportion of neurons expressing ChAT following treatment with neurotrophic factors. All factors tested showed statistically significant effect on the proportion of ChAT+ neurons. *P < 0.05; **P < 0.001 as compared to control. Values are expressed as mean ± S.E.M., n= 6. (E) Immunostaining of ChAT (red) in NT-3-differentiated neurons at day 18 (at 20×).
To examine which signalling mechanisms are activated in hES cell-derived neuronal cells following exposure to neurotrophic factors, we analysed ERK protein expression, which is known to stimulate neurotrophin responses and neurite outgrowth [22]. After 15 min. stimulation, all factors examined induced phosphorylation of ERK (Fig. S4). Activation of Trk receptors by NT-3 induced the highest p-ERK expression and this induction of p-ERK expression was inhibited by 200 nM K252a, an inhibitor of tyrosine protein kinase activity. These findings suggest that neurotrophic factors were able to activate pathways that induce differentiation in these cells.
To investigate cholinergic characteristics of the hES-cell derived neuronal cells, we analysed the expression of ChAT and cholinergic receptors. Transcripts encoding ChAT were detected and the expression of ChAT was increased following BDNF, NT-3, CNTF and NGF exposure for 18 days (Fig. 2A). Consistent with the results of RT-PCR analysis, we detected expression of ChAT by immunocytochemistry (Fig. 2E). The proportion of neurons expressing ChAT was significantly increased (P < 0.05) after stimulation with BDNF (69 ± 6.9%), NT-3 (69 ± 8.9%), CNTF (78 ± 5.0%) and NGF (77 ± 9.9%) compared with control (40 ± 4.8%) in late differentiated cells (Fig. 2D). In contrast, no expression of ChAT was detected in early differentiated cells (data not shown). The expression of the homeodomain proteins Nkx2.1 and Islet-1, which is associated with the development of forebrain cholinergic neurons, was co-localized with hES cell-derived ChAT+ neurons, demonstrating a forebrain identity (Fig. 2B and C). As virtually all cholinergic cells also were immunoreactive to the p75 receptor (Fig. S5), it is suggested that basal forebrain cholinergic neurons are derived rather than striatal neurons.
Human ES-cell derived neurons express various subtypes of cholinergic receptors
We examined the expression of cholinergic receptors and found transcripts encoding α3, α4 and α7 nAChR subunits and M1, M2 and M3 mAChR subtypes (Fig. 3A). Exposure with BDNF, NT-3, CNTF and NGF increased the α3 nAChR subunit in late differentiated cells. In contrast, a small reduction was observed for α4 nAChR subunit following BDNF and CNTF treatment, whereas no substantial effect was observed as regards the α7 nAChR subunit. The α7 nAChR subtype was expressed in 5–10% of the cells (>600 cells counted) and it was co-localized with expression of the neuronal marker βIII-tubulin (Fig. 3B). Furthermore, BDNF exposure also induced NMDA receptors in these cells (Fig. 3A). Together these findings suggest that BDNF, NT-3, CNTF and NGF may have multiple functions during the progression of neuronal differentiation of hES cells. We also detected transcripts encoding EAAT3, a Na+/glutamate transporter localized mainly in neurons, GAD67 and tyrosine hydroxylase, enzymes synthesizing GABA and dopamine, respectively (data not shown).
Fig 3.
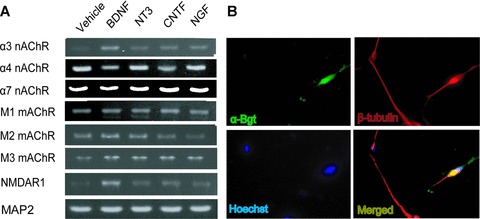
Expression of cholinergic receptors in neurons from hES cells. (A) RT-PCR analysis of hES cell-derived neurons in late differentiated cells indicate expression of various subunits for both nAChRs and mAChRs. (B) Co-localization of α-bungarotoxin (green) and βIII-tubulin (red) (magnification 40×). Nuclei were stained with Hoechst 33342 (blue).
Functional properties of cholinergic receptors
To examine the functionality of cholinergic receptors expressed in hES cell-derived neuronal cells, cytosolic calcium was monitored by Fluo-3/Fura-Red imaging. Increased cytosolic calcium evoked by exposure to ACh was observed in a subpopulation of cells (Fig. 4A). To define an ACh-responding cell, we used an increase of over 50% of the basal fluorescence as a cut-off limit. Using this criterion, the proportion of ACh-responding cells increased in a dose-dependent manner. Concentrations higher than 1 μM evoked a calcium response in a maximum range of 50% to 60% of imaged cells (Fig. 4B). We then investigated the source of the ACh-evoked cytosolic calcium. ACh (10 μM) was applied first to identify the responding cells, and this was then followed by a second application of ACh in calcium-free conditions (Fig. 4C). The removal of extracellular calcium was ineffective in abolishing the ACh-evoked calcium increase, suggesting an intracellular origin. In addition, the mAChR antagonist scopolamine (10 μM) abolished a second calcium response (Fig. 4D), suggesting the presence and involvement of mAChRs in the mobilization of calcium from intracellular stores. In contrast, the α7 nAChR subtype antagonist methyllycaconitine (10 nM) was ineffective in inhibiting the ACh-evoked calcium response (data not shown).
