Abstract
Impaired adipogenesis has been shown to predispose to disturbed adipocyte function and development of metabolic abnormalities. Previous studies indicate that polyamines are essential in the adipogenesis in 3T3-L1 fibroblasts. However, the specific roles of individual polyamines during adipogenesis have remained ambiguous as the natural polyamines are readily interconvertible inside the cells. Here, we have defined the roles of spermidine and spermine in adipogenesis of 3T3-L1 cells by using (S’)- and (R’)- isomers of α-methylspermidine and (S,S’)-, (R,S)- and (R,R’)-diastereomers of α,ω-bismethylspermine. Polyamine depletion caused by α-difluoromethylornithine (DFMO), an irreversible inhibitor of ornithine decarboxylase, prevented adipocyte differentiation by suppressing the expression of its key regulators, peroxisome proliferator-activated receptor γ and CCAAT/enhancer binding protein α. Adipogenesis was restored by supplementation of methylspermidine isomers but not of bismethylspermine diastereomers. Although both spermidine analogues supported adipocyte differentiation only (S)-methylspermidine was able to fully support cell growth after extended treatment with α-DFMO. The distinction between the spermidine analogues in maintaining growth was found to be in their different capability to maintain functional hypusine synthesis. However, the differential ability of spermidine analogues to support hypusine synthesis did not correlate with their ability to support differentiation. Our results show that spermidine, but not spermine, is essential for adipogenesis and that the requirement of spermidine for adipogenesis is not strictly associated with hypusine modification. The involvement of polyamines in the regulation of adipogenesis may offer a potential application for the treatment of dysfunctional adipocytes in patients with obesity and metabolic syndrome.
Keywords: adipogenesis, fibroblast, polyamine, hypusine
Introduction
Committed preadipocytes from mesenchymal stem cells differentiate into mature adipocytes during adipogenesis [1]. In human beings, formation of adipocytes begins during embryogenesis, their number and size are substantially increased rapidly after birth and contrary to the popular belief, adipogenesis occurs also in adult adipose tissue throughout the life. The regulation of adipogenesis is crucial to maintain normal metabolic functions of the body because both severely reduced adipose tissue, lipodystrophy and an excess of adipose tissue, obesity, lead to profound metabolic dysfunctions like insulin resistance [2].
Adipogenesis is a complex process that involves coordinated expression and activation of two main groups of transcription factors, peroxisome proliferator-activated receptor γ (PPAR-γ)2 and CCAAT/enhancer binding protein (C/EBP) family [3]. These transcription factors activate other transcription factors and several genes needed in adipocyte maturation. As adipocytes differentiate, they acquire a spherical and monolocular shape, insulin-responsive glucose uptake, a coordinated control of lipid storage and utilization and a capability to synthesize and secrete adipokines. Typical marker genes for mature adipocytes are, e.g. glucose transporter 4 (Glut 4), fatty acid synthase (FAS) and hormone-sensitive lipase (HSL) [4]. Glut 4 is the main insulin-regulated glucose transporter isoform in mature adipocytes, FAS catalyses one of the critical steps in triglyceride synthesis and HSL functions as the rate-limiting enzyme in lipolysis by mediating the hydrolysis of stored triacylglycerol to diacylglycerol.
The naturally occurring polyamines, spermidine and spermine and their precursor putrescine are required for normal cell growth and differentiation. However, the role of polyamines and the mechanisms by which they are involved in cell differentiation depends on the cell type. In several cell types (e.g. keratinocytes and chondrocytes), polyamines induce cell differentiation and maturation. In other cell types, as in many tumour cells, differentiation process is inhibited by polyamines [5]. It has been reported that polyamines are essential during adipogenesis of 3T3-L1 cells and increased cellular levels of spermidine are detected during adipogenesis [6, 7]. In addition, depletion of cellular polyamines by treatment with a inhibitor of polyamine biosynthesis, α-difluoromethylornithine (DFMO), has been noticed to prevent adipogenesis in 3T3-L1 by unknown mechanism and this inhibitory effect of DFMO is reverted by supplementation of putrescine or spermidine. However, specific roles of individual polyamines in adipogenesis have remained ambiguous as the natural polyamines are quickly metabolized further by the cells.
We have recently synthesized stable α-methylated polyamine analogues as single isomers and enantiomers: (S)- and (R)- isomers of methylspermidine (MeSpd) and (S,S)- and (R,R)-diastereomers of α,ω-bismethylspermine (Me2Spm). They are not N-acetylated by spermidine/spermine N1-acetyltransferase (SSAT) and MeSpd is a poor substrate for spermine synthase. Otherwise these polyamine analogues seem to fulfil many of the physiological roles of natural polyamines [8]. However, we have recently shown that only (S)-MeSpd, but not (R)-MeSpd, can be converted to hypusine in DU145 prostate carcinoma cells [9]. Hypusine is an unusual amino acid which is an integral part of functional eukaryotic translation initiation factor 5A (eIF5A) and is known to be essential for normal cell growth [10]. Spermidine has been shown to serve as the sole biosynthetic precursor in hypusine synthesis [11]. In this paper, we have investigated the importance of individual polyamines in adipogenesis. Moreover, the possible role of hypusinated eIF5A during adipogenesis was evaluated.
