Abstract
Patients treated for cancer therapy using ionizing radiation (IR) have delayed tissue repair and regeneration. The mechanisms mediating these defects remain largely unknown at present, thus limiting the development of therapeutic approaches. Using a wound healing model, we here investigate the mechanisms by which IR exposure limits skin regeneration. Our data show that induction of the stromal cell-derived growth factor 1α (SDF-1α) is severely impaired in the wounded skin of irradiated, compared to non-irradiated, mice. Hence, we evaluated the potential of bone marrow-derived multipotent stromal cells (MSCs), which secrete high levels of SDF-1α, to improve skin regeneration in irradiated mice. Injection of MSCs into the wound margin led to remarkable enhancement of skin healing in mice exposed to IR. Injection of irradiated MSCs into the wound periphery of non-irradiated mice delayed wound closure, also suggesting an important role for the stromal microenvironment in skin repair. The beneficial actions of MSCs were mainly paracrine, as the cells did not differentiate into keratinocytes. Specific knockdown of SDF-1α expression led to drastically reduced efficiency of MSCs in improving wound closure, indicating that SDF-1α secretion by MSCs is largely responsible for their beneficial action. We also found that one mechanism by which SDF-1α enhances wound closure likely involves increased skin vascularization. Our findings collectively indicate that SDF-1α is an important deregulated cytokine in irradiated wounded skin, and that the decline in tissue regeneration potential following IR can be reversed, given adequate microenvironmental support
Keywords: SDF-1α, ionizing radiation, tissue regeneration, MSCs, microenvironment
Introduction
Cancer treatment using ionizing radiation (IR) alone or in combination with different types of drugs leads to short- and long-term side-effects [1, 2]. These defects are wide-ranging, but often involve defective tissue growth and regeneration. One such example is delayed wound healing following surgical resection of an irradiated tumour [3, 4]. Wound healing is a complex biological process involving diverse cytokines implicated in varying stages, from the initial phase of inflammation to migration and proliferation of infiltrating or resident cells [5]. To date, effective therapies for chronic non-healing wounds in diabetic or cancer-treated patients are lacking, being mostly restricted to intense wound care. Direct supplementation of different cytokines at the injured site via either topical application of recombinant proteins or viral delivery improves wound healing in mice. For instance, sonic hedgehog, vascular endothelial growth factor (VEGF), and stromal derived cell factor-1α (SDF-1α or CXCL12), augmented wound healing mainly by enhancing angiogenesis [6–8]. Importantly, the efficiency of these cytokines was evaluated in diabetic mice with impaired microcirculation. Because the molecular mechanism(s) involved in IR-based skin regeneration defects are unknown at present, it is uncertain whether cytokine-based angiogenesis therapy would be effective on irradiated tissues of a cancer-treated patient.
IR induces irreversible damages to the stroma, reducing by as much as 90% the colony forming unit capacity of the bone marrow or skin derived stromal cells [9–11]. Importantly, this reduction in the number of stromal progenitor cells is long-lasting as their number does not recover for several years [9, 12, 13]. Hence, we suggested that IR-induced prolonged stromal damage may be responsible for delayed skin repair. Interestingly, injection of bone marrow derived multipotent stromal cell (MSCs) in the immediate periphery of wounded skin accelerates healing in diabetic mice [14–16]. MSCs can be easily expanded in vitro, and display the potential to differentiate into several cell types (such as adipocytes, osteocytes or chondrocytes) [17–20], making MSCs attractive candidates for cellular therapy. The exact mechanisms by which MSCs accelerate wound closure remain ambiguous, especially as MSC ability to differentiate into keratinocytes is controversial [21–23]. In earlier studies, negligible differentiation into keratinocytes was observed (0.0001% to 0.1%), even in situations where MSC homing to the injured site was not required (i.e. when MSCs were injected in the vicinity of the wound margin) [14, 15, 21, 24]. Hence, MSCs may provide paracrine factors that foster skin regeneration independently of the ability of MSCs to transdifferentiate. In support of this hypothesis, conditioned media collected from MSCs and injected within the wound periphery facilitated skin healing; the factors responsible were not identified [25]. However, the issue of whether MSCs or their secretome have therapeutic effects on irradiated wounded skin containing damaged cells, likely diminished in their regeneration potential, remains to be clarified.
In this study, we focus on two important topics. Initially, the mechanism responsible for delayed healing following exposure to IR was analysed. Deficiency in SDF-1α induction was identified as an important cause of wound repair defects in irradiated skin. Subsequent results showed that injection of MSCs rescued long-term IR damage, which was mainly attributed to the specific secretion of SDF-1α. Based on these findings, we suggest that the decreased regeneration potential of irradiated skin is somewhat reversible and may be restored with adequate stromal support.
Materials and methods
Isolation, purification and characterization of MSCs
Bone marrow was collected by flushing the tibias, femurs and iliacs from C57BL/6 female mice with α-MEM containing 2% FBS (Wisent, St. Bruno, QC, Canada). MSCs were then isolated based on their property to attach to polystyrene tissue culture dishes after 7–10 days in α-MEM containing 15% FBS and 1% penicillin/streptomycin. MSCs were cultured for 2–4 extra passages (corresponding to 6–12 population doublings) after which characterization by flow cytometry confirmed they were negative for CD31, CD45 and positive for CD44, CD90, CD105 markers. Cells were further characterized by their ability to differentiate into adipocytes following incubation with medium containing 1 μM dexamethazone, 10 μg/ml insulin, 0.5 μM isobutyl methylxantine and 50 μM indomethacine for 1 week. Osteogenic differentiation potential was also confirmed after incubation of the cells with medium containing 100 nM dexamethasone, 50 μM ascorbate-2-phosphate (vitamin C), 50 nM vitamin D3 and 10 mM β-glycerophosphate for 2 weeks. Differentiation in adipocytes and osteocytes was revealed by staining with Oil Red O (Sigma, St. Louis, MO, USA) and Alizarin Red S (Sigma), respectively.