Fig 4.
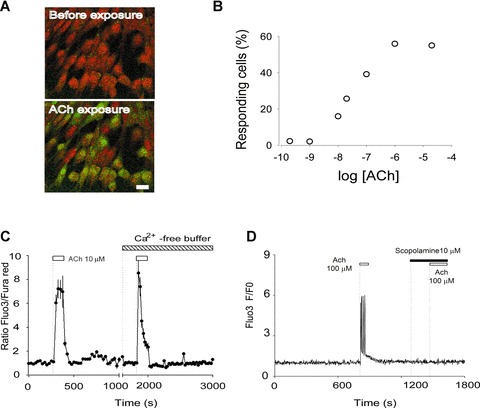
Cholinergic receptors mediate the calcium increase evoked by acetylcholine. (A) Image from cells loaded with the calcium-sensitive dyes Fluo-3 and Fura-Red showing a frame taken before application of 100 μM ACh (upper panel). Upon exposure to ACh the fluorescence that came from Fluo-3 increased, whereas that from Fura-Red diminished (lower panel). In the same frame, a cytosolic calcium increase turned the responding cells green, whereas non-responding cells remained red. The bar indicates 20 μm. (B) The fraction of responding cells was plotted against the logarithm of the concentration of ACh (the first application of ACh in each experiment). Each point corresponds to the counting of at least 50 cells (two to six experiments). (C) The responding cells were exposed to ACh (10 μM) two times. The first application was done in a physiological buffer supplemented with 20 mM glucose (KRH-glc). The second time, ACh was applied in calcium-free buffer (see ‘Materials and methods’). The trace corresponds to an average of 11 cells and is a representative of three experiments. (D) Cells were exposed twice to ACh (100 μM). The first application shows the calcium increase elicited by ACh. The second application of ACh was performed in the presence of the mAChR antagonist scopolamine (10 μM).
Discussion
In the embryonic telencephalon, neuroepithelial cells and radial glial cells serve as progenitors of neurons and glial cells [23]. In an earlier study we demonstrated that cells from six hES cell lines (HS181, HS237, HS293, HS306, HS346, HS382) form neuroepithelial cells which then further developed into neurons using both adherent and suspension cultures in serum- and feeder-free conditions. All six hES cell lines had similar growth and differentiation characteristics at different passages [17]. Here we have shown that radial glial cells were derived from bFGF/EGF-expanded neurospheres, subsequently giving rise to neuronal cells.
Several signalling pathways have been implicated in the control of self-renewal and commitment of stem cells and progenitors to a neuronal fate including FGF, Wnt and Notch signalling [23]. Consistent with a previous report [7], we found that hES cells express neurotrophin receptors. Stimulation with NT-3, NGF and CNTF increased the cell proliferation following 18 days exposure. These growth factors also have regulatory roles in neuronal differentiation and neurotransmitter phenotype specification [16, 24, 25]. In the current study, neurotrophic factors induced a differential effect on the transcripts encoding Lhx6, Lhx8, ChAT, the α3 and α4 nAChR subunits and the M3 mAChR subtype.
Although, the roles of the LIM homeodomain genes Lhx6 and Lhx8 in early neuronal development are not completely understood, earlier studies reported that these genes may have important functions in the determination of cholinergic and GABAergic cell fates in the developing forebrain [26, 27]. The expression of Lhx8, specifically expressed in MGE areas of the embryonic forebrain and oral mesenchyme, was induced in hES cell-derived neuronal cells by BDNF and NGF, whereas Lhx6 expression, which marks other subdomains of the MGE, was induced by CNTF. On the basis of the analysis of homeodomain transcription factors Nkx2.1, Islet-1 and Bf-1, which specify the identity of progenitors and neuronal cells in the developing forebrain, we suggest that this culture system offers a way to generate specifically basal forebrain cholinergic neuronal populations. The robust cholinergic differentiation, also in the absence of neurotrophic factors, indicates a default induction for telencephalic neurons. Previous studies have identified neural progenitors with an anterior identity from hES cells [28, 29]. Derivation of forebrain specific neural progenitors from mouse ES cells using serum-free, floating cultures of embryoid body-like aggregates has also been reported [30].