Materials and methods
Chemicals
The synthesis of (R)- and (S)-isomers of α-MeSpd and (R,R)- and (S,S)-enantiomers of α,ω-Me2Spm has been described earlier [12]. Insulin, 3-isobuthyl-1-methylxanthine (IBMX), dexamethasone, aminoguanidine, spermidine and spermine were purchased from (Sigma Aldrich, St. Louis, MO, USA). The polyamine oxidase inhibitor MDL72527 [N,N′-bis(2,3-butadienyl)-1,4-butanediamine] was a generous gift from Hoechst-Roussel.
Cell cultures
3T3-L1 cells (CL-173) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen, UK) supplemented with 10% foetal bovine serum (Gibco) and 50 μg/ml gentamycin. Cells were cultured on 100 mm plates at +37°C in 95% air and 5% carbon dioxide.
Throughout the adipogenesis experiments, 5 mM DFMO alone or in combination with 100 μM spermidine, spermine or polyamine analogues was added to the medium at every medium change until harvest. Cultures containing natural polyamines were supplemented with 1 mM aminoguanidine to inhibit polyamine degradation by serum-derived oxidases. Cells were plated on six-well plates (80,000 cells/well) with the above-mentioned supplementation, cultured until confluence and then differentiated into adipocytes as described earlier [13]. Briefly, 2 days after the confluence (differentiation day 0, 6 days after plating), cells were first grown in medium supplemented with insulin, IBMX and dexamethasone for 48 hrs whereafter (from differentiation day 2) in medium supplemented only with insulin for the next 48 hrs. After that the medium was changed every other day (on differentiation days 4 and 6). Long-term adipogenesis studies were performed with 3, 5, 7 or 9 days subculture in the presence of 5 mM DFMO and 100 μM (R)- or (S)-MeSpd on 100 mm plates as a pre-treatment before transferring cells to the six-well plates and induction of adipogenesis as described above. In all the experiments, cells were harvested on the differentiation days 0, 4 and 8 by scraping the cells to buffer (25 mM Tris pH 7.4, 1 mM DTT, 0.1 mM EDTA), sonicated and homogenized with a pestle. For the cell proliferation studies, cells were plated on 100 mm plates (250,000 cells/plate) or on six-well plates (45,000 cells/well) in the presence of 100 μM polyamine analogues with or without 5 mM DFMO. Every third day, cells were harvested by trypsinization, counted (Coulter particle counter, Beckham, Fullerton, CA, USA) and passaged again, until day 12.
Analytical methods
Polyamines and their α-methylated analogues were quantified by using high performance liquid chromatography as described earlier [14]. Protein concentrations were determined by using Bradford Protein Assay Reagent (BioRad Laboratories, Hercules, CA, USA). DNA contents were determined by modified Burton’s method [15]. Different isoforms of eIF5A were analysed by two-dimensional electrophoresis and immunoblotting as described earlier [16, 17]. A total of 20 μg of protein were used for each analysis. Samples from the gels were blotted onto an Immobilon FL membrane (Millipore, Billerica, MA, USA), probed with eIF5A antibody (BD Biosciences, San Jose, CA, USA) and scanned with a Typhoon Variable Mode Imager (GE Healthcare, Fairfield, CT, USA). Differentiation was evaluated by measuring the accumulation of the fat by Oil Red O staining and detecting expression of Glut 4 protein by Western blotting. Oil Red O staining was performed essentially as described earlier [18] except that absorbance was measured in 540 nm. Western blotting was performed according to a laboratory manual, Current Protocols in Molecular Biology[19]. Shortly, 10 μg of protein was used for SDS-PAGE gel electrophoresis and transferred onto the PVDF membranes. Membranes were blocked with 5% dry milk in phosphate-buffered saline containing 0.1% Tween and probed with 1:10,000 dilution of Glut 4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). HRP-conjugated secondary antibody (Santa Cruz Biotechnology) was used in 1:10,000 dilution. Antibody-bound protein was detected by chemiluminescence reaction (ECL Plus Western Blotting detection System, GE Healthcare).
Quantitative RT-PCR
The standard adipogenesis protocol was used as previously described. A total of 5 mM DFMO alone or in combination with 100 μM spermidine, spermine or polyamine analogues were added to the medium at every medium change until harvested on the differentiation day 4. Total RNA from 3T3L1 cells was isolated using Tri Reagent (Sigma Aldrich). Total RNA was treated with DNA-free™ Kit (Applied Biosystems, Foster City, CA, USA). The absence of genomic DNA in the RNA samples was verified by a lack of PCR amplification with primers recognizing numerous Gapdh pseudogenes. Total RNA was transcribed to cDNA using High Capacity cDNA Archive kit (Applied Biosystems). Quantitative RT-PCRs were performed with 12 ng (RNA equivalents) of cDNA as template, gene-specific primers and probe, and Taqman reagents from Applied Biosystems in ABI BRISM 7700 and 7000 (Applied Biosystems). Running conditions were 2 min. at 50°C, 10 min. at 95°C and 40 cycles of 15 sec. at 95°C and 1 min. at 60°C. Primers and probes were designed using Assay-by-Design system (Applied Biosystems). Data were normalized to cyclophilin mRNA expression.
Statistical analyses
The data are expressed as the mean ± S.D. One-way or two-way ANOVA with Bonferroni’s post hoc test for multiple comparisons were used. A P-value less than 0.05 was considered significant. Analyses were performed by using GraphPad Prism 4.03 software package (GraphPad Software, Inc., San Diego, CA, USA).
