Abstract
The aim of the present study was to develop and validate a good manufacturing practice (GMP) compliant procedure for the preparation of bone marrow (BM) derived CD133+ cells for cardiovascular repair. Starting from available laboratory protocols to purify CD133+ cells from human cord blood, we implemented these procedures in a GMP facility and applied quality control conditions defining purity, microbiological safety and vitality of CD133+ cells. Validation of CD133+ cells isolation and release process were performed according to a two-step experimental program comprising release quality checking (step 1) as well as ‘proofs of principle’ of their phenotypic integrity and biological function (step 2). This testing program was accomplished using in vitro culture assays and in vivo testing in an immunosuppressed mouse model of hindlimb ischemia. These criteria and procedures were successfully applied to GMP production of CD133+ cells from the BM for an ongoing clinical trial of autologous stem cells administration into patients with ischemic cardiomyopathy. Our results show that GMP implementation of currently available protocols for CD133+ cells selection is feasible and reproducible, and enables the production of cells having a full biological potential according to the most recent quality requirements by European Regulatory Agencies.
Keywords: GMP, validation, CD133, cell therapy, angiogenesis, ischemia
Introduction
The efficacy of stem/progenitor cells clinical application is currently still limited by several knowledge gaps. In fact, the lack of a complete understanding of molecular mechanisms driving progenitor cell differentiation, the lack of well-defined criteria to identify the best cell type for a given pathology and to select patients, still represent major bottlenecks in stem cells-based regenerative medicine. In addition, the absence of validated, safe and reproducible procedures for stem/progenitor cells production and quality testing makes results from diverse clinical trials often incomparable. This makes extremely difficult to raise valid and general conclusions about efficacy of cells to regenerate organs affected by degenerative disorders.
In the cardiovascular field, the use of adult bone marrow cells (BMCs) having angiogenic activity and myocardial regeneration potential has yielded promising results (reviewed in [1, 2]). Several clinical trials have been performed worldwide and, today, more than 1000 patients with acute and chronic cardiac ischemia, or peripheral artery disease, have been treated using autologous BMCs. The results obtained in trials with acute myocardial infarction (MI) or ischemic heart failure patients have been analysed in meta-analysis studies [3–7]. Although based on different study selection criteria, these analyses were in agreement that administration of autologous cells in the heart is safe, and that it causes an improvement, although modest, in primary clinical end-points such as ejection fraction, end-systolic volume and infarct scar size.
A possible explanation for the null or modest effect of BMCs administration in patients may arise from the use of non-selected cellular fractions [8]. In fact, it has been suggested that administration of non-selected BMCs may have an antagonistic, ‘Janus-like’, effect resulting from the secretion of cytokines having a pro-vasculogenic, but also pro-inflammatory and pro-atherogenic effects [9]. Thus, while BMCs injection has been reported to better preserve heart function compared to other progenitor cells types such as mesenchymal stem cells, skeletal myoblasts and fibroblasts [10], the use of selected progenitors may prevent possible adverse consequences such as inflammatory and atherogenic response (discussed in [11]).
Endothelial progenitors cells (EPCs) have been found in the human umbilical cord blood (UCB), peripheral blood and the adult bone marrow (BM) (reviewed in [12]). Recently, two distinct EPCs populations have been identified and characterized, the endothelial colony forming cells (ECFCs) (also named endothelial outgrowing cells, OECs, or ‘late’ EPCs) that do not express the CD45 pan-haematopoietic marker, and CD45+CD133+CD34+-derived EPCs that have been named colony forming unit-endothelial cells (CFU-ECs or ‘early’ EPCs) [13–17]. The two cell types differ in their clonogenic capacity as well as their ability to contribute to neo-vascularization process in vivo; while ECFCs have high colonogenic activity and participate to formation of blood vessels [16, 18], CFU-ECs have modest or null proliferation ability and stimulate blood vessels formation in a paracrine way [18], by secreting an array of pro-angiogenic and pro-inflammatory cytokines [19].
A definitive consensus about the clinical use of one or the other EPC type has not been reached. In fact, despite preclinical studies have highlighted lineage distinctions and different biological properties of CFU-ECs and ECFCs, the CD34 and CD133 antigens remain the reference markers to be used to isolate EPCs for clinical trials, irrespective of their CD45 expression (discussed in [20]). In fact, the benefits of isolated human CD34+ and CD133+ cells administration have been validated in MI [21–25] and hind limb ischemia or cutaneous wounds [26–30] animal models. Furthermore, compared to un-fractionated cells, purified CD34+ and/or CD133+ cells have a higher and dose-dependent angiogenic effect and myocardial regeneration potential [8, 31] and a higher selectivity for myocardial homing after intracoronary administration into patients [32–34] compared to unselected cells. Finally, significant clinical improvements by purified CD133+ cells administration have been reported in relatively small cohorts of patients affected by acute or chronic myocardial ischemia [35–40].
The use of purified stem cell populations in therapy needs a careful evaluation concerning safety, quality and efficacy of cells. To provide an answer to the growing need for improved standardization of cell preparation protocols, the major regulatory agencies worldwide, i.e. the US Food and Drug Administration (FDA) and the European Medicines Agency (EMEA), have established regulatory frameworks to ensure high safety standards and biological quality of cell-based medicinal products (CBMPs), that must comply to good manufacturing practice (GMP) specifications.
In a recent study, F. Seeger and coworkers [41] have shown that slight modifications in preparation protocols and the choice of cell storage media have important consequences on the efficacy of BMCs transplantation into ischemic tissues, by significantly affecting their migratory ability and their in vivo angiogenic potential. This example suggests that definition of clear and standardized procedures to prepare stem cells for cardiovascular cell therapy represents a key issue to obtain optimal clinical results.
The goal of the present study was to develop and test GMP-compliant conditions to obtain purified CD133+ cells (called here CD133 CBMPs) to be used in patients with cardiac or lower limb ischemia. We established standard operating procedures (SOPs) to purify human Cord Blood (CB) CD133+ cells using CliniMACS and applied these procedures to obtain BM-derived CD133 cells for an ongoing clinical trial in patients affected by chronic cardiac ischemia. Here we thus describe a fully GMP-compliant CD133 CBMPs production and quality control process. We also provide a demonstration that manipulated cells maintain a full biological potency using in vitro and in vivo assays.
Materials and methods
Samples
UCB collection was performed after written approval by mothers. The age of neonates ranged between 36 and 42 weeks of gestation. BM samples were obtained from patients undergoing cardiac surgery upon approval of the study by local ethical committee and Italian national regulatory agencies (Istituto Superiore di Sanità, authorization no 63163-PRE.21–869, 30-03-2006).