Wound healing model and analysis
Eight-week-old female C57BL/6 mice (Charles River, Saint-Constant, Qc, Canada) were γ-irradiated at the sub-lethal dose of 8 Gy (0.25 Gy/sec.) using a Gammacell 220 and cobalt 60 as a source. Mice suffered severe leucopoenia for a period of approximately 2 weeks after irradiation with no sign of any other side effects. Eighteen to 22 weeks after irradiation, mice were anaesthetized with 2% isoflurane, hair was removed on their dorsal surface and one or two 4-mm full-thickness punch biopsy wounds were created. Twenty-four hours later, mice were placed under anaesthesia and received four 15 μl intradermal injections totalling 1 million MSCs or mouse embryonic fibroblasts (MEFs) in PBS. Wound area was measured every other day for the first week, then every day until complete closure. Photographs of wounds were taken to record evolution of healing. The percentage of wound closure was determined as: (area of wound day 0 – area of wound day X)/area of wound day 0 × 100. All procedures were approved by the CHU Ste-Justine’ Animal Care Committee and animals were handled in accordance with institutional guidelines.
Lentiviral gene regulation
Using RNA collected from murine MSCs, the SDF-1α cDNA was amplified with the QuantiTect Reverse Transcription Kit (Qiagen, Montreal, Canada) and standard PCR method using the following primers (forward: 5′-CGGGAATTCCTTGCTGTCCAGCTCTGCAG-3′: reverse: 5′-CGACGTCGACGGTACCGTCCTTTGGGCTGTTGTGCT-3′). The SDF-1α amplicon was confirmed by sequencing and, together with the enhanced green florescent protein (EGFP), cDNA was introduced into a lentiviral vector backbone under the control of the human CMV promoter. A set of five lentivectors expressing shRNAs targeting the murine SDF-1α were purchased from Open Biosystems (Huntsville, AL, USA) (cat nb: RMM3981- NM_011331). Validation experiments demonstrated that the lentivector expressing the shRNA targeting nucleotides 207–227 (NM_013655) was the most effective (see Fig. S1). A lentivector expressing a shRNAs against the human p16 gene, with no homology to murine sequences, was used as negative control [26]. Briefly, lentivector stocks were prepared by transfecting overnight 293T cells using Lipofectamine 2000 (Invitrogen, Burlington, Canada) with the required transfer vector plasmid, the pMD.Lg/pRRE packaging plasmid, the pMD2.VSV-G envelope-encoding plasmid, and pRSV-Rev. The following morning, the media was replaced with fresh media and then supernatant collected 30 hrs later. Media containing lentivectors was either used directly or concentrated by ultracentrifugation (50,000 ×g in a SW28 rotor for 2 hrs) and frozen at –80°C. Quantification of the virus was done using a commercial enzyme-linked immunobsorbent assay (ELISA) kit detecting p24 levels (ZeptoMetrix Corporation, Buffalo, NY, USA).
RNA isolation and quantitative real-time PCR
Excised wounds together with epidermal margins were mechanically dissociated in 500 μl trizol reagent using a power-driven homogenizer (OMNI International, Marietta, GA, USA), and total RNA (0.1–0.5 μg RNA/mg tissue) was extracted using the RNeasy lipid tissue Mini Kit (Qiagen). One microgram of RNA was then reverse-transcribed using the QuantiTect Reverse Transcription Kit (Qiagen). Quantitative differences in gene expression were determined by real-time quantitative PCR using SYBR-Green PCR master mix (Qiagen) and a spectrofluorometric thermal cycler (Mx3000P from Stratagene, La Jolla, CA, USA). A list of primers used to detect murine SDF-1α, metalloproteinase gene (MMP)-3, MMP-9, MMP-13, VEGF, PAI-1, FGF-2 and 18S is provided as supporting information (see Table S1). Values are presented as the ratio of target mRNA to 18S rRNA obtained using the relative standard curve method of calculation.
Protein analysis by ELISA
Proteins were isolated following three cycles of freeze and thaw from homogenized wounded skin (2–5 μg/mg tissue) in PBS containing a protease inhibitor cocktail (Sigma). Total protein concentration was determined by the Bradford protein assay and SDF-1α specific level was determined by ELISA according to the manufacturer’s instructions (RayBiotech, Norcross, GA, USA). Levels of SDF-1α protein were normalized for the total amount of protein isolated from wounded skin samples. Determination of SDF-1α protein concentration in conditioned media from MSCs or MEFs was normalized to the total number of cells present at the time of collection.
Histological and immunological examination
Mouse skin samples were snap-frozen in OCT compound (Sakura Finetek, Torrance, CA, USA) and cryosections were stained in haematoxylin for histological evaluation. For immunohistochemical staining, cryosections were fixed with paraformaldehyde 4%, stained using antibodies against the von Willebrand Factor (vWF) endothelial cell marker (RB-281-A; NeoMarker, Fremont, CA, USA) or the pan-leucocyte CD45 antibody (BD Pharmingen) and then detected with a peroxidase kit (SK-4100) from Vector Laboratories. Vascularization was determined by counting the number of vWF positive vessels in two random fields per section between the wound edges. Counts were confirmed by a second double-blind investigator. For immunofluorescence stainings, cryosections were fixed and incubated with an antibody against epidermal cytokeratin subunits (Zo622, Dako, Mississauga, Canada) and subsequently by a goat anti-rabbit 594 TxRED-conjugated antibody (Invitrogen). Sections were stained with DAPI and mounted with mounting medium (Vectashield H-1000, Burlington, Canada).