Results
Effect of DFMO and polyamine analogues on adipogenesis
3T3-L1 cells were treated simultaneously with DFMO and polyamine analogues in order to inhibit de novo polyamine synthesis and to replace the natural polyamines with their methylated counterparts, respectively. The intracellular concentrations of polyamines and their analogues in cells treated with DFMO and polyamine analogue isomers are presented in Table 1. DFMO treatment depleted natural polyamines almost completely and only remains of spermine were detected. High levels of spermidine and spermine were detected in the untreated control cells and cells treated with DFMO plus natural spermidine. Spermidine levels were highest on the differentiation day 0 after which they declined during differentiation. Spermine levels did not change significantly during adipogenesis within given treatments. Cells exposed to DFMO with (S)- or (R)-MeSpd contained very low levels of natural spermine, and traces of natural spermidine were detected only with (S)-MeSpd treatment. Both isomers of MeSpd were metabolized to some extent to monomethylated spermine. DFMO in combination with either Me2Spm diastereomer depleted natural polyamines completely. (S,S)-Me2Spm was slightly converted to MeSpd, whereas (R,R)- Me2Spm was not.
Table 1.
Polyamine and analogue concentrations in 3T3-L1 cells. After plating, the cells were treated with 5 mM DFMO (D) and with 100 μM spermidine or polyamine analogues throughout the experiment. The data, expressed as pmol/μg DNA, are presented as the mean ± S.D, n= 3. Control, untreated cells; nd., not detected
| Put | Spd | MeSpd | Spm | MeSpm | Me2Spm | |
|---|---|---|---|---|---|---|
| Differentiation day 0 | ||||||
| Control | 59 ± 6 | 400 ± 35 | nd. | 69 ± 11 | nd. | nd. |
| DFMO | nd. | nd. | nd. | 4 ± 0.7 | nd. | nd. |
| D+Spd | nd. | 478 ± 39 | nd. | 62 ± 5 | nd. | nd. |
| D+(S)-MeSpd | nd. | nd. | 589 ± 106 | 22 ± 5 | 109 ± 2 | nd. |
| D+(R)-MeSpd | nd. | nd. | 542 ± 22 | 20 ± 0.9 | 91 ± 6 | nd. |
| D+(S,S)-Me2Spm | nd. | nd. | 69 ± 9 | nd. | nd. | 506 ± 82 |
| D+(R,R)-Me2Spm | nd. | nd. | nd. | nd. | nd. | 569 ± 5 |
| Differentiation day 4 | ||||||
| Control | nd. | 183 ± 19 | nd. | 98 ± 17 | nd. | nd. |
| DFMO | nd. | nd. | nd. | 4 ± 0.4 | nd. | nd. |
| D+Spd | 32 ± 4 | 347 ± 12 | nd. | 68 ± 3 | nd. | nd. |
| D+(S)-MeSpd | nd. | 0.7 ± 1 | 500 ± 17 | 11 ± 0.5 | 8 ± 0.2 | nd. |
| D+(R)-MeSpd | nd. | nd. | 532 ± 17 | 12 ± 6 | 20 ± 9 | nd. |
| D+(S,S)-Me2Spm | nd. | nd. | 19 ± 0.9 | nd. | nd. | 301 ± 22 |
| D+(R,R)-Me2Spm | nd. | nd. | nd. | nd. | nd. | 287 ± 25 |
| Differentiation day 8 | ||||||
| Control | 15 ± 1 | 133 ± 13 | nd. | 101 ± 8 | nd. | nd. |
| DFMO | nd. | nd. | nd. | 2 ± 0.2 | nd. | nd. |
| D+Spd | 19 ± 0.6 | 240 ± 12 | nd. | 67 ± 3 | nd. | nd. |
| D+(S)-MeSpd | nd. | 4.0 ± 0.2 | 385 ± 9 | 14 ±3 | 12 ± 1 | nd. |
| D+(R)-MeSpd | nd. | nd. | 379 ± 7 | 16 ± 3 | 36 ± 2 | nd. |
| D+(S,S)-Me2Spm | nd. | nd. | 21 ± 0.8 | nd. | nd. | 251 ± 22 |
| D+(R,R)-Me2Spm | nd. | nd. | nd. | nd. | nd. | 301 ± 27 |
Microscopic views of Oil Red O stained cells after 8 days of differentiation are shown in Fig. 1. Untreated cells differentiated completely (Fig. 1A) whereas DFMO treatment totally prevented adipogenesis (Fig. 1B). DFMO-treated cells remained at confluent state and maintained fibroblast-like appearance. In fact, they were poorly visible in the phase contrast microscopy due to flatness of the cells. Natural spermidine (not shown) and both (S)- and (R)-MeSpd enabled the cells to differentiate in the presence of 5 mM DFMO (Fig. 1C and D, respectively). Differentiated cells became spherical in shape and they accumulated fat droplets in their cytoplasm. Contrary to spermidine isomers, (S,S)- and (R,R)- Me2Spm did not support adipogenesis in the presence of DFMO (Fig. 1E and F, respectively). Only very few cells had differentiated whereas most of the cells remained at preadipocyte state.
Fig 1.