Isolation of CD133 CBMPs
Cord blood was recovered in ethylenediaminetetraacetic acid containing bags immediately after delivery. Blood samples from at least three different donors were pooled and used in each experiment. UCB mononuclear cell fraction was obtained by Ficoll–Histopaque density gradient. Isolation of CD133+ cells was performed by CliniMACS™ (Miltenyi, Bergisch Gladbach, Germany) system using the direct CD133 isolation kit (Miltenyi, catalogue no. 177–01) according to manufacturer’s instructions. Briefly, after recovery from gradients, mononuclear cells were washed using Dulbecco’s phosphate-buffered saline containing 1% (v/v) human serum albumin (DPBS-HSA). This was followed by two centrifugations at 600 ×g for 10 min. Cells were subsequently resuspended into 100 ml volume with DPBS-HSA containing 7.5 ml of CliniMACS™ CD133 reagent (Miltenyi Biotech) and 1.5 ml of human IgG and incubated for 45 min. at room temperature under gentle agitation. After two washing steps (550 ×g, 10 min.), mononuclear cells were resuspended in 100 ml of DPBS-HSA and thereafter loaded into a column into CliniMACS device, where CD133+ cells were separated and collected. Experiments performed to assess KDR expression in CD133+ cells were performed with cells isolated from small BM aliquots (5–7 ml) of patients undergoing heart surgery using miniMACS™ device (Miltenyi) and the indirect CD133+ cells isolation kit (Miltenyi), according to manufacturer’s instructions.
GMP process and quality control (QC) testing
A detailed description of our GMP program and QC testing of CD133 CBMPs is described in the supplementary online section.
In vitro experiments
CD133 CBMPs transported from the cell factory were stored in different solutions: saline containing 1 mg/ml human albumin, Stem Span (Stem Cells Technologies, Vancouver, Canada) or X-VIVO-15 medium (Lonza, Basel, Switzerland). Shortly after arrival, cells were counted. They were seeded in Stem Span containing a cytokine mixture supplemented with interleukin (IL)-3 and IL-6 (both at 20 ng/ml), Flt3-Ligand and Stem cell factor (both at 100 ng/ml) to allow cell proliferation. In these experiments cells were seeded at 105/well in 96-well plates. After 5 days under these conditions, cells were counted. To assess endothelial differentiation, CD133 CBMPs expanded for 5 days were seeded onto Fibronectin (Sigma-Aldrich, St. Louis, MO, USA)-coated dishes using M199 medium (Gibco, Carlsbad, CA, USA) supplemented with 20% FBS, 100 units/ml penicillin/streptomycin and 2 mM L-Glutamine and cultured for 7 days [42]. After 7 days in these differentiation-promoting conditions, cells were fixed with 4% paraformaldehyde (Sigma) for 20 min. and incubated overnight with 2 μg/ml of dioctadecyl-tetramethylindo-carbocyanine perchlorate (DiI)-labelled acetylated LDL (DiIAcLDL; Biomedical Technologies, Firenze, Italy). After washing with PBS, cells were stained with 40 μg/ml of FITC-labelled Lectin from Ulex europaeus I (Lectin UEA I; Sigma-Aldrich) for 1 hr. Nuclei were stained with Hoechst 33258 (Sigma-Aldrich). For vWF immunofluorescence, cells were fixed with 4% paraformaldehyde and permeabilized using PBS containing 0.2% Triton-X 100 and 1% bovine serum albumin. Anti-human vWF antibody (Chemicon, Billerica, MA, USA) was diluted at 10 μg/ml in the same medium. Cells were incubated with primary antibody overnight at 4°C and, following extensive washing, were incubated with Alexa-488 conjugated anti-rabbit secondary antibody for 1 hr at room temperature. Cells were observed under a Zeiss (Zeiss-Italy, Arese, Italy) Axio Observer.Z1 fluorescence microscope equipped with Apotome image deconvolution hardware. Immunophenotyping by flow cytometry was performed as described in the online Supporting Information.
Cytokine quantification by multiplex analysis
Angiogenic cytokines expression in CFU-ECs obtained from BM-derived CD133 CBMPs was measured by analyzing their concentration (expressed as pg/ml/105 cells) in conditioned medium. M199 containing 20% FBS was conditioned for 24 hrs. The concentration was measured by Bio-Plex Pro-Angiogenesis assay (Bio-Rad-Italy, Segrate, Italy). As a negative control the same, non-conditioned, medium was used.
In vivo experiments
Swiss CD1 male mice, 2 months old (Charles River, Italy), were used in this study. Immunosuppression was performed by injecting cyclosporin-A (Cs-A) at 20 mg/kg weight for 2 days before, and daily after surgery, for the entire period of the experiment. To produce hind limb ischemia, the left femoral artery was excised with an electrocoagulator from its proximal origin as a branch of the external iliac artery till the bifurcation into saphenous and popliteal arteries as described [43]. Mice were anesthetized with an intra-peritoneal injection of 2.5% Avertin (Sigma) (100% Avertin: 10 g 2,2,2-tribromoethyl alcohol in 10 ml tert-amyl alcohol). Injection of CD133 CBMPs (105/animal) resuspended in saline solution (105 cells /50 μl) was performed at the time of surgery; 10 μl of cell containing solution were injected at five levels (from proximal to distal) into the adductor muscle, along the femoral artery site after its removal. Laser Doppler Perfusion Imaging (LDPI) was used to monitor tissue immediately after and at 7 and 14 days after surgery.
Histology and morphometric analysis
Anesthetized mice were perfused with saline solution containing 1000 U/ml heparin (Roche Molecular Biochemical, Monza, Italy) followed by 10% buffered formalin for 10 min. via the left ventricle at 100 mm Hg. Adductor muscles were removed, fixed in formalin for 48 hrs and embedded in paraffin (Bio-plast special; melting point 52–54°C). Sections from each sample were cut at a 3 μm thickness and both capillary number and arteriole number were analysed as described [44, 45].
Statistical analysis
All data are expressed as mean ± S.E.M.; 2-tailed unpaired Student’s t-test was performed with GraphPad statistical software, and a probability value of P < 0.05 was considered statistically significant.
Results
From purification to quality control and certification of CB CD133 CBMPs production procedure
Implementation of currently available laboratory procedures to large scale GMP setting into the cell factory required appropriate validations in order to: (1) establish detailed SOPs, (2) identify and monitor critical steps during the production process and (3) define final product release specifications. In order to develop SOPs for CD133+ cells isolation from human cord blood we used laboratory protocols already established during our previous experience.
Magnetic purification by CliniMACS was chosen as it is performed with clinical grade reagents that are routinely used for purification of haematopoietic stem cells in haematological transplantation settings [46, 47], and because it was safely used in previous studies to produce CD133+ cells for the treatment of patients with cardiac ischemia [35, 38, 40].
The GMP-compliant CD133 CBMPs production process was organized and accomplished according to the scheme presented in Fig. 1. In this scheme, the QC testing actions, the locations of each action and the responsibility of the actions at each step are represented. Table 1 describes the tests, the methods according to European Pharmacopeia and the product specifications that were utilized to proceed with CD133 CBMPs lot certification and release. Specifications were intended as threshold values to be met for lot release. Figure 2 represents a typical example of flow cytometric assessment of CD133 CBMP vitality and purity. To this purpose a hierarchical gating strategy was followed; specifically, cell purity was evaluated by first gating the CD45+ cells and then by determining the percentage of CD45+/PI− living cells, to which was finally applied the appropriate gate to identify CD133+/CD34+ cells. Results obtained by this testing are shown for each CB-derived CD133 CBMPs lot in Table 2. It is to be noted that in all these preparations, threshold values were successfully met. Finally, as shown in Table 3, sterility, endotoxin and mycoplasma tests were negative in all CD133 CBMPs lots preparation. Altogether, these results show high reproducibility of the CD133 CBMPs production process under aseptic conditions.
Fig 1.