Chimeric mice and GFP imaging
Wild-type GFP chimeric mice were generated by the retro-orbital venous plexus injection of 1 × 106 bone marrow cells harvested from 8–10 week old donor mice ubiquitously expressing enhanced GFP (C57BL/6-Tg(CAGEGFP) 1Osb/J, The Jackson Laboratory, Bar Harbor, ME, USA) as described elsewhere [24]. Upon complete haematopoietic reconstitution (8 weeks), wounds were created and chimeras received daily retro-orbital injections of 40 μg (1 μg /1 μl) of an SDF-1α blocking antibody (MAB310, R&D Systems), as reported previously [24, 27–29] or 40 μl saline starting immediately after wounding (n= 7 mice in each group). Mice were killed on day 7, wounds harvested and GFP expression determined using a rabbit anti-GFP (Abcam, Cambridge, UK) as previously described [30]. Each wound section was imaged in three different areas immediately adjacent to the wound with an inverted epifluorescent microscope and the proportion of GFP infiltrating cells determined by counting and averaging the number of pixels in each image using the ‘Histogram’ tool in Adobe Photoshop 7.
Statistical analysis
All results are expressed as means ± standard error of the mean (S.E.M.). The statistical differences were assessed using the Student’s t-test and ANOVA followed by Tukey–Kramer’s post-test. Statistical significance was set at P < 0.05.
Results
Long-term impairment of wound healing after exposure to IR
A significant proportion of cells exposed to IR undergo persistent DNA damage and concomitant inhibition of cell cycle progression in vitro. Consequently, we suggested that tissue regeneration could be altered for an extended period of time upon exposure to IR. Indeed, up to a minimum of 4 months following exposure to a single sub-lethal dose of 8 Gy (total body irradiation), C57BL/6 mice displayed deficiencies in their ability to heal a full-thickness 4 mm skin punch biopsy (Fig. 1A). Cytokeratin immunostaining and histology confirmed that re-epithelization was delayed by over 25% at 10–13 days after wound induction in irradiated mice, compared to their non-irradiated counterparts (Fig. 1A and B). Interestingly, both groups responded early after injury, as delayed wound closure became evident only about 1 week after wounding. No differences in the levels of infiltrating leucocytes within the wound periphery were observed between the irradiated and non-irradiated groups at day 3 or day 7 after injury (Fig. 1C and data not shown). Hence, while exposure to IR does not fully prevent the healing response, it generates long-lasting damage to tissues which can delay skin re-epitalization.
Fig 1.
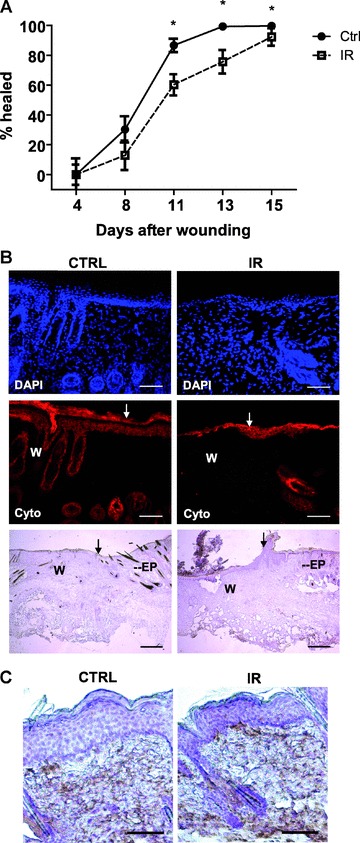
Wound healing is permanently impaired in mice exposed to IR. Full-thickness wound biopsies were created on the dorsal surface of mice exposed or not to total body irradiation at the sub-lethal dose of 8 Gy. Mice were allowed to recover from IR for a minimum of 4 months prior to wound induction. (A) Measurement of wound size at different times after injury. Wound surface area is presented as the mean percent healed ± S.E.M. (n= 15–16 wounds in each group). (B) Representative immunofluorescence and histological stainings showing delayed re-epithalization of skin previously exposed to irradiation. Wound boundaries are indicated with arrows. W, wound; Ep, epidermis. Cryosections shown were immunostained with an antibody against pan-cytokeratin (in red) and nuclei with DAPI (in blue) 11 days after wounding. Scale bar = 100 μm. Cryosections used for histology were counterstained with haematoxylin. Scale bar = 500 μm. (C) Leucocytes infiltration in the wound area at day 3 after wounding as determined by immunohistochemistry with an anti-CD45 antibody. A representative section from four independent wounds analysed per group is shown. Scale bar = 250 μm. *P < 0.01).
Absence of SDF-1 induction in wounded skin previously exposed to IR
Previous studies showed that expression of several transcription factors, cytokines, and proteases, increased during the response to the wound. One such factor, SDF-1α, is induced at 4–7 days after injury [31, 32], immediately preceding the period during which wound healing defects became significant in irradiated mice (Fig. 1A). Using quantitative real-time PCR, we analysed the levels of several genes implicated in the wound response. Expression of SDF-1α was increased (∼2-fold) in wounded tissue from control mice, but not in mice previously exposed to IR (Fig. 2A). Protein levels assessed by ELISA on lysates obtained from homogenized wound margins were consistent with this observation (Fig. 2B). The deficiency in SDF-1α expression at 3 days after injury is unlikely to be a general defect in gene expression, in view of the finding that induction of VEGF, another cytokine, was not significantly affected in either control mice or those previously exposed to IR (Fig. 2A). Similarly, expression of the plasminogen activator inhibitor (PAI-1), an important negative regulator of the wound response [33], was not significantly affected (Fig. 2A). Specific MMPs that play key roles in tissue remodelling after injury were additionally analysed [34, 35]. As expected, compared to levels observed at day 0, control mice displayed markedly increased levels of MMP-3 (3.7-fold), MMP-9 (9.5-fold), and MMP-13 (14.3-fold) 3 days after wounding (Fig. 2A). Mice previously exposed to IR also displayed significant up-regulation of MMP-3 (4.3-fold), MMP-9 (10.2-fold), and MMP-13 (14.1-fold). Despite no clear differences in the -fold induction of all three MMPs analysed, the absolute amounts of MMP-9 and MMP-13 expressed were considerably lower in wounds from mice previously exposed to IR (Fig. 2A). We are uncertain whether this variation in the absolute amounts of MMP-9 and MMP-13 is important in the rate of wound repair, given the robust increases in MMP-9 and MMP-13 levels in contrast to the change in SDF-1α level.