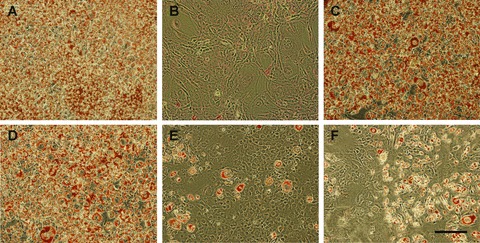
Morphological changes and fat accumulation in 3T3-L1 cells after 8 days of differentiation. During differentiation, cells were untreated (A), treated with 5 mM DFMO alone (B) or in combination with 100 μM (S)-MeSpd (C), (R)-MeSpd (D), (S,S)-Me2Spm (E) or (R,R)-Me2Spm (F). The cells were stained with Oil Red O on the differentiation day 8. Scale bar, 200 nm.
In order to measure the amount of fat quantitatively, Oil Red O stain was extracted from the cells and the absorbance of the extracts was measured at 540 nm. Fat accumulation is presented in Fig. 2. Cells treated with DFMO and (S)- or (R)-MeSpd accumulated fat as well as the untreated control cells or cells treated with DFMO and natural spermidine. If anything, accumulation of fat in the cells treated with spermidine isomers was slightly delayed when compared with the control cells on the differentiation day 4. In contrast, the fat accumulation was significantly decreased in the cells treated with DFMO and either (S,S)- or (R,R)-Me2Spm. However, cells treated with the (S,S)-diastereomer accumulated fat somewhat better than the cells treated with the (R,R)-diastereomer.
Fig 2.

Fat accumulation in 3T3-L1 cells during adipogenesis. After plating, the cells were treated with 5 mM DFMO in combination with 100 μM spermidine or a polyamine analogue throughout the experiment. On the differentiation days 0, 4 and 8 days cells were stained with Oil Red O stain. For quantitative analysis of fat accumulation, Oil Red O stain was extracted and quantified spectrophotometrically at 540 nm. The data, expressed as absorbance per well, are presented as the mean ± S.D., n= 3, ***=P < 0.001 as compared with untreated cells (Contr). S, (S)-MeSpd, R, (R)-MeSpd, SS, (S,S)-Me2Spm, RR, (R,R)-Me2Spm.
Differentiation was also assessed by quantifying the amount of Glut 4 that is considered as a marker protein for mature adipocytes. Results of Glut 4 protein expression supported those from Oil Red O staining. On the differentiation day 4, the level of Glut 4 protein was slightly lower in the cells treated with spermidine isomers when compared with untreated control cells (Fig. 3). On the differentiation days 6 and 8, equal amounts of Glut 4 were expressed in the control cells and in cells treated with DFMO plus (S)- or (R)-MeSpd. Cells treated with DFMO in combination with Me2Spm diastereomers did not express Glut 4 protein at any time-point (Fig. 3).
Fig 3.

Expression of Glut 4 protein. After plating, the cells were untreated (Cont) or treated with 5 mM DFMO in combination with 100 μM spermidine or a polyamine analogue. Glut 4 protein expression was determined on differentiation days 4, 6 and 8. Ten micrograms of protein were used for Western blotting analysis. Equal loading was confirmed by Ponceau staining (not shown). Molecular size as kD is shown on the right. S, (S)-MeSpd, R, (R)-MeSpd, SS, (S,S)-Me2Spm, RR, (R,R)-Me2Spm.
Hypusine is an integral part of functional eIF5A that is essential for normal cell growth. As spermidine has been shown to serve as the sole biosynthetic precursor in hypusine synthesis, the presence of hypusinated eIF5A and its precursor forms in differentiating 3T3-L1 cells were studied by using 2D electrophoresis and immunoblotting (Fig. 4). Only hypusinated eIF5A was detected in the untreated control cells on differentiation days 0 and 8. Similarly, it was the prevalent form in the cells treated with DFMO and natural spermidine or (S)-MeSpd. Treatment with DFMO and (R)-MeSpd or either Me2Spm isomer only partially supported hypusine synthesis leading to accumulation of precursor isoforms of eIF5A. On differentiation day 8 some hypusinated eIF5A was present when cells were treated with DFMO and (R)-MeSpd or (S,S)-Me2Spm whereas it was barely detectable in cells treated with DFMO and (R,R)-Me2Spm (Fig. 4).
Fig 4.
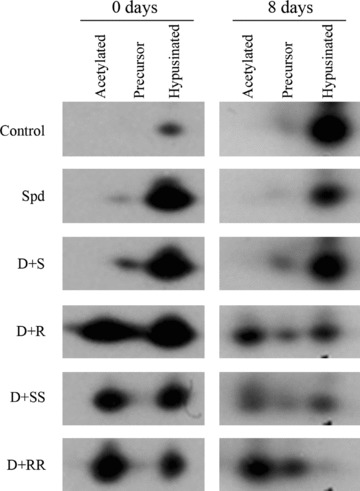
Effect of MeSpd and Me2Spm isomers on the level of hypusinated eIF5A in differentiating 3T3-L1 cells. During differentiation, cells were untreated (control) or treated with 5 mM DFMO in combination with 100 μM (S)-MeSpd (S), (R)-MeSpd (R), (S,S)-Me2Spm (SS) or (R,R)-Me2Spm (RR). Twenty micrograms of protein from each sample were separated by two-dimensional gel electrophoresis followed by immunoblotting and detection with eIF5A antibody.