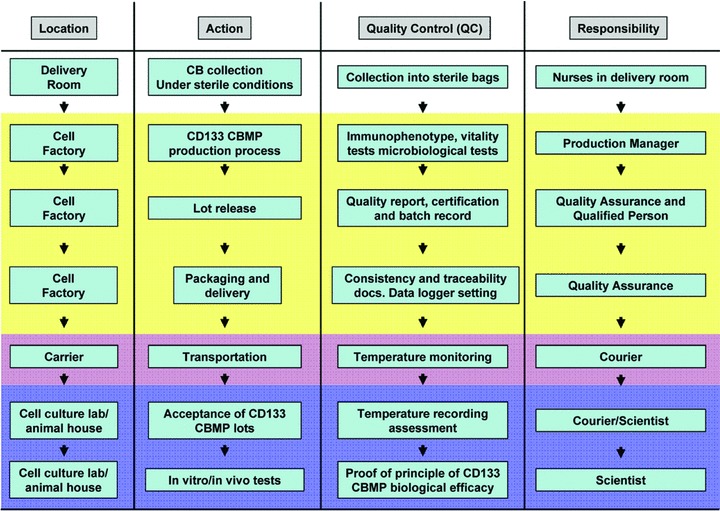
Scheme representing the main actions that were performed during the validation procedure adopted in the present study. The locations, the QC testing steps, and the main responsibilities are shown.
Table 1.
QC testing thresholds adopted for CD133 CBMP production process under GMP conditions
| Test | Method | Specification |
|---|---|---|
| Purity | Flow cytometry (%CD133+ cells) | ≥50% |
| Vitality | Flow cytometry (propidium iodide staining) | ≥70% |
| Number of total nucleated cells | Automated/manual cell counting | ≥106 |
| Sterility | Sterility test (European Pharmacopeia) | Negative |
| Mycoplasma | Cultural test (European Pharmacopeia) | Negative |
| Endotoxin | Limulus amebocyte lysate test (European Pharmacopeia) | <0.5 EU/ml |
Table 2.
Vitality (before and after isolation) of nucleated cells and CD133+ cells. Purity and recovery for each of the 10 CD133 CBMP lots produced under GMP conditions
| Lot # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean ± S.D. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Viability before isolation | 89.76% | 90.00% | 95.00% | 92.00% | 95.90% | 92.76% | 84.80% | 92.30% | 92.00% | 97.18% | 92.17%± 3.53% |
| Viability after isolation | 78.70% | 85.44% | 80.00% | 93.00% | 91.00% | 90.90% | 93.54% | 87.70% | 98.48% | 99.32% | 89.81%± 6.96% |
| Number of nucleated cells recovered from CliniMACS | 4.32 × 106 | 4.40 × 106 | 1.65 × 106 | 5.80 × 106 | 4.20 × 106 | 2.39 × 106 | 11.20 × 106 | 16.32 × 106 | 2.11 × 106 | 2.61 × 106 | 5.50 × 106± 4.69 × 106 |
| CD133+ cells purity | 66.90% | 84.70% | 82.70% | 83.50% | 82.50% | 61.74% | 90.40% | 64.90% | 77.97% | 75.28% | 77.06%± 9.59% |
| CD133+ cells recovery | 67.30% | 90.00% | 58.87% | 48.40% | 58.90% | 28.80% | 90.00% | 71.10% | 65.00% | 80.73% | 65.91%± 18.83% |
Table 3.
Sterility, endotoxin and mycoplasma screening in each of the 10 CD133 CBMP lots produced under GMP conditions
| Lot # | Sterility | Endotoxin | Mycoplasma |
|---|---|---|---|
| 1 | Neg | <0.24 EU | Neg |
| 2 | Neg | <0.24 EU | Neg |
| 3 | Neg | <0.24 EU | Neg |
| 4 | Neg | <0.24 EU | Neg |
| 5 | Neg | <0.24 EU | Neg |
| 6 | Neg | <0.24 EU | Neg |
| 7 | Neg | <0.24 EU | Neg |
| 8 | Neg | <0.24 EU | Neg |
| 9 | Neg | <0.24 EU | Neg |
| 10 | Neg | <0.24 EU | ND |
Proof of principle of CB CD133 CBMPs biological quality maintenance (1): in vitro experiments
A key issue to be addressed in the assessment of CBMPs quality is, according to EMEA guidelines (Guideline on Human Cell-Based Medicinal Products, doc. ref. emea/chmp/410869/2006), the demonstration of ‘proofs of principle’ that CBMPs maintain a full biological potential by appropriate ‘potency’ tests. This ensures that the production process does not modify the ability of stem cells to engraft in recipient tissues and that they maintain the expected and desired regenerative effect.
We thus performed in vitro experiments according the scheme shown in Fig. 3(A). Briefly, the cells were grown in culture with mitogenic cytokines [29] for a period of 5 days, after which they were counted. After counting, cells were plated under differentiation conditions to assess the formation of EPC (CFU-EC) early colonies [15, 42, 48]. According to Seeger et al.[49], the choice of the storage medium represents an important step toward the optimization of cells to be transplanted. In our in vitro assays, we tested different storage media in order to identify the condition that best maintains fully viable and biologically active CD133 CBMPs for 18 hrs, i.e. the time which would elapse between lot release and delivery of the cellular product at the clinical centre.
Fig 3.
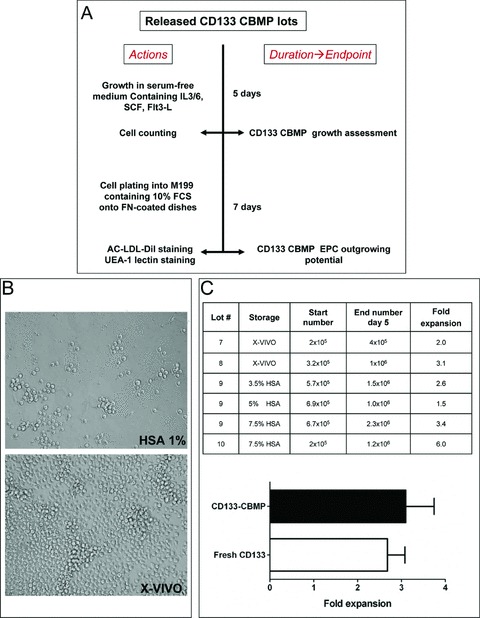
In vitro CB-derived CD133 CBMPs proliferation. (A) shows the experimental scheme that was followed for in vitro testing. After an initial 5 days in proliferation medium, cells were counted to assess growth. (B) shows the appearance of cells after 5 days in proliferation medium. Cells that were stored in 1% HSA containing saline proliferated very poorly, while cells stored in X-VIVO formed large clusters of proliferating cells. (C) shows the results of cell counting at 5 days in proliferation medium. The fold expansion of each cellular lot stored under different storage conditions is shown. The bar graph indicates the comparison between proliferation of GMP produced CD133 CBMP and freshly isolated CD133+ cells.