Fig 2.
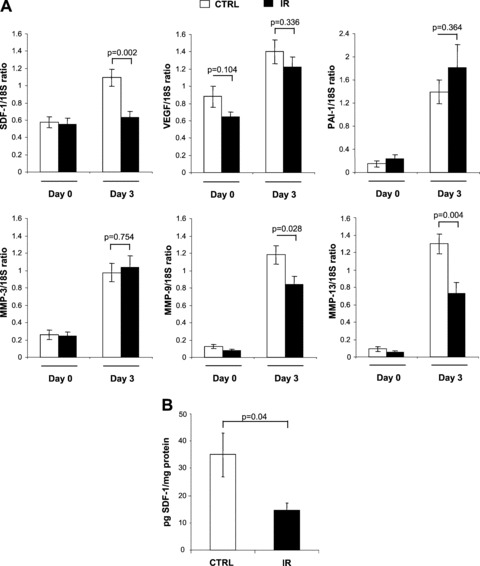
Absence of SDF-1α induction at the RNA and protein levels in wounded skin previously exposed to IR. (A) Total RNA was collected from skin (day 0) or from wounded tissues together with surrounding margins (day 3), and the ratios of SDF-1α, VEGF, PAI-1, MMP-3, MMP-9 and MMP-13 RNA relative to 18S ribosomal RNA were determined by quantitative real-time PCR. Shown is the expression levels as detected in mice previously exposed to IR (black bar) compared to control non-irradiated mice (white bar). (B) Amount of SDF-1α protein was determined by ELISA on wound lysates obtained from irradiated mice (black bar) compared to non-irradiated mice (white bar) 3 days after skin injury. Results are expressed as pg of SDF-1α protein per mg of total protein isolated per wound. Data are mean ± S.E.M.; (n= 9 mice per group, where each RNA and protein sample is composed of an average of two wounds per mouse).
MSC injection influences healing of irradiated skin
We analysed the possibility of overcoming permanently impaired (over 4 months) skin regeneration induced by IR. Initially, we attempted to compensate for the deficiency in SDF-1α by injecting bone marrow-derived MSCs expressing high levels of SDF-1α (Fig. 3A). Interestingly, injection of 1 × 106 total MSCs directly into the vicinity of the wound margin was very efficient in improving skin healing of irradiated, but not control non-irradiated, mice (Fig. 3A, and data not shown). In fact, at 13 days after wounding, 100% of wounds (15/15) of non-irradiated mice receiving PBS displayed over 90% closure (hereafter referred to as ‘healed’) in comparison to only 13% of wounds (2/16) from mice exposed to IR and injected with PBS. In contrast, 72% of wounds (13/18) from irradiated mice injected with MSCs were healed (Fig. 3A). This remarkable capacity of MSCs to improve healing was only partially reproduced by MEFs, which may be explained by the fact that MEFs do not secrete detectable levels of SDF-1α (Fig. S2). Supporting the importance of an intact microenvironment, we also have found the injection of irradiated/senescent MSCs (S-MSC) to delay wound healing of non-irradiated skin (Fig. 3A). Injections using MSCs genetically engineered to express EGFP revealed no or limited migration away from the injection site or differentiation into keratinocytes at the time of analysis (Fig. 3B–D). Although we cannot eliminate the possibility that MSCs have the potential to differentiate into keratinocytes under specific conditions, high-magnification analysis of newly formed epithelium failed to demonstrate co-localization of EGFP and cytokeratin doubly positive cells (Fig. 3B–D). Our results suggest that at least early on during the wound response, the action of MSCs on irradiated skin is largely paracrine, as the cells are not directly involved in wound remodelling and/or closure.
Fig 3.
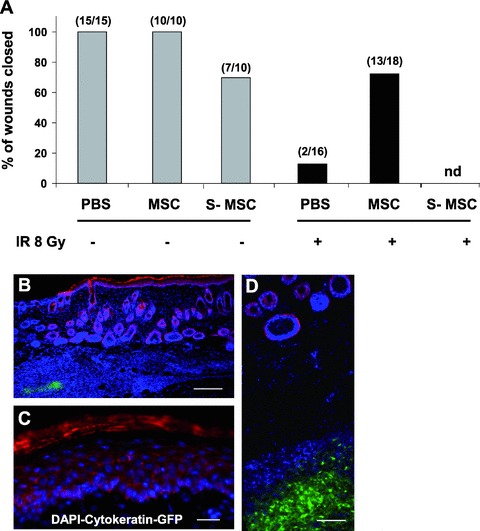
Local injection of MSCs improves skin wound healing without transdifferentiation. (A) Full-thickness biopsies were created on the dorsal surface of mice previously exposed to IR or not, and the effect of intradermal injection of PBS, MSCs or MSCs exposed to 8 Gy IR in vitro (S-MSC), four injection sites per wound (1 × 106 cells total), on wound healing were analysed. Shown are the proportions of wounds over 90% closed at day 13. (B) Absence of colocalization signal between cytokeratin (in red) and MSCs expressing EGFP (in green) in newly formed epithelium collected 11 days after wounding. Cryosections were stained with DAPI (in blue). Scale bar = 250 μm. (C)–(D) Higher magnification images of epidermis and dermis stained as in (B), respectively. Scale bar = 100 μm, 250 μm.