The effect of natural spermine on adipogenesis in 3T3-L1 cells was studied in similar conditions as described above. On differentiation day 4, the cells treated with DFMO alone contained virtually no spermidine but a normal level of spermine (Table 2). Cells treated with the combination of DFMO and spermine contained spermine at concentrations twice as high as those found in untreated or DFMO-treated cells. In addition, they contained spermidine at levels of about half of those in untreated cells at early phase of differentiation (days 0 and 2) indicating some backconversion of spermine to spermidine. Adipogenesis was prevented in DFMO-treated cells, as observed before, but it proceeded nearly normally in cells supplemented with spermine. Parallel cultures were additionally treated with 10 μM MDL72527, an inhibitor of polyamine oxidase and spermine oxidase (SMO), to prevent catabolism of spermine. With the addition of MDL72527, we were able to deplete spermidine by day 4 and prevent the differentiation virtually completely. The trace amount of spermidine, close to the limit of detection, found at early phase of differentiation in cells treated with DFMO, spermine and MDL72527 (Table 2) was not sufficient to support differentiation, analogously to the situation seen in cells treated with (S,S)-Me2Spm that was converted to MeSpd to some extent (Table 1, Fig. 1).
Table 2.
Effect of MDL72527 on polyamine concentrations of the 3T3-L1 cells treated with DFMO and natural spermine. After plating, the cells were treated with 5 mM DFMO and 100 μM spermine and/or 20 μm MDL72527 (MDL) as indicated throughout the experiment. The data, expressed as pmol/μg DNA, are presented as the mean ± S.D., n= 4. Control, untreated cells; nd., not detected
| Put | Spd | Spm | ||
|---|---|---|---|---|
| Differentiation day 0 | ||||
| Control | 14 ± 5 | 234 ± 17 | 72 ± 13 | |
| DFMO | nd. | nd. | 107 ± 15 | |
| DFMO +Spermine | nd. | 165 ± 11 | 113 ± 10 | |
| DFMO+ MDL + Spermine | nd. | 50 ± 16 | 237 ± 20 | |
| Differentiation day 1 | ||||
| Control | nd. | 340 ± 20 | 83 ± 4 | |
| DFMO | nd. | nd. | 105 ± 19 | |
| DFMO +Spermine | nd. | 150 ± 10 | 135 ± 13 | |
| DFMO+ MDL + Spermine | nd. | 45 ± 9 | 195 ± 19 | |
| Differentiation day 4 | ||||
| Control | 19 ± 2 | 413 ± 20 | 137 ± 5 | |
| DFMO | nd. | nd. | 96 ± 34 | |
| DFMO +Spermine | nd. | 57 ± 3 | 226 ± 19 | |
| DFMO+ MDL + Spermine | nd. | nd. | 247 ± 14 | |
Effect of long-term exposure to DFMO and polyamine analogues on cell proliferation and differentiation of 3T3-L1 cells
We investigated the ability of polyamine analogues to maintain cell proliferation of undifferentiated 3T3-L1 cells grown in the absence or presence of 5 mM DFMO. Relative cell growth during a 12-day culture is depicted in Fig. 5. In the absence of DFMO (Fig. 5A), polyamine analogues did not dramatically affect the cell growth. However, after 9 days with (R)-MeSpd or (R,R)-Me2Spm, cell growth was slower than that of the untreated cells. DFMO treatment efficiently prevented cell growth and a total growth arrest was obtained already at 6 days (Fig. 5B). All used isomers of polyamine analogues were able to rescue cells from DFMO-related growth inhibition at least to some extent. (S)-MeSpd was the most effective and the only one that maintained cell growth during the whole 12-day experiment. With (R)-MeSpd and (S,S)-Me2Spm, the rate of cell growth was dramatically decreased during experiment. (R,R)-Me2Spm partially rescued cells from growth inhibition for 6 days where after cell proliferation ceased totally.
Fig 5.
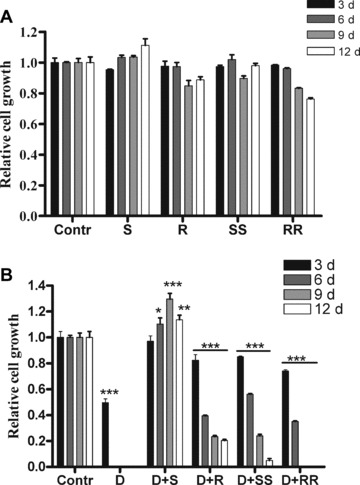
Effect of MeSpd and Me2Spm isomers on cell proliferation of 3T3-L1 cells in the presence or absence of DFMO. Cells were cultured in the absence or presence of 100 μM polyamine analogue without (A) or with (B) 5 mM DFMO. The cells were harvested, counted and passaged every third day until day 12. The data, expressed as increase in cell number per well relative to untreated cultures (Contr), are presented as the mean ± S.D., n= 3. *, P < 0.05, **, P < 0.01 and ***, P < 0.001 as compared with untreated cells. S, (S)-MeSpd, R, (R)-MeSpd, SS, (S,S)-Me2Spm, RR, (R,R)-Me2Spm.