As a storage medium during initial tests, we used saline solution supplemented with human albumin (HSA) at a 1% concentration (HSA 1%). CD133 CBMPs, stored overnight into HSA 1% were cultured in serum free-medium containing mitogenic cytokines (see ‘Materials and methods’). To our surprise, we found that purified cells stored in this medium failed to proliferate (Fig. 3B), likely due to cell damage during the overnight storage, despite PI exclusion tests, performed before and after the overnight period, did not indicate substantial cell death (not shown). To better preserve CD133 CBMPs vitality, we thus set different storage strategies: we used X-VIVO-15 (X-VIVO) without serum and saline solution containing different amounts of human albumin (HSA 3.5%, HSA 5% and HSA 7.5%). The results (Fig. 3B, C) showed that CD133 CBMPs stored in these media had a proliferation rate that was comparable to that shown by CB-derived freshly isolated CD133+cells under the same culture conditions (Fig. 3C).
Both in animal models [21] and in patients [35, 39], CD133+ cells have been found to have an angiogenic function. As a preliminary in vitro screen for CD133 CBMPs angiogenic activity, we tested formation of CFU-EC colonies by plating pre-expanded CD133 CBMPs (stored into X-VIVO or HSA 7.5%) into fibronectin-coated wells in high serum conditions [15, 42]. The results showed the development of large cellular clusters taking up Ac-LDL-DiI and stained with UEA-1 lectin [48] (Fig. 4), typically resembling CFU-EC colonies [15].
Fig 4.
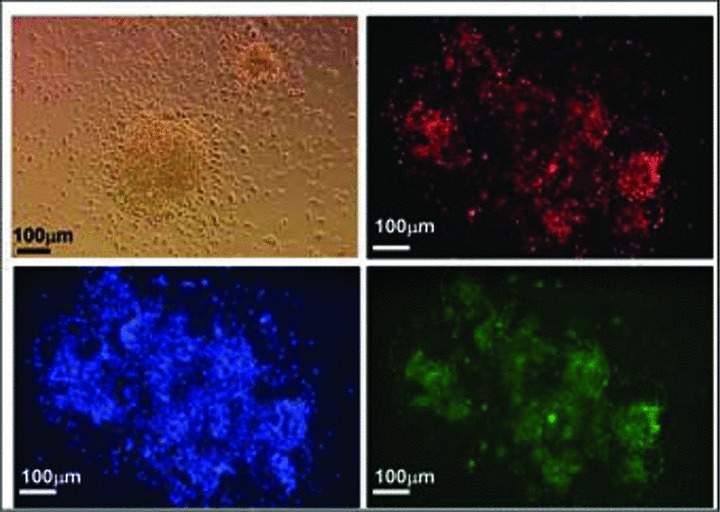
In vitro CD133 CBMPs differentiation. After 5 days in expansion medium, X-VIVO or 7.5% HSA saline solution stored cells were plated onto fibronectin-coated dishes to promote the formation of CFU-EC EPC colonies. (A) shows the morphologic appearance of a CFU-EC cluster and the histochemical detection by fluorescence microscopy of AC-LDL-DiI uptake (red fluorescence), UEA-1-FITC lectin (green fluorescence) staining and nuclear staining (blue fluorescence) by Hoechst 33258.
Proof of principle of CB CD133 CBMP biological quality maintenance (2): Induction of angiogenesis in ischemic limbs
Previous studies have shown the ability of human CD34+ and CD133+ cells to induce myocardial repair following infarction in animal models of MI or injury [21–25]. Additionally, we and others have shown the pro-vasculogenic effect of CD34+ progenitor cells using hind limb ischemia models in mice [26–29]. In the present study the latter approach was used to demonstrate that GMP-produced CD133 CBMPs maintain a pro-vasculogenic potential and contribute to ischemic tissue repair, thus providing direct evidence that GMP production process of these cells preserves their biological activity.
The ability of CD133 CBMPs stored in X-VIVO and 7.5% HSA to improve recovery of limb perfusion was evaluated by LDPI and by capillaries and arterioles density determination. As shown in Fig. 5(A), LDPI imaging showed that in all animals blood flow was drastically reduced immediately after femoral artery dissection. In saline-injected animals, a progressive recovery was detected between day 7 and day 14 after femoral artery dissection. When CD133 CBMPs were injected in the ischemic limbs, a significant increase in perfusion ratio compared to saline-injected mice was observed at the same time-points. Morphometric analysis was performed on adductor muscle sections at day 14 after femoral artery dissection. Both the capillary and the arterioles density (Fig. 5B and C, respectively) were significantly increased by CD133 CBMP injection.
Fig 5.
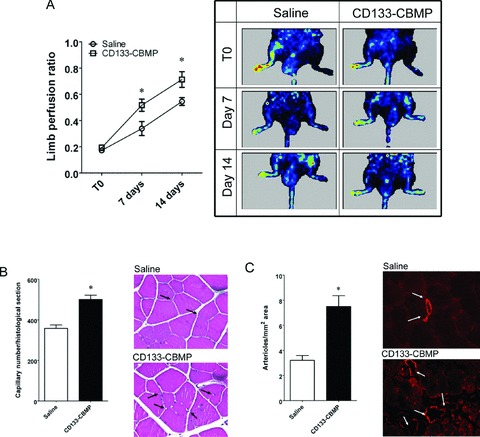
In vivo potency test of CB-derived CD133 CBMPs. (A) shows the increase in perfusion ratio in saline and CD133 CBMP-injected limbs. The LDPI imaging sequences show the increase in perfusion of the same saline-injected or CD133 CBMP-injected mice at the three times considered (T0, 7 and 14 days after removing the femoral artery). Plot shows the quantitative evaluation of the limb perfusion ratio in saline- (n= 8) and CD133 CBMP-(n= 11) injected mice at the three times considered. * indicates P < 0.05 by two ways unpaired t-test. (B) shows the capillary density in ischemic limbs injected with saline solution (n= 13) and CD133 CBMPs (n= 16). Insets show the capillaries in injected adductor muscles. * indicates P < 0.05 by two ways unpaired t-test. (C) shows the increase in the number of arterioles per histological section, as detected by smooth muscle actin staining in the same animals. Insets show the arterioles stained by α-actin antibody. * indicates P < 0.05 by two ways unpaired t-test.
Translation of isolation procedures to BM samples for autologous transplantation in patients affected by myocardial ischemia: release quality control and in vitro proofs of principle
SOPs established to produce CB-derived CD133 CBMPs were translated to production of clinical grade CD133 CBMPs from BM of three patients undergoing a phase II clinical trial of CD133+ cells intramyocardial administration, which has been recently approved by Italian Regulatory Agency. This trial is currently being carried at Centro Cardiologico Monzino.