Specific secretion of SDF-1α by MSCs enhances wound closure
To further define the role of SDF-1α in wound healing, we genetically engineered MSCs to have impaired SDF-1α secretion. A lentiviral vector was used to stably express shRNA directed against SDF-1α (the cells are designated ‘MSC-shSDF-1’). MSCs were effectively transduced using VSV-G pseudotyped lentivirus, with modification of nearly 100% of cells after a single round of virus exposure (∼100 ng of p24/105 cells, data not shown). This remarkable transduction efficacy permits knockdown of SDF-1α expression without the need to select for a clonal population using antibiotics. As a result, ELISA showed over 90% reduction in SDF-1α secretion in MSC-shSDF-1 cells, compared to the control population transduced with non-specific shRNA (Fig. 4B). The shRNA targeting nucleotides 207–227 was the most effective among the five sequences examined (Fig. S1). Using the skin punch model, we evaluated the efficacy of MSC-shSDF-1 cells (secreting only ∼40 pg SDF-1α/104 cells) in improving wound closure. Injection of 1 × 106 MSC-shSDF-1 cells led to only a slight improvement in skin repair, confirming the extremely high dependence of skin wound healing on SDF-1α. Specifically, only 25% of the wounds (2/8) from mice injected with these cells and 13% (2/16) of wounds injected with PBS were healed by day 13, far off the 72% (13/18) of wounds healed following the injection of control MSCs secreting over 500 pg of SDF-1α/104 cells (Fig. 4A and B). Our findings strongly suggest that the beneficial paracrine effect of MSCs is largely mediated by the action of SDF-1α. In view of these results, we explored the possibility of delivering supraphysiological amounts of SDF-1α with the aim of further improving wound healing. MSCs were modified, using lentiviruses, to secrete >4000 pg of SDF-1α/104 cells, which is 8-fold greater than the normal amount of SDF-1α (the cell line is designated MSC-SDF-1) (Fig. 4B). Surprisingly, injured mice injected with 1 × 106 MSC-SDF-1 did not display significantly faster regeneration than control MSC-implanted animals. Specifically, 70% (7/10) of wounds were healed by day 13, compared to 72% (13/18) wounds in mice injected with control MSCs (Fig. 4A). This observation is consistent with our previous findings that MSC therapy is not beneficial for non-irradiated mice in which perhaps sufficient amounts of SDF-1α are induced at the injured site.
Fig 4.
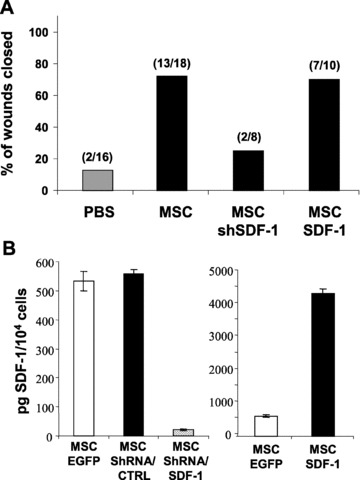
Specific secretion of SDF-1α by MSCs enhanced wound healing. (A) Full-thickness wound biopsies were created on the dorsal surface of mice previously exposed to IR and wound healing was determined following the intradermal injection (4 injections sites per wound) of purified populations of MSC, MSC-ShSDF-1, MSC-SDF-1 or PBS. As in Fig. 3, the proportions of wounds over 90% closed at day 13 are shown. (B) Amount of secreted SDF-1α in conditioned medium from MSCs genetically modified to express EGFP, shSDF-1, shCTR or SDF-1 was determined by ELISA. Data are expressed as means ± S.E.M. of three independent measurements.
MSC injection increases wound vascularization
To clarify the mechanisms by which various populations of MSCs improved wound closure, we measured differences in vascularization, based on the well-known angiogenic and vasculogenic properties of SDF-1α. Initially, we observed that wound beds from irradiated mice contained a significantly lower number of blood vessels than controls (P≤ 0.001), indicating a vascularization defect in skin previously exposed to IR (Fig. 5). Injection of MSCs virally transduced with EGFP, SDF-1 or shSDF-1, in the vicinity of the wound margin, enhanced the blood vessel density in proportion to the amount of SDF-1α secreted (Fig. 5B). Notably, the number and size of blood vessels was considerably larger upon delivery of supraphysiological levels of SDF-1α using MSC-SDF-1 (Fig. 5A). MSC-shSDF-1 cells induced an increase in the blood vessel density to a level equal to or greater than that observed in non-irradiated wound beds, suggesting the presence of pro-angiogenic molecules other than SDF-1α with the ability to augment wound vascularization. Furthermore, using GFP chimeric mice, we observed that bone marrow derived cells are recruited at the wound periphery, but that inhibition of SDF-1 with a blocking antibody did not significantly reduce the infiltration of GFP+ marrow-derived cells (Fig. 5C). Altogether, our results imply that while SDF-1α can restore vascularization of irradiated wound beds, SDF-1α-regulated mechanisms other than blood vessel density are important in wound closure.
Fig 5.
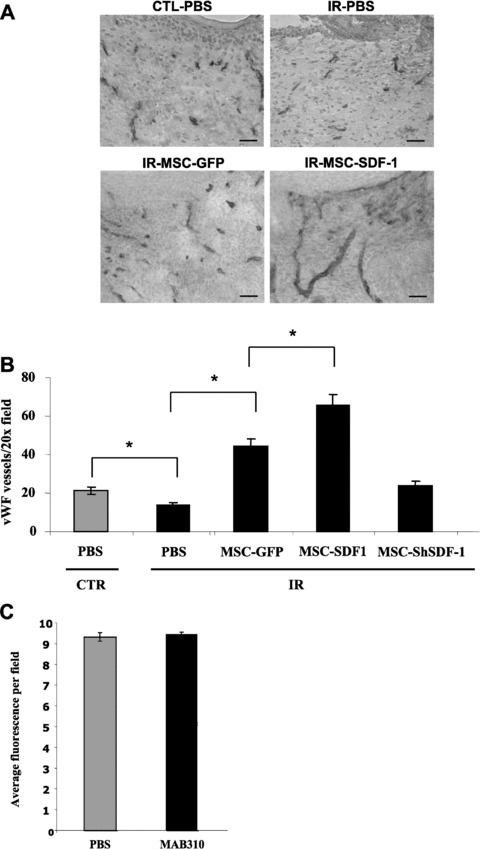
Increased wound vascularization by stromal cell injection. Full-thickness wound biopsies were created on the dorsal surface of mice, and purified populations of MSC-EGFP, MSC-ShSDF-1, MSC-SDF-1 in PBS or PBS alone were injected intradermally the following day (as described in Figs 3 and 4). Wound vascularization density, as determined by vWF stainings, was analysed 15 days after injury. Representative pictures of cryosections counterstained with haematoxylin are shown. Scale bar = 250 μm. (B) Quantification of the number of vWF positive vessels per 20× field for each group. Data are mean ± S.E.M.; (n= 6 to 12 sections from n= 3 to 5 mice; *P < 0.001). (C) Quantification of the proportion of GFP+ bone marrow-derived cells infiltrating the periphery of wounds injected daily with PBS or 40 μg of MAB310 (SDF-1α blocking antibody) at day 7 after injury (shown is the average of three photos per wound, each collected from n= 7 mice per group).