Long-term exposure to DFMO and (S)- or (R)-MeSpd isomers was performed also to further study the utilization of spermidine isomers in hypusine synthesis and the role of hypusination in adipogenesis. In order to deplete cellular contents of natural spermidine-derived hypusinated eIF5A before the induction of adipogenesis, cells were pre-treated with DFMO and (S)- or (R)-MeSpd for 3, 5, 7 or 9 days before plating. Results from all performed experiments were similar and only the results from an experiment with 3 days pre-treatment are presented here. Polyamine and polyamine analogue concentrations are presented in Table 3. Generally, levels of polyamines and polyamine analogues were similar to those in the adipogenesis experiment without pre-treatment (Table 1). As a difference, some natural spermidine was detected and the level of natural spermine was substantially decreased in cells treated with either MeSpd isomer (Table 3). All cells treated with (S)-MeSpd grew and differentiated similarly to the cells in previous experiments. In contrast, long-term treatment with (R)-MeSpd affected cell growth and viability, and a number of cells detached from plates and apparently died. Yet, cells remaining on the plates differentiated and accumulated fat as well as the (S)-MeSpd-treated cells. Therefore, fat accumulation per well was higher in (S)-MeSpd-treated cells (Fig. 6A) but when fat accumulation was normalized to DNA content representing cell number, (S)- and (R)-MeSpd-treated cells accumulated fat equally (Fig. 6B). As in previous experiments, (S)-MeSpd-treated cells had only hypusinated eIF5A and (R)-MeSpd-treated cells contained both the hypusinated form and precursor isoforms of eIF5A (results not shown).
Table 3.
Polyamine and analogue concentrations in 3T3-L1 cells during differentiation after extended pre-treatment with DFMO and polyamine analogues. Cells were treated 3 days before, and during differentiation with 5 mM DFMO and 100 μM MeSpd analogues. The data, expressed as pmol/μg DNA, are presented as the mean ± S.D., n= 3. Control, untreated cells; nd., not detected
| Put | Spd | α-MeSpd | Spm | α-MeSpm | |
|---|---|---|---|---|---|
| Differentiation day 0 | |||||
| Control | 26 ± 5 | 240 ± 22 | nd. | 47 ± 7 | nd. |
| D+(S)-MeSpd | nd. | 15 ± 2 | 412 ± 17 | 2 ± 0.2 | 20 ± 0.6 |
| D+(R)-MeSpd | nd. | 12 ± 3 | 383 ± 14 | 0.1 ± 0.1 | 38 ± 7 |
| Differentiation day 4 | |||||
| Control | 27 ± 8 | 238 ± 12 | nd. | 137 ± 2 | nd. |
| D+(S)-MeSpd | nd. | 4 ± 2 | 524 ± 37 | 2 ± 2 | 24 ± 4 |
| D+(R)-MeSpd | nd. | 4 ± 0.7 | 452 ± 19 | 0.4 ± 0.4 | 34 ± 2 |
| Differentiation day 8 | |||||
| Control | 34 ± 1 | 179 ± 6 | nd. | 129 ± 4 | nd. |
| D+(S)-MeSpd | nd. | 3 ± 0.6 | 424 ± 11 | 4 ± 0.1 | 25 ± 2 |
| D+(R)-MeSpd | nd. | 6 ± 0.5 | 358 ± 9 | 0.4 ± 0.6 | 42 ± 5 |
Fig 6.

Fat accumulation in 3T3-L1 cells during differentiation after extended pre-treatment with DFMO and polyamine analogues. Cells were pre-treated for 3 days before plating and thereafter during differentiation with 5 mM DFMO in combination with 100 μM (S)- or (R)-MeSpd. On the differentiation days 0, 4 and 8 days, the cells were stained with Oil Red O. For quantitative analysis of fat accumulation, Oil Red O was extracted and quantified spectrophotometrically at 540 nm. The data, expressed as relative absorbance per well (A) or per DNA (B), are presented as the mean ± S.D., n= 3. **, P < 0.01 and ***, P < 0.001 as compared with untreated cells. S, (S)-MeSpd, R, (R)-MeSpd.
Effects of DFMO, polyamine analogues and natural polyamines on expression of adipocyte specific genes
To determine the mechanism(s) via which DFMO and polyamine analogues contribute to adipogenesis, gene expression studies were performed. As Fig. 7 shows, DFMO treatment blocked almost completely expression of PPAR-γ and C/EBP-α, the critical factors regulating adipogenesis, on the differentiation day 4. Expression of PPAR-γ in cells treated with (S)-MeSpd, (R)-MeSpd or natural spermine was reduced about 30 to 40% whereas in cells treated with (S,S)-Me2Spm, (R,R)-Me2Spm or natural spermine plus MDL72527, a decline in the expression was about 70–80% (Fig. 7). In contrast, natural spermidine sustained the expression of this transcription factor at the control level. Expression of C/EBP-α in cells treated with (S)-MeSpd, (R)-MeSpd or natural spermidine was near to the control level whereas treatment with (S,S)-Me2Spm, (R,R)-Me2Spm or natural spermine plus MDL72527 resulted in about 90% reduction in the expression. In contrast, cells treated with natural spermine without MDL72527 exhibited about 55% reduced expression of C/EBP-α. A similar expression pattern between the treatments was also observed for a lipolytic gene, HSL, and a late adipocyte differentiation marker, FAS, on the differentiation day 4 (Fig. 7). The only exception was that (R)-MeSpd -treated cells, similarly to (S,S)-Me2Spm, (R,R)-Me2Spm and natural spermine plus MDL72527 -treated cells, had dramatically reduced expression of FAS.