Translation of GMP procedures to obtain BM-derived CD133 CBMPs from CB was not immediate and required suitable validation runs. In fact, differences in the stem cells sources may cause (1) a different recovery of MNCs after Ficoll gradient centrifugation and (2) a different purity of yielded cells due to differences in the CD133 expression level in BM compared to CB stem cells. To address these issues we calculated the percentage of MNCs loss after CB and BM Ficoll gradient centrifugation. As shown in Table S1, a higher cellular loss was observed using BM compared to CB. The difference in MNCs recovery was not statistically significant and a high variability was observed between different samples. We also calculated the mean fluorescence intensity (MFI) relative to CD133 expression in CB and BM stem cells by flow cytometry; Fig. S1 shows a relatively lower MFI in BM compared to CB stem cells; again comparison of the MFI values did not reveal a statistically significant difference. Finally, to assess whether lower MNCs recovery after Ficoll gradient centrifugation and CD133 expression affect the quality of the final products, we compared CB and BM CD133 CBMPs release quality parameters such as purity of CD133+ cells (mean ± S.D.: 73.52%± 20.22%versus 77.06%± 9.59%, BM versus CB; P > 0.05, Student’s t-test), recovery of nucleated cells after cliniMACS™ (mean ± S.D.: 5.70 × 106± 3.18 × 106 cells versus 5.50 × 106± 4.69 × 106 cells BM versus CB; P > 0.05, Student’s t-test) and post-isolation vitality (mean ± S.D.: 98.77%± 1.02%versus 89.91%± 6.96%, BM versus CB; P > 0.05, Student’s t-test). All these comparisons did not reveal statistically significant differences (see also Fig. 6, Tables 2 and 4), showing that translation of SOPs developed for CB CD133+ cells to GMP production of BM-derived CD133 CBMPs produced similar results to those obtained using CB.
Fig 6.
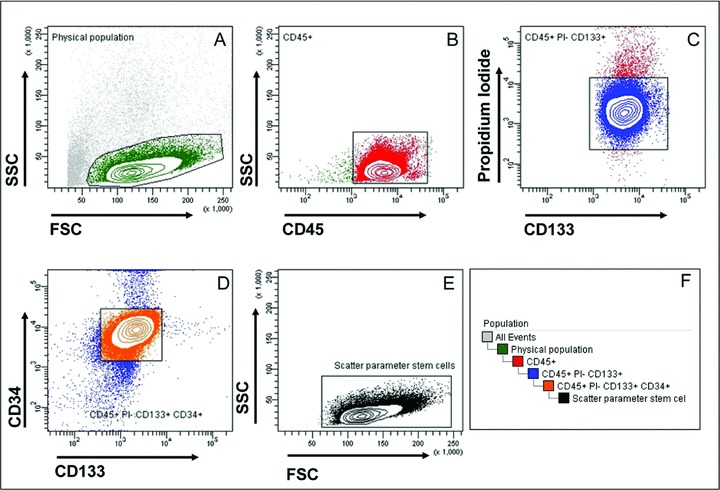
Example of vitality and phenotype testing of CD133 CBMP from BM of one patient affected by chronic ischemic disease using four colour analysis by flow cytometry. These cells were injected into the ischemic heart of the same patient. Gating hierarchy and panels description is as in Fig. 2.
Table 4.
Translation of production procedures from CB to BM from three patients. QC testing showing the compliance to thresholds established during step 1 validation procedure using CB
| Test | Method | Specification | Result | (Mean ± S.D.) | ||
|---|---|---|---|---|---|---|
| Patient #1 | Patient #2 | Patient #3 | ||||
| Purity | Flow cytometry (% of CD133+) | ≥50% | 51.31% | 90.87% | 78.38% | 73.52 ± 20.22 |
| Viability after isolation | Flow cytometry (propidium iodide staining) | ≥70% | 98.37% | 99.93% | 98.02% | 98.77 ± 1.02 |
| Number of nucleated cells recovered from CliniMACS | Automated/manual cell counting | ≥106 | 4.46 × 106 | 9.31 × 106 | 3.32 × 106 | 5.70 × 106± 3.18 × 106 |
| Sterility | Sterility test (European Pharmacopeia) | Negative | Negative | Negative | Negative | - |
| Mycoplasma | Cultural test (European Pharmacopeia) | Negative | Negative | Negative | Negative | - |
| Endotoxin | Limulus amebocyte lysate test (European Pharmacopeia) | <0.5 EU/ml | <0.24 EU/ml | <0.24 EU/ml | <0.24 EU/ml | <0.24 EU/ml |
Fig 2.

Example of vitality and phenotype testing of CD133 CBMP using four colour analysis by flow cytometry. (A) shows the initial gating that was established on the scatter plot to identify cells having lymphocyte-like dimensions and complexity within the cellular population eluted from CliniMACS. (B) shows the CD45 expression in cells gated on the basis of physical parameters in (A). This gating allowed to identify CD45+ cells in this population. (C) shows propidium iodide (PI) staining of the cells gated on the basis of CD45 expression. This gate allowed to discriminate between living (PI−) and dead cells (PI+) CD45+ cells. (D) shows the expression of CD34 and CD133 in CD45+ PI− cells. This analysis shows that the majority of GMP-produced CD133+ cells shared the expression of the other stem cell marker CD34+. (E) shows the physical paramenter of living CD45+/CD133+/CD34+ cells isolated during the GMP production procedure. (F) illustrates the hierarchical gating strategy that was adopted in flow cytometry to hierarchically identify living CD45+/CD133+/CD34+ cells obtained by GMP-compliant selection procedure.
A second step was to provide the suitable proofs of principle that BM-derived CD133 CBMPs have a similar phenotype to that of CB-CD133+ cells. As an initial biological quality control test, patients-derived CD133+ cells first tested for the expression of KDR antigen, an important marker defining progenitors having an in vivo angiogenic activity [23, 28] (Fig. 7). The biological quality of BM CD133 CBMPs was then functionally assessed by in vitro tests that were performed according to the procedure established for CB-derived CD133+ shown in Fig. 3(A). In these experiments it was found that, analogous to CB, BM-derived CD133 CBMPs formed CFU-EC colonies (not shown). The endothelial-like phenotype of cells composing these colonies was confirmed by detection of the endothelial marker vWF by immunofluorescence (Fig. 8A) and by multicolour flow cytometry analysis, which revealed that these cells were CD45+ and expressed, although at a lesser extent, CD31+ and CD105+ endothelial cells markers (Fig. 8B and C). By contrast, the expression of CD144 and CD146 antigens, that are expressed in terminally differentiated endothelial cells, was expressed in a minority of cells (Fig. 8B and C). Cells were also assayed for expression of monocyte marker CD14, the pre-B/pre-T antigen CD48 and CD133 and KDR stem cell markers (Fig. S2). Finally, to functionally characterize the putative pro-angiogenic activity of BM-derived CD133 CBMPs, we tested the expression of angiogenic cytokines in CFU-EC conditioned media by multiplex analysis. Results showed that these cells expressed HGF, IL-8, PDGF-BB and VEGF cytokines (Fig. 8D).
Fig 7.
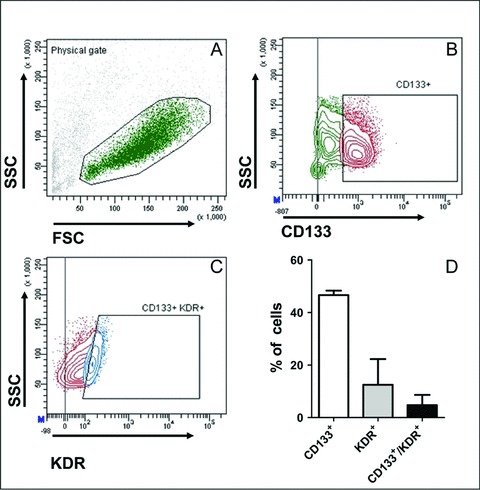
Expression of VEGFR-2/KDR receptor in BM-derived CD133 obtained from patients. (A) and (B) show the cellular population that was gated in this analysis and its CD133 expression. (C) shows KDR expression in the CD133+ cells population gated in (C). (D) shows the markers quantification in three independent BM cells preparations. In (B) and (C), gating was established on the basis of negative staining in the presence of isotype control antibodies conjugated to the respective fluorochromes.