Discussion
Tissue regeneration defects are common, and constitute a major problem in cancer-treated survivors. The molecular origin of these defects remains largely unknown, and thus development of an adapted therapy is essential. Here, we establish that SDF-1α plays a role in wound healing following exposure to IR. SDF-1α expression at the mRNA and protein levels was impaired in irradiated, compared to non-irradiated, wounded skin, in contrast to other cytokines that were induced in both groups (Fig. 2). A deficit in SDF-1α was additionally reported in wounded skin from diabetic mice, confirming an important role of this cytokine in wound healing [31]. The mechanism of IR-mediated prevention of SDF-1α induction up to several months after exposure remains to be determined. One possibility is that cells exposed to IR undergo severe DNA damage, consequently entering a state of senescence [36, 37]. In contrast to apoptotic cells that are rapidly eliminated after damage, senescent cells persist in tissues and may alter the microenvironment for extended periods [38, 39]. Unexpectedly, exposure to a sub-lethal dose of IR (8 Gy) led to the persistent accumulation of damaged cells in several tissues, including skin, for up to at least 4 months after irradiation (Le et al., manuscript in preparation).
A recent study showed that activation of p53, a hallmark of damaged cells, led to attenuation of SDF-1α expression in some mouse and human fibroblast cell lines in vitro[40]. This observation supports the hypothesis that damaged skin cells with constitutively activated p53 have a reduced capacity to produce SDF-1α during the wound response. Additionally, we previously reported that senescent human fibroblasts are impaired in their ability to accumulate the hypoxia inducible factor-1 (HIF-1) under hypoxic conditions in vitro[41]. The SDF-1α promoter contains HIF-1 binding sites, and is regulated by oxygen tension [42]. Therefore, another possibility is that damaged/senescent cells are unable to induce SDF-1α expression in response to hypoxia.
Another reported regulator of SDF-1α is fibroblast growth factor 2 (FGF-2), a cytokine involved in cell growth and other key roles, including wound healing [43, 44]. FGF-2 down-regulates SDF-1α expression in MSCs in a post-transcriptional manner. Interestingly, we observed no differences in FGF-2 expression between control and irradiated skin (Fig. S3). Moreover, we cannot rule out the possibility that the inability of irradiated wounded skin to induce SDF-1α expression can be attributed to a reduction in the number of recruited cells expressing SDF-1α. However, this is unlikely, given the comparable levels of infiltrating leucocytes in irradiated versus non-irradiated wounds (Fig. 1C), and because SDF-1α secretion was shown to occur through residing stromal and endothelial cells [32]. Nonetheless, fewer blood vessels were observed in irradiated than in non-irradiated wounded skin (Fig. 5). Hence, a lower number of residing SDF-1α-producing endothelial cells in irradiated wounded skin may account for the difference in SDF-1α expression.
Next, we showed that delayed healing as a consequence of IR exposure is almost completely rescued by paracrine secretion of SDF-1α from MSCs injected in the wound periphery. MSC injection has been successfully applied as a therapeutic approach to treat skin wound healing in diabetic mice [15, 16]. However, MSC effects on irradiated mice are somewhat surprising, given our initial hypothesis that IR-induced damage would eliminate or permanently impair skin regenerative potential. Under these conditions, it is expected that regeneration will not respond to paracrine factors, but rather depend on the differentiation of an external source of progenitor cells. Our results suggest the skin can preserve his autonomous regenerative potential following IR, assuming in the presence of an appropriate microenvironment (i.e. healthy stromal support). This hypothesis is further supported by our observation that cultured MSCs irradiated in vitro and then injected at the wound margin of a non-irradiated mouse, could delay would closure (Fig. 3A). Surprisingly, while the amount of SDF-1α secreted by irradiated MSCs was not changed, evidence suggest its functionality is reduced following irradiation-induced proteases activity (Carbonneau et al., submitted). Unexpectedly, injection of MSCs was not beneficial in control non-irradiated mice, inconsistent with previous reports [14, 15]. These discrepancies may result from the use of different mouse strains (BalbC versus C57Bl/6) or the method of MSC delivery (i.e. intravenous as opposed to intradermal). Alternatively, we believe the difference in the wound size (10 mm versus 4 mm in our experiments) likely explains this discrepancy based on the fact that bigger wounds heal more slowly and thus are more likely to benefit from cell therapy, especially in a control non-irradiated background. Finally, unintentional variability within experimental conditions such as distance from the wound margin and deepness of MSCs injections may explain why a low proportion of mice did not respond to cell therapy.