Fig 7.

Effects of DFMO, polyamine analogues and natural polyamines on expression of adipocyte-specific genes. Results are presented relative to the expression level in untreated cells (control, dashed line). Cells were cultured in the presence of 100 μM polyamine or analogue with or without 5 mM DFMO (D) and collected on the differentiation day 4. A total of 20 μM MDL72527 (MDL) were added into a set of cells treated with DFMO and spermine to prevent the backconversion of spermine to spermidine. The expression of selected genes was analysed by quantitative RT-PCR. Data are means ± S.D. of four cultures per group. *, P < 0.05, **, P < 0.01 and ***, P < 0.001 as compared with untreated cells. PPAR-γ, peroxisome proliferator-activated receptor γ; C/EBP-α, CCAAT/enhancer binding protein α; HSL, hormone-sensitive lipase and FAS, fatty acid synthase. S, (S)-MeSpd, R, (R)-MeSpd, SS, (S,S)-Me2Spm, RR, (R,R)-Me2Spm.
Discussion
Obesity is a growing epidemic worldwide. During positive caloric intake, excess fat is stored in adipocytes which are increased by their size (hyperplasia) and number (hypertrophy) [2]. If normal adipogenesis is disturbed, excess fat is stored through adipocyte hypertrophy. Exceeding of the storage capacity of adipocytes causes dysfunction of the adipocytes and accumulation of fat in non-adipose tissues which in turn leads to the development of insulin resistance and metabolic syndrome. Therefore, a direct reduction of adipogenesis does not seem to have a beneficial effect on metabolism. In fact, it has been noted that PPAR-γ agonists thiazolidinediones, are able to increase recruitment, proliferation and differentiation of preadipocytes leading ultimately to improved adipose tissue function and control of glucose homeostasis [20]. Thus, it is important to delineate factors that support adipocyte differentiation, growth and metabolism.
In adipogenesis, the timely concerted action of different transcription factors facilitates the differentiation of preadipocytes to fully mature adipocytes. The exact role of polyamines, spermidine in particular, in this process has remained unclear. Here, we were able to confirm by using methylated polyamine analogues that spermidine is the key polyamine for adipogenesis to occur, as was previously suggested [6, 7]. In present study spermidine and its α-methylated analogues (S)-MeSpd or (R)-MeSpd were able to rescue the cells from DFMO induced differentiation blockage and the cells accumulated fat and expressed a mature adipocyte marker, Glut4, like the control cells. In contrast, the cells treated with either (S,S)-Me2Spm or (R,R)-Me2Spm isomers together with DFMO showed markedly reduced differentiation capability. Moreover, the inability of added natural spermine to support adipogenesis was verified in conditions where formation of spermidine both via de novo synthesis and conversion from spermine was prevented.
To address the question of the role of spermidine in adipogenesis, we studied the presence of hypusinated eIF5A in analogue-treated cells during differentiation. In the present study, we did not achieve complete depletion of hypusinated eIF5A with any treatment. It is possible that even in 3T3-L1 cells treated for an extended time with (R)-MeSpd, complete depletion of eIF5A was prevented by a limited metabolism of (R)-MeSpd to (R)-MeSpm and potentially back to both spermidine and (R)-MeSpd by the action of SMO. It is also possible that (R)-MeSpd could be used directly as a substrate for deoxyhypusine synthase more efficiently in mouse 3T3-L1 cells than in human DU145 cells [9]. Nevertheless, our results on two-dimensional gel electrophoresis showed a clear shift towards the acetylated immature form of eIF5A in the cells treated with the analogues that did not fully support differentiation and that also reduced cell proliferation. The mature hypusinated eIF5A is necessary for cell proliferation, and reflecting that, only 3 days pre-treatment with (R)-MeSpd in combination with DFMO caused cell death during adipogenesis. Thus, the impaired synthesis of hypusinated eIF5A in analogue-treated cells influenced more distinctly proliferation than differentiation.
Our studies revealed for the first time the mechanism by which DFMO prevents adipogenesis: mRNA levels of key differentiation transcription factors (PPAR-γ and C/EBP-α.) were markedly decreased in DFMO-treated cells. As could be expected, the expression of late adipocyte marker genes (FAS and HSL) was also suppressed. The supplementation of spermidine and MeSpd analogues restored the expression of all these genes whereas Me2Spm analogues or spermine, especially in the presence of MDL72527, were not able to support the expression. The fact that the expression levels were lower in cells treated with spermine plus MDL72527 than in cells treated with spermine alone tends to indicate that the partial capability of spermine to restore gene expression was due to its backconversion to spermidine. The down-regulation of HSL in the cells treated with DFMO and Me2Spm analogues verified that reduced fat accumulation in these cells was mediated by impaired adipogenesis and not by increased lipolysis. In addition, our gene expression results demonstrated that spermidine seems to promote the expression of PPAR-γ, C/EBP-α, FAS and HSL. The interaction of polyamines and especially spermidine with genes involved in adipocyte metabolism has previously been poorly understood. On the other hand, it is known that enhanced polyamine catabolism, but not polyamines themselves, can alter homeostatic control of white adipose tissue mass through depleting cellular ATP and acetyl-CoA pools [21, 22]. In the present study we have obtained clear evidence that spermidine plays an important role in the maintenance of the expression of some adipocyte specific genes. Whether the mechanism is direct or indirect requires further investigation.