The detection of transcripts for cholinergic receptors prompted further functional analysis of these cells. Interestingly, functional nAChRs have previously been reported in primary cultures of stem and progenitor cells from embryonic mouse cortex [31]. In the present study, the hES cell-derived neuronal cells represent a heterogeneous cellular system in which we could distinguish between cells responding to ACh and cells that did not respond to ACh. On the basis of the observation that ACh-evoked calcium responses were obtained at a similar magnitude after the removal of calcium from the extracellular medium, we suggest that calcium permeability was not a major contributor to the hES cell-derived neuronal cell response. In contrast to scopolamine, no substantial effect on the calcium response to ACh was observed by blocking with the α7 nAChR antagonist methyllycaconitine. Together, these findings suggest that the cytosolic calcium increase was mainly mediated by mAChRs. A nicotinic contribution in augmentation of cellular calcium was not observed in our experimental conditions, which may reflect the different kinetics of the ACh-evoked calcium responses mediated by mAChRs and nAChRs. It is also possible that some subtypes of receptors remain in intracellular compartments, with negligible presence on the plasma membrane.
Our findings suggest that telencephalic cholinergic neuronal cells, derived from hES, express various subtypes of both nAChRs and mAChRs. This is the first report demonstrating expression of nAChRs and mAChRs in hES cell-derived neuronal cells. Detection of ChAT+ cells obtained from foetal human neural stem cells has been reported earlier [32]. Increasing the number of cholinergic neurons generated from hES cells is of particular interest in developmental biology and for the development of cell-based replacement therapies for neurodegenerative diseases where the cholinergic system is compromised.
In an earlier study, we demonstrated that treatment with the novel drug (+)-phenserine, a drug modulating amyloid production in the brain, combined with transplantation of human neural stem cells to Alzheimer (APP23) transgenic mice, stimulated differentiation of human neural stem cell progenitors into neurons [33]. We therefore suggest that generation of hES cell-derived cholinergic neuronal cells will facilitate further investigations into the mechanisms of cholinergic function during development and synaptic plasticity. Functional neurons, obtained from hES cells, and expressing cholinergic receptors may be extremely useful for screening pharmacological targets for treating neurodegenerative disorders.
Acknowledgments
We are grateful to Ann-Marie Strömberg for assistance with hES cultures and Ahmadul Kadir for help with figure editing. The financial support by Swedish Research Council (project no. 05817 [A.N.], project no. 50434701 [O.H.]), Gun and Bertil Stohnes Foundations, Alzheimer Foundation Sweden, The Old Servant Foundation, The Brain Foundation, ALF agreement between Stockholm City Council and Karolinska Institutet (A.N., O.H.) is highly appreciated.
Supporting Information
Fig. S1 RT-PCR analysis of Trk receptors in hES cell-derived neurons on day 18.
Fig. S2 Co-localization of the homeodomain protein Bf-1 and the nuclear staining in hES cell-derived cells, demonstrating basal forebrain identity.
Fig. S3 Co-localization of the homeodomain protein Nkx2.1 and the nuclear staining in hES cell-derived cells, demonstrating telencephalic identity of cells derived from the lines HS293 and HS346.
Fig. S4 Phosphorylation of ERK (pERK) induced by neurotrophins in hES cell-derived neurospheres. The induction of pERK was inhibited by K252a, an inhibitor of Trk signaling.