Fig 8.
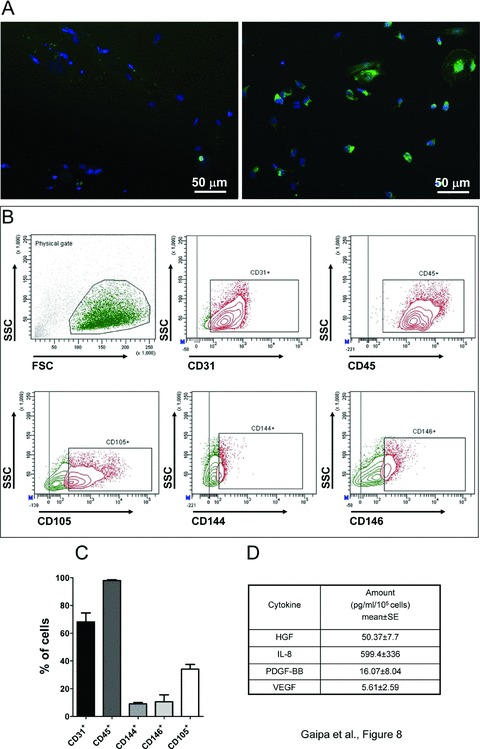
In vitro testing of BM-derived CD133 CBMP biological potency. (A) shows immunofluorescence analysis of human vWF in CFU-ECs obtained from CD133 CBMPs. Left panel shows the control staining that was performed omitting the incubation with the primary antibody, while right panel shows the staining performed in the presence of primary and secondary antibody. (B) shows the expression of endothelial cell-specific markers in BM CD133 CBMPs-derived CFU-ECs by flow cytometry. Each panel indicates in the boxed areas the specific staining for each marker. Gating to calculate percentage of marker positive cells was calculated on negative control staining with isotype antibodies conjugated with the respective fluorochromes. The population that was considered in the marker analysis is indicated as ‘physical gate’ in the upper left panel. (C) shows the quantification of specific expression of each endothelial marker expressed in CFU-ECs. Results in the bar graph are the mean ± SE of three independent experiments. (D) shows quantification of angiogenic cytokines produced in the CFU-EC culture medium in a conditioning period of 24 hrs. The expression of cytokines was determined by Multiplex assay.
Discussion
Therapeutic use of EPCs has been suggested as a promising approach to induce neo-vascularization in ischemic diseases [50]. Despite a large number of preclinical reports describing marked EPCs beneficial effect on ischemic tissues perfusion, data obtained from meta-analyses of clinical trials have provided evidence of only a modest clinical benefit [4–7, 51]. So far, the reason for such a modest increase in heart function following stem/progenitor cells administration is not clear. In fact, it is still a matter of debate whether it may be related to a decreased biological function of cells obtained from patients with CVD risk factors (diabetes, hypercholesterolemia, hypertension) and/or to a low survival/engraftment of the cells due to a hostile microenvironment such as that present into the ischemic myocardium (discussed in [1]).
Different possible strategies to overcome the limited biological function of EPCs from individuals at risk have been suggested. A first option is provided by the possibility to use ex vivo manipulation strategies such as culture in the presence of factors that enhance ability of EPCs to migrate, proliferate/survive and adhere [52–54] (also discussed in [41, 55, 56]). A second possibility, not necessarily alternative to the previous, is the use of strategies that maximize CBMPs reproducibility, safety and efficacy [49].
From GMP compliance to proofs of principle: ‘release’ and ‘potency’ validation levels for standardization of CBMPs production process for cardiovascular therapy
Today, GMP compliance requirement is one of the major tasks in translational application of stem cells in cardiovascular disease. A central component of GMP is the establishment of standard operational procedures for clinical grade manufacturing of cells, ranging from the systematic checking and traceability of materials to microbiological and efficacy validation of final products. Guidelines for GMP application in stem cell therapy have been established by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The European Community has adopted these guidelines to introduce GMP regulations in the EU Member States. According to the Directive 2006/86/EC ‘validation means establishing documented evidence that provides a high degree of assurance that a specific process, piece of equipment or environment will consistently produce a product meeting its predetermined specifications and quality attributes; a process is validated to evaluate the performance of a system with regard to its effectiveness based on intended use’.
From these considerations CBMPs certification for use in ischemic patients essentially requires a two-step validation program (Fig. 9).
Fig 9.
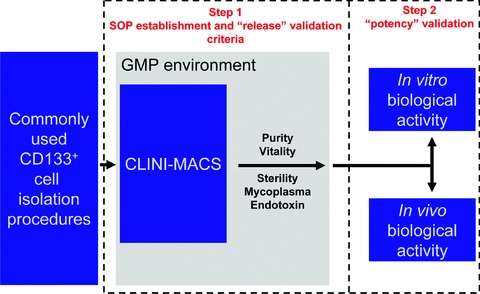
The two-step validation program necessary for the testing of the CD133 CBMP production process adopted in the present study. The scheme illustrates the activities that were accomplished to achieve the certification of ‘clinical grade’ CD133 CBMP for cardiovascular use. During step 1 definition of SOPs was performed followed by translation into the GMP environment based on available laboratory protocols and experience. During this step, SOPs refinement was performed in cell factory. This activity enabled us to reach sufficient reproducibility of the production process by CLINI-MACS (purity and vitality of CD133+ cells) and fulfil the pharmacopeia requirements (sterility, absence of mycoplasma and endotoxin contamination) to proceed with lot release. In the second step, the proof of principle of CD133 CBMP product potency was demonstrated by suitable in vitro/in vivo experiments.
Step 1. During a preliminary step of GMP compliant CBMP preparation procedure setup, tests are performed in order to check for sterility and absence of mycoplasma and endotoxin contamination and to set CBMPs purity/vitality thresholds. The execution of these experiments enabled us to establish criteria to ensure the reproducibility of the purification procedure based on the CliniMACS device (purity and vitality) and the safety of manipulation procedures in sterility controls (mycoplasma, endotoxin and sterility tests), to establish quality thresholds for the production process of CD133 CBMPs as well as the lot-to-lot consistency. They also led us to the important result of defining media (X-VIVO-15 or 7.5% HSA containing saline) for an optimal storage of the cells.
Step 2. A second but not less important validation step is the assessment of CBMPs biological properties. These studies demonstrate the CBMP potency (biological quality) in a suitable model in vitro or in vivo. The demonstration provided by step 2 is necessary to demonstrate that stem cells produced according to the GMP-compliant procedure maintain the intended biological activity which should be related to the expected clinical effect. While step 1 validation tests are necessary at every CBMP lot release, potency validation tests should not to be repeated. In fact, once validated and suitably performed without amendments, the procedure used for CBMPs preparation is recognized itself as a compliant procedure to meet requirements needed for CBMP human use. The experiments performed during this second phase in the present study showed that the isolation procedure followed by storage and transport under optimal conditions, allowed to maintain a biological potency of CB CD133+ cells similar to that supposed for native cells (angiogenic potential) anticipated in several preclinical studies.