Chen and coworkers reported that unidentified factors secreted by MSCs enhance wound healing [25]. SDF-1α is a strong candidate factor, as specific down-regulation of its expression in MSCs severely impairs MSCs capacity to rescue wound closure (Fig. 4). Our results demonstrate that wound beds vascularization is linked to SDF-1α secretion and that increased blood vessel density fosters wound healing. We also found that SDF-1α effect on vascularization is likely independent of its capacity to induce vasculogenesis as SDF-1α inhibition using a blocking antibody did not interfere with bone marrow-derived cells recruitment (Fig. 5C). This suggests that other cytokines secreted at the wound site (i.e. VEGF) may be capable of recruiting cells but less capable at generating full blood vessel formation in absence of sufficient amount of SDF-1α. Moreover, while wound closure efficacy relies, at least in part, on SDF-1α, wound closure does not seem to be exclusively dependent on vascularization. For example, wounds injected with MSC-shSDF-1α display similar levels of blood vessels compared to non-irradiated PBS-injected wounds, but exhibit severely impaired healing. Furthermore, MSC-SDF-1α cells, secreting SDF-1α at a level over 8-fold the basal amount, did not display a superior ability to accelerate wound closure, despite generating more and larger blood vessels than did non-engineered MSCs (Figs 4 and 5). In this case, a threshold level of SDF-1α may be sufficient to assure optimal wound closure, and provision of more SDF-1α does not further improve healing. This theory is supported by our finding that MSC injection does not reduce the healing time of non-irradiated mice displaying normal SDF-1α expression after wounding. Our results indicate that the therapeutic effect of MSC injection is almost exclusively dependent on secretion of SDF-1α, and that wound healing is only partially dependent on increased vascularization. We speculate that the MSC secretome could also accelerate wound closure by exerting a mitogenic effect on residing cells mainly through SDF-1α. Indeed, SDF-1α promotes keratinocyte proliferation in vitro[45]. Another possibility entails that SDF-1α could alter skin fibroblasts contraction, en event that take place in the early phase of the wound response [46]. However, as skin wound healing occurs predominately by contraction in mice and by re-epithelization in human beings, extrapolation on the role of SDF-1α on IR-induced delayed wound healing in cancer-treated patients should be made with great care.
In summary, our findings provide an insight into the underlying mechanisms of the diminished regeneration capability of irradiated skin, and possibly other tissues, up to several months following exposure to IR. Fortunately, this deficiency appears reversible with appropriate stromal support, indicating that MSCs or cytokine-based therapies should be effective in improving wound healing in patients treated for cancer.
Acknowledgments
We thank Dr. F. Rodier for providing MEFs, as well as members of Drs. J. Galipeau, E. Haddad and N. Heveker’s respective laboratories for their critical comments. We also want- to acknowledge D. Carrier and S. Faubert for their expertise in setting up the skin wound model. This work was supported by a grant from the Canadian Institute of Health Research (CIHR, MOP-79317 to C.M.B.). Y.L. and C.B. have been, respectively, supported by a studentship from the Fondation de l’Hôpital Sainte-Justine and by the Health Research Foundation Rx&D (Health Research Foundation)/CIHR (Canadian Institute of Health Research (CIHR)) young investigator award.
Supporting Information
Fig S1. Efficacy of lentivectors expressing differentshRNA targeting SDF-1. Briefly, MSCs were transfected overnightwith shRNA expression vectors (obtained from Open Biosystem) usingLipofectamine 2000 and clones were selected with puromycin (2.5μg/ml). Amount of secreted SDF-1α in conditioned mediumfrom genetically modified MSCs was determined by ELISA. Data areexpressed as means ± S.E.M. of two independent measurements.
Fig. S2. MEFs increase wound healing independently ofSDF-1α. (A) Full-thickness biopsies were created onthe dorsal surface of mice previously exposed to IR or not, and theeffect of intradermal injection of PBS or MEFs (four injectionssites per wound, 1 × 106 cells total) on wound healing wereanalysed. Shown are the proportions of wounds over 90%closed at day 13. (B) Wound vascularization density, asdetermined by vWF stainings, analysed 15 days after injury.Quantification of the number of vWF positive vessels per 20×field for each group is shown. Data are mean ± S.E.M.;(n = 6 sections from n = 3 mice; *P = 0.001).(C) MEFs secrete undetectable level of SDF-1α inconditioned medium compared to MSCs as determined by ELISA. Dataare expressed as means ± S.E.M. of two independent measurements. *Below detection levels.
Fig. S3. Level of FGF-2 is unchanged in irradiated skin.Total RNA was collected from wounds together with surroundingmargins (day 3), and the ratios of FGF-2 RNA relative to 18Sribosomal RNA were determined by quantitative real-time PCR. Shownis the expression levels as detected in mice previously exposed toIR (black bar) compared to control non-irradiated mice (white bar).Data are mean ± S.E.M.; (n = 6).