Fig. S5 Expression of p75 receptors on cholinergic neurons indicates basal forebrain cholinergic neurons rather than striatal neurons.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Muotri AR, Nakashima K, Toni N, et al. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci USA. 2005;102:18644–8. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bally-Cuif L, Hammerschmidt M. Induction and patterning of neuronal development, and its connection to cell cycle control. Curr Opin Neurobiol. 2003;13:16–25. doi: 10.1016/s0959-4388(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 3.Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol. 2001;11:43–9. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 4.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–9. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 5.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 6.Schuldiner M, Yanuka O, Itskovitz-Eldor J, et al. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2000;97:11307–312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–50. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 8.Van der Zee EA, Luiten PG. Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: a review of immunocytochemical localization in relation to learning and memory. Prog Neurobiol. 1999;58:409–71. doi: 10.1016/s0301-0082(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 9.Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 10.Falk L, Nordberg A, Seiger A, et al. The alpha7 nicotinic receptors in human fetal brain and spinal cord. J Neurochem. 2002;80:457–65. doi: 10.1046/j.0022-3042.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- 11.Kihara T, Shimohama S, Sawada H, et al. Alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem. 2001;276:13541–6. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 12.Nordberg A. Nicotinic receptor abnormalities of Alzheimer’s disease: therapeutic implications. Biol Psychiatry. 2001;49:200–10. doi: 10.1016/s0006-3223(00)01125-2. [DOI] [PubMed] [Google Scholar]
- 13.Eriksdotter-Jonhagen M, Nordberg A, Amberla K, et al. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:246–57. doi: 10.1159/000017069. [DOI] [PubMed] [Google Scholar]
- 14.Tuszynski MH, Thal L, Pay M, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–5. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 15.Inzunza J, Gertow K, Stromberg MA, et al. Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells. 2005;23:544–9. doi: 10.1634/stemcells.2004-0201. [DOI] [PubMed] [Google Scholar]
- 16.Nilbratt M, Friberg L, Mousavi M, et al. Retinoic acid and nerve growth factor induce differential regulation of nicotinic acetylcholine receptor subunit expression in SN56 cells. J Neurosci Res. 2007;85:504–14. doi: 10.1002/jnr.21156. [DOI] [PubMed] [Google Scholar]
- 17.Nat R, Nilbratt M, Narkilahti S, et al. Neurogenic neuroepithelial and radial glial cells generated from six human embryonic stem cell lines in serum-free suspension and adherent cultures. Glia. 2007;55:385–99. doi: 10.1002/glia.20463. [DOI] [PubMed] [Google Scholar]
- 18.Bulfone A, Puelles L, Porteus MH, et al. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J Neurosci. 1993;13:3155–72. doi: 10.1523/JNEUROSCI.13-07-03155.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 20.Grigoriou M, Tucker AS, Sharpe PT, et al. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development. 1998;125:2063–74. doi: 10.1242/dev.125.11.2063. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Marin O, Hermesz E, et al. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci USA. 2003;100:9005–10. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greene LA, Kaplan DR. Early events in neurotrophin signalling via Trk and p75 receptors. Curr Opin Neurobiol. 1995;5:579–87. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 23.Guillemot F. Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr Opin Cell Biol. 2005;17:639–47. doi: 10.1016/j.ceb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Brodski C, Schaubmar A, Dechant G. Opposing functions of GDNF and NGF in the development of cholinergic and noradrenergic sympathetic neurons. Mol Cell Neurosci. 2002;19:528–38. doi: 10.1006/mcne.2001.1093. [DOI] [PubMed] [Google Scholar]
- 25.Brodski C, Schnurch H, Dechant G. Neurotrophin-3 promotes the cholinergic differentiation of sympathetic neurons. Proc Natl Acad Sci USA. 2000;97:9683–8. doi: 10.1073/pnas.160080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Mori T, Takaki H, et al. Comparison of the expression patterns of two LIM-homeodomain genes, Lhx6 and L3/Lhx8, in the developing palate. Orthod Craniofac Res. 2002;5:65–70. doi: 10.1034/j.1600-0544.2002.02198.x. [DOI] [PubMed] [Google Scholar]
- 27.Manabe T, Tatsumi K, Inoue M, et al. L3/Lhx8 is involved in the determination of cholinergic or GABAergic cell fate. J Neurochem. 2005;94:723–30. doi: 10.1111/j.1471-4159.2005.03261.x. [DOI] [PubMed] [Google Scholar]
- 28.Pankratz MT, Li XJ, La Vaute TM, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–20. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkabetz Y, Panagiotakos G, Al Shamy G, et al. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–65. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K, Kamiya D, Nishiyama A, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–96. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 31.Atluri P, Fleck MW, Shen Q, et al. Functional nicotinic acetylcholine receptor expression in stem and progenitor cells of the early embryonic mouse cerebral cortex. Dev Biol. 2001;240:143–56. doi: 10.1006/dbio.2001.0453. [DOI] [PubMed] [Google Scholar]
- 32.Wu P, Tarasenko YI, Gu Y, et al. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neurosci. 2002;5:1271–8. doi: 10.1038/nn974. [DOI] [PubMed] [Google Scholar]
- 33.Marutle A, Ohmitsu M, Nilbratt M, et al. Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proc Natl Acad Sci USA. 2007;104:12506–11. doi: 10.1073/pnas.0705346104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 RT-PCR analysis of Trk receptors in hES cell-derived neurons on day 18.
Fig. S2 Co-localization of the homeodomain protein Bf-1 and the nuclear staining in hES cell-derived cells, demonstrating basal forebrain identity.
Fig. S3 Co-localization of the homeodomain protein Nkx2.1 and the nuclear staining in hES cell-derived cells, demonstrating telencephalic identity of cells derived from the lines HS293 and HS346.
Fig. S4 Phosphorylation of ERK (pERK) induced by neurotrophins in hES cell-derived neurospheres. The induction of pERK was inhibited by K252a, an inhibitor of Trk signaling.
Fig. S5 Expression of p75 receptors on cholinergic neurons indicates basal forebrain cholinergic neurons rather than striatal neurons.