Release and potency validation steps of BM CD133 CBMPs for cardiovascular repair
The use of CD133 CBMPs in patients affected by myocardial ischemia required translation to BM samples of isolation procedures set using CB. Validation of procedures translated from CB to BM firstly required appropriate testing in order to ensure that product specification and minimal threshold parameters established during CB isolation runs were met. This testing led us to establish minimal starting quality thresholds (Table 5) such as (i) a vitality higher than 90%, (ii) a starting number of mononuclear cells higher than 5 × 109, (iii) a total number of CD133+ cells higher than 15 × 106 and (iv) execution of CD133+ cells purification process within 24 hrs after BM collection.
Table 5.
Range of vitality, total number of nucleated cells, total number of CD133+ cells and time delay from collection to GMP processing, as recorded during validation runs using CB (n= 10) and BM (n= 3) in our study. These parameters are the basis to establish suggested minimal thresholds that we advice to take into account to ensure feasibility of BM-derived CD133 CBMP GMP production process
| Test | CB (our study) n= 10 | BM (our study) n= 3 | Suggested parameter for BM |
|---|---|---|---|
| Cell vitality (range) | 84– 96% | 93– 96% | >90% |
| Number of total nucleated cells (range) | 1.0 – 3.4 × 109 | 5.1 – 5.9 × 109 | >5 × 109 |
| Number of total CD133+ cells (range) | 2.9 – 16.7 × 106 | 28.0 – 71.0 × 106 | >15 × 106 |
| Time delay from collection to processing | ≤48 hrs | ≤24 hrs | ≤24 hrs |
We then considered possible major deviations that may arise from the different cellular composition of BM compared to CB and possible differences in the expression levels of the CD133 stem cell marker, which may affect recovery and purity of the final cellular product. Comparison of MNCs loss after Ficoll centrifugation and the analysis of MFI relative to CD133 expression in CB and BM cells did not reveal statistically significant differences even if, as shown in Fig. S1 and Table S1, for both parameters we noticed a possible deviation that might reach statistically significance by increasing the experimental replicates. On the other hand, it has to be noted that purity, number of MNCs recovered from cliniMACS™ and vitality of CD133+ cells did not show the same trend, suggesting that translation of isolation procedures from CB to BM was feasible with a comparable efficiency. In this respect, the adoption of improved strategies to minimize cellular loss during Ficoll centrifugation is under study.
A final step required to assess the feasibility of BM-derived CD133 CBMPs GMP production was to demonstrate the biological potency of these cells by providing suitable proofs of principle. Due to the limited amounts of BM that we could use for this purpose, we performed only in vitro tests aimed at demonstrating the phenotype and the potential pro-angiogenic function of these cells. These tests showed the endothelial-like phenotype of CFU-ECs grown from these cells in culture and, in line with existing literature, the mixed endothelial/monocytic/ myeloid phenotype of these cells [15, 16, 57–61]. Furthermore, these cells produced, although at different levels, hepatocyte growth factor (HGF), IL-8, platelet-derived growth factor-BB (PDGF-BB) and vascular endothelial growth factor (VEGF) cytokines. Altogether, these data suggest that BM-derived CD133 CBMPs possess a pro-angiogenic paracrine activity.
In summary, our work shows that GMP implementation of currently available protocols to produce CD133+ stem cells for cardiovascular repair is feasible and that it maintains the innate angiogenic properties of these cells. This work represents the first example of protocol standardization that goes in the still unexplored direction of optimizing quality and enhancing reproducibility of cellular products application into patients affected by ischemic heart disease.
Acknowledgments
This work has been in part supported by EU FP6 funded project ‘Ulcer Therapy’, contract no. LSHB-CT-2005–512102) issued to M.C.C. and M.P., by EU funded project FP6 ‘Thercord’, contract no. LSHB-CT-2005–018817) to M.P., by Regione Lombardia (Decreto n 7917 del 10.07.2006) to A.B. and E.B., and by Comitato Stefano Verri. G.G. and A.B. are supported by Fondazione M. Tettamanti. The authors thank Dr. Francesca Prandi and Dr. Paolo Devanna for help in preparation of BM-derived CD133+ cells for KDR expression assessment, and Dr. Benedetta Cabiati for her support in the GMP quality control activities.
Supporting Information
Comparison between the MNC loss using CB and BM samples. Unpaired t-test was used to calculate statistical significance.
Fig. S1 Comparison between the CD133 MFI in CB- andBM-derived CD133 CBMP cells, as evaluated by flow cytometry.(A) shows the histogram plots of CD133 fluorescence spectrumin CB-derived (upper panel) and BM-derived (lower panel) CD133CBMPs. Dotted lines indicate the putative mean fluorescence in thetwo histograms. (B) shows quantification of CD133 MFI in CB(n ∇ 10) and BM (n ∇ 3) CD133 CBMPs. Itis evident the lower MFI in BM-derived CD133 CBMPs. The differencedid not reach statistical significance (unpaired Student’st-test; P ∇ 0.06; n ∇ 10 for CB andn ∇ 3 for BM).
Fig. S2 Expression of myeloid (CD14), pre-B/Pre-T cells (CD48), and endothelial progenitor cells (CD133, KDR) markers in CFU-ECs obtained from CD133 CBMPs in culture as assessed by flow cytometry analysis.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 2.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–9. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 3.Kajiguchi M, Kondo T, Izawa H, et al. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J. 2007;71:196–201. doi: 10.1253/circj.71.196. [DOI] [PubMed] [Google Scholar]
- 4.Kang S, Yang YJ, Li CJ, et al. Effects of intracoronary autologous bone marrow cells on left ventricular function in acute myocardial infarction: a systematic review and meta-analysis for randomized controlled trials. Coron Artery Dis. 2008;19:327–35. doi: 10.1097/MCA.0b013e328300dbd3. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Rendon E, Brunskill SJ, Hyde CJ, et al. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–18. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Rendon E, Brunskill S, Doree C, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2008:CD006536. doi: 10.1002/14651858.CD006536.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto A, Iwasaki H, Kusano K, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–9. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 9.Epstein SE, Stabile E, Kinnaird T, et al. Janus phenomenon: the interrelated tradeoffs inherent in therapies designed to enhance collateral formation and those designed to inhibit atherogenesis. Circulation. 2004;109:2826–31. doi: 10.1161/01.CIR.0000132468.82942.F5. [DOI] [PubMed] [Google Scholar]
- 10.van der Bogt KE, Sheikh AY, Schrepfer S, et al. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–9. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekiguchi H, Ii M, Losordo DW. The relative potency and safety of endothelial progenitor cells and unselected mononuclear cells for recovery from myocardial infarction and ischemia. J Cell Physiol. 2009;219:235–42. doi: 10.1002/jcp.21672. [DOI] [PubMed] [Google Scholar]
- 12.Liew A, Barry F, O’Brien T. Endothelial progenitor cells: diagnostic and therapeutic considerations. Bioessays. 2006;28:261–70. doi: 10.1002/bies.20372. [DOI] [PubMed] [Google Scholar]