Table S1 Murine primers for quantitative real-time PCR.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–15. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Bujko K, Suit HD, Springfield DS, Convery K. Wound healing after preoperative radiation for sarcoma of soft tissues. Surg Gynecol Obstet. 1993;176:124–34. [PubMed] [Google Scholar]
- 4.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 5.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 6.Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–47. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai J, Takenaka H, Kusano KF, et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113:2413–24. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 8.Badillo AT, Chung S, Zhang L, et al. Lentiviral gene transfer of SDF-1alpha to wounds improves diabetic wound healing. J Surg Res. 2007;143:35–42. doi: 10.1016/j.jss.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 9.Galotto M, Berisso G, Delfino L, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27:1460–6. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 10.Paterson MC, Sell BM, Smith BP, et al. Impaired colony-forming ability following gamma irradiation of skin fibroblasts from tuberous sclerosis patients. Radiat Res. 1982;90:260–70. [PubMed] [Google Scholar]
- 11.Hill RP, Kaspler P, Griffin AM, et al. Studies of the in vivo radiosensitivity of human skin fibroblasts. Radiother Oncol. 2007;84:75–83. doi: 10.1016/j.radonc.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piersma AH, Brockbank KG, Ploemacher RE, et al. Recovery of hemopoietic stromal progenitor cells after lethal total-body irradiation and bone marrow transplantation in mice. Transplantation. 1985;40:198–201. doi: 10.1097/00007890-198508000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–70. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–7. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 16.Badillo AT, Redden RA, Zhang L, et al. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res. 2007;329:301–11. doi: 10.1007/s00441-007-0417-3. [DOI] [PubMed] [Google Scholar]
- 17.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–57. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 18.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 19.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 20.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–8. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 21.Fan Q, Yee CL, Ohyama M, et al. Bone marrow-derived keratinocytes are not detected in normal skin and only rarely detected in wounded skin in two different murine models. Exp Hematol. 2006;34:672–9. doi: 10.1016/j.exphem.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Borue X, Lee S, Grove J, et al. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol. 2004;165:1767–72. doi: 10.1016/S0002-9440(10)63431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brittan M, Braun KM, Reynolds LE, et al. Bone marrow cells engraft within the epidermis and proliferate in vivo with no evidence of cell fusion. J Pathol. 2005;205:1–13. doi: 10.1002/path.1682. [DOI] [PubMed] [Google Scholar]
- 24.Aghi M, Chiocca EA. Contribution of bone marrow-derived cells to blood vessels in ischemic tissues and tumors. Mol Ther. 2005;12:994–1005. doi: 10.1016/j.ymthe.2005.07.693. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beausejour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–22. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler JM, Guthrie SM, Koc M, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petty JM, Sueblinvong V, Lenox CC, et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–57. doi: 10.4049/jimmunol.178.12.8148. [DOI] [PubMed] [Google Scholar]
- 29.Fierro FA, Brenner S, Oelschlaegel U, et al. Combining SDF-1/CXCR4 antagonism and chemotherapy in relapsed acute myeloid leukemia. Leukemia. 2009;23:393–6. doi: 10.1038/leu.2008.182. [DOI] [PubMed] [Google Scholar]
- 30.Mace KA, Hansen SL, Myers C, et al. HOXA3 induces cell migration in endothelial and epithelial cells promoting angiogenesis and wound repair. J Cell Sci. 2005;118:2567–77. doi: 10.1242/jcs.02399. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–59. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toksoy A, Muller V, Gillitzer R, et al. Biphasic expression of stromal cell-derived factor-1 during human wound healing. Br J Dermatol. 2007;157:1148–54. doi: 10.1111/j.1365-2133.2007.08240.x. [DOI] [PubMed] [Google Scholar]
- 33.Chan JC, Duszczyszyn DA, Castellino FJ, et al. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am J Pathol. 2001;159:1681–8. doi: 10.1016/S0002-9440(10)63015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund LR, Romer J, Bugge TH, et al. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 1999;18:4645–56. doi: 10.1093/emboj/18.17.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madlener M. Differential expression of matrix metalloproteinases and their physiological inhibitors in acute murine skin wounds. Arch Dermatol Res. 1998;290:S24–9. doi: 10.1007/pl00007450. [DOI] [PubMed] [Google Scholar]
- 36.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 37.Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35:7475–84. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aoshiba K, Tsuji T, Nagai A. Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J. 2003;22:436–43. doi: 10.1183/09031936.03.00011903. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Schulte BA, LaRue AC, et al. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–66. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moskovits N, Kalinkovich A, Bar J, et al. p53 Attenuates cancer cell migration and invasion through repression of SDF-1/CXCL12 expression in stromal fibroblasts. Cancer Res. 2006;66:10671–6. doi: 10.1158/0008-5472.CAN-06-2323. [DOI] [PubMed] [Google Scholar]
- 41.Coppe JP, Kauser K, Campisi J, et al. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–74. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 42.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 43.Nakayama T, Mutsuga N, Tosato G. FGF2 posttranscriptionally down-regulates expression of SDF1 in bone marrow stromal cells through FGFR1 IIIc. Blood. 2007;109:1363–72. doi: 10.1182/blood-2006-06-028217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenhalgh DG, Sprugel KH, Murray MJ, et al. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–46. [PMC free article] [PubMed] [Google Scholar]
- 45.Florin L, Maas-Szabowski N, Werner S, et al. Increased keratinocyte proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts. J Cell Sci. 2005;118:1981–9. doi: 10.1242/jcs.02303. [DOI] [PubMed] [Google Scholar]
- 46.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Efficacy of lentivectors expressing differentshRNA targeting SDF-1. Briefly, MSCs were transfected overnightwith shRNA expression vectors (obtained from Open Biosystem) usingLipofectamine 2000 and clones were selected with puromycin (2.5μg/ml). Amount of secreted SDF-1α in conditioned mediumfrom genetically modified MSCs was determined by ELISA. Data areexpressed as means ± S.E.M. of two independent measurements.
Fig. S2. MEFs increase wound healing independently ofSDF-1α. (A) Full-thickness biopsies were created onthe dorsal surface of mice previously exposed to IR or not, and theeffect of intradermal injection of PBS or MEFs (four injectionssites per wound, 1 × 106 cells total) on wound healing wereanalysed. Shown are the proportions of wounds over 90%closed at day 13. (B) Wound vascularization density, asdetermined by vWF stainings, analysed 15 days after injury.Quantification of the number of vWF positive vessels per 20×field for each group is shown. Data are mean ± S.E.M.;(n = 6 sections from n = 3 mice; *P = 0.001).(C) MEFs secrete undetectable level of SDF-1α inconditioned medium compared to MSCs as determined by ELISA. Dataare expressed as means ± S.E.M. of two independent measurements. *Below detection levels.
Fig. S3. Level of FGF-2 is unchanged in irradiated skin.Total RNA was collected from wounds together with surroundingmargins (day 3), and the ratios of FGF-2 RNA relative to 18Sribosomal RNA were determined by quantitative real-time PCR. Shownis the expression levels as detected in mice previously exposed toIR (black bar) compared to control non-irradiated mice (white bar).Data are mean ± S.E.M.; (n = 6).
Table S1 Murine primers for quantitative real-time PCR.