- 13.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–31. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 14.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 15.Prater DN, Case J, Ingram DA. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–9. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 16.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 17.Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572–9. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 18.Sieveking DP, Buckle A, Celermajer DS, et al. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–8. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Ingram DA, Murphy MP, et al. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2009;296:H1675–82. doi: 10.1152/ajpheart.00665.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmermans F, Plum J, Yoder MC, et al. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2008;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma N, Ladilov Y, Moebius JM, et al. Intramyocardial delivery of human CD133+ cells in a SCID mouse cryoinjury model: bone marrow vs. cord blood-derived cells. Cardiovasc Res. 2006;71:158–69. doi: 10.1016/j.cardiores.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Agbulut O, Vandervelde S, Al Attar N, et al. Comparison of human skeletal myoblasts and bone marrow-derived CD133+ progenitors for the repair of infarcted myocardium. J Am Coll Cardiol. 2004;44:458–63. doi: 10.1016/j.jacc.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 23.Botta R, Gao E, Stassi G, et al. Heart infarct in NOD-SCID mice: therapeutic vasculogenesis by transplantation of human CD34+ cells and low dose CD34+KDR+ cells. FASEB J. 2004;18:1392–4. doi: 10.1096/fj.03-0879fje. [DOI] [PubMed] [Google Scholar]
- 24.Leor J, Guetta E, Feinberg MS, et al. Human umbilical cord blood-derived CD133+ cells enhance function and repair of the infarcted myocardium. Stem Cells. 2006;24:772–80. doi: 10.1634/stemcells.2005-0212. [DOI] [PubMed] [Google Scholar]
- 25.Yeh ET, Zhang S, Wu HD, et al. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–3. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 26.Schatteman GC, Hanlon HD, Jiao C, et al. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000;106:571–8. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awad O, Dedkov EI, Jiao C, et al. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol. 2006;26:758–64. doi: 10.1161/01.ATV.0000203513.29227.6f. [DOI] [PubMed] [Google Scholar]
- 28.Madeddu P, Emanueli C, Pelosi E, et al. Transplantation of low dose CD34+KDR+ cells promotes vascular and muscular regeneration in ischemic limbs. FASEB J. 2004;18:1737–9. doi: 10.1096/fj.04-2192fje. [DOI] [PubMed] [Google Scholar]
- 29.Pesce M, Orlandi A, Iachininoto MG, et al. Myoendothelial differentiation of human umbilical cord blood-derived stem cells in ischemic limb tissues. Circ Res. 2003;93:e51–62. doi: 10.1161/01.RES.0000090624.04507.45. [DOI] [PubMed] [Google Scholar]
- 30.Sivan-Loukianova E, Awad OA, Stepanovic V, et al. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–77. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–25. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 32.Goussetis E, Manginas A, Koutelou M, et al. Intracoronary infusion of CD133+ and CD133-CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells. 2006;24:2279–83. doi: 10.1634/stemcells.2005-0589. [DOI] [PubMed] [Google Scholar]
- 33.Schots R, De Keulenaer G, Schoors D, et al. Evidence that intracoronary-injected CD133+ peripheral blood progenitor cells home to the myocardium in chronic postinfarction heart failure. Exp Hematol. 2007;35:1884–90. doi: 10.1016/j.exphem.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 35.Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112:I178–83. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 36.Boyle AJ, Whitbourn R, Schlicht S, et al. Intra-coronary high-dose CD34+ stem cells in patients with chronic ischemic heart disease: a 12-month follow-up. Int J Cardiol. 2006;109:21–7. doi: 10.1016/j.ijcard.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–72. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 38.Pompilio G, Steinhoff G, Liebold A, et al. Direct minimally invasive intramyocardial injection of bone marrow-derived AC133+ stem cells in patients with refractory ischemia: preliminary results. Thorac Cardiovasc Surg. 2008;56:71–6. doi: 10.1055/s-2007-989351. [DOI] [PubMed] [Google Scholar]
- 39.Stamm C, Westphal B, Kleine HD, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–6. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 40.Stamm C, Kleine HD, Choi YH, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–25. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 41.Seeger FH, Zeiher AM, Dimmeler S. Cell-enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med. 2007;4:S110–3. doi: 10.1038/ncpcardio0734. [DOI] [PubMed] [Google Scholar]
- 42.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 43.Couffinhal T, Silver M, Zheng LP, et al. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–79. [PMC free article] [PubMed] [Google Scholar]
- 44.Emanueli C, Salis MB, Pinna A, et al. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–62. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- 45.Zaccagnini G, Martelli F, Fasanaro P, et al. p66ShcA modulates tissue response to hindlimb ischemia. Circulation. 2004;109:2917–23. doi: 10.1161/01.CIR.0000129309.58874.0F. [DOI] [PubMed] [Google Scholar]
- 46.Perseghin P, Gaipa G, Dassi M, et al. CD34+ stem cell recovery after positive selection of “overloaded” immunomagnetic columns. Stem Cells Dev. 2005;14:740–3. doi: 10.1089/scd.2005.14.740. [DOI] [PubMed] [Google Scholar]
- 47.Gaipa G, Dassi M, Perseghin P, et al. Allogeneic bone marrow stem cell transplantation following CD34+ immunomagnetic enrichment in patients with inherited metabolic storage diseases. Bone Marrow Transplant. 2003;31:857–60. doi: 10.1038/sj.bmt.1704024. [DOI] [PubMed] [Google Scholar]
- 48.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 49.Seeger FH, Tonn T, Krzossok N, et al. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–72. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 50.Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc Med. 2008;18:33–7. doi: 10.1016/j.tcm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lipinski MJ, Biondi-Zoccai GG, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–7. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 52.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zemani F, Silvestre JS, Fauvel-Lafeve F, et al. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644–50. doi: 10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 54.Carmona G, Chavakis E, Koehl U, et al. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood. 2008;111:2640–6. doi: 10.1182/blood-2007-04-086231. [DOI] [PubMed] [Google Scholar]
- 55.Yoon CH, Hur J, Park KW, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–27. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 56.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514–22. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Rohde E, Malischnik C, Thaler D, et al. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–67. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 58.Rohde E, Bartmann C, Schallmoser K, et al. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–52. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 59.Urbich C, Heeschen C, Aicher A, et al. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–6. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 60.Romagnani P, Annunziato F, Liotta F, et al. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–22. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 61.Hur J, Yang HM, Yoon CH, et al. Identification of a novel role of T cells in postnatal vasculogenesis: characterization of endothelial progenitor cell colonies. Circulation. 2007;116:1671–82. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison between the MNC loss using CB and BM samples. Unpaired t-test was used to calculate statistical significance.
Fig. S1 Comparison between the CD133 MFI in CB- andBM-derived CD133 CBMP cells, as evaluated by flow cytometry.(A) shows the histogram plots of CD133 fluorescence spectrumin CB-derived (upper panel) and BM-derived (lower panel) CD133CBMPs. Dotted lines indicate the putative mean fluorescence in thetwo histograms. (B) shows quantification of CD133 MFI in CB(n ∇ 10) and BM (n ∇ 3) CD133 CBMPs. Itis evident the lower MFI in BM-derived CD133 CBMPs. The differencedid not reach statistical significance (unpaired Student’st-test; P ∇ 0.06; n ∇ 10 for CB andn ∇ 3 for BM).
Fig. S2 Expression of myeloid (CD14), pre-B/Pre-T cells (CD48), and endothelial progenitor cells (CD133, KDR) markers in CFU-ECs obtained from CD133 CBMPs in culture as assessed by flow cytometry analysis.