Interestingly, polyamines have also been shown to modulate the response of adipose tissue-derived stem cells to mechanical loading [23] and 1,25-dihydroxyvitamin-D3, a stimulant of osteogenic differentiation [24]. The stem cells responded to these stimuli with an increase of SSAT gene expression. Whether there was a consequent induction of SSAT activity leading to changes in cellular polyamine pools was not reported. However, exogenously added spermine prevented the bone cell-like responses, such as nitric oxide production and Cox-2 gene expression, in the former system [23] but increased the expression of osteogenic differentiation markers, such as runx-2 and osteopontin, in the latter system [24]. The findings suggest that polyamines could be used for manipulation of mesenchymal stem cells towards osteogenic differentiation. While adipose tissue-targeted inhibition of polyamine synthesis could inhibit adipogenesis according to our findings, it might facilitate osteogenic differentiation of adipose stem cells through the action of spermine. This should be taken into consideration in designing therapeutic approaches based on modulation of polyamine metabolism.
In summary, we have demonstrated by using methylated spermidine analogues that spermidine is essential for adipogenesis. Because recruitment of newly formed adipocytes is crucial for the capability to store excess fat, it is tempting to speculate that the maintenance of normal polyamine homeostasis and spermidine concentrations during positive energy intake is critical for the normal adipose tissue function. In the future, it would be interesting to investigate spermidine levels in white adipose tissue of obese patients. However, the present data as such introduce spermidine as a novel regulator of adipocyte formation and therefore, polyamine metabolism should be considered as a potential target pathway for the drug development for obesity.
Acknowledgments
We thank Tuula Reponen, Anne Karppinen and Sisko Juutinen for skilful technical assistance, and Emilia Kansanen for supervision in preadipocyte cell culture. This work was supported by grants from the Academy of Finland.
References
- 1.Feve B. Adipogenesis: cellular and molecular aspects. Best Prac Res Clin Endocrinol Metab. 2005;19:483–99. doi: 10.1016/j.beem.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Bays H, Blonde L, Rosenson R. Adiposopathy: how do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients. Expert Rev Cardiovasc Ther. 2006;4:871–95. doi: 10.1586/14779072.4.6.871. [DOI] [PubMed] [Google Scholar]
- 3.Rosen ED, Walkey CJ, Puigserver P, et al. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–307. [PubMed] [Google Scholar]
- 4.Vazquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39:715–28. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Teti D, Visalli M, McNair H. Analysis of polyamines as markers of (patho)physiological conditions. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:107–49. doi: 10.1016/s1570-0232(02)00669-4. [DOI] [PubMed] [Google Scholar]
- 6.Bethell DR, Pegg AE. Polyamines are needed for the differentiation of 3T3-L1 fibroblasts into adipose cells. Biochem Biophys Res Commun. 1981;102:272–8. doi: 10.1016/0006-291x(81)91517-5. [DOI] [PubMed] [Google Scholar]
- 7.Erwin BG, Bethell DR, Pegg AE. Role of polyamines in differentiation of 3T3-L1 fibroblasts into adipocytes. Am J Physiol. 1984;246:C293–300. doi: 10.1152/ajpcell.1984.246.3.C293. [DOI] [PubMed] [Google Scholar]
- 8.Keinänen TA, Järvinen A, Uimari A, et al. Alpha-methylated polyamines as potential drugs and experimental tools in enzymology. Mini Rev Med Chem. 2007;7:813–20. doi: 10.2174/138955707781387867. [DOI] [PubMed] [Google Scholar]
- 9.Hyvönen MT, Keinänen TA, Cerrada-Gimenez M, et al. Role of hypusinated eukaryotic translation initiation factor 5A in polyamine depletion-induced cytostasis. J Biol Chem. 2007;282:34700–6. doi: 10.1074/jbc.M704282200. [DOI] [PubMed] [Google Scholar]
- 10.Park MH, Wolff EC, Lee YB, Folk JE. Antiproliferative effects of inhibitors of deoxyhypusine synthase. Inhibition of growth of Chinese hamster ovary cells by guanyl diamines. J Biol Chem. 1994;269:27827–32. [PubMed] [Google Scholar]
- 11.Park MH, Cooper HL, Folk JE. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci USA. 1981;78:2869–73. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigorenko NA, Khomutov AR, Keinänen TA, Järvinen A, Alhonen L, Jänne J, Vepsäläinen J. Synthesis of novel optical isomers of α-methylpolyamines. Tetrahedron. 2007;63:2257–63. [Google Scholar]
- 13.Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980;255:4745–50. [PubMed] [Google Scholar]
- 14.Hyvönen T, Keinänen TA, Khomutov AR, et al. Monitoring of the uptake and metabolism of aminooxy analogues of polyamines in cultured cells by high-performance liquid chromatography. J Chromatogr. 1992;574:17–21. doi: 10.1016/0378-4347(92)80093-6. [DOI] [PubMed] [Google Scholar]
- 15.Giles KW, Myers A. The role of nucleic acids in the growth of the hypocotyl of Lupinus albus under varying light and dark regimes. Biochim Biophys Acta. 1964;87:460–77. doi: 10.1016/0926-6550(64)90118-5. [DOI] [PubMed] [Google Scholar]
- 16.Taylor CA, Sun Z, Cliche DO, et al. Eukaryotic translation initiation factor 5A induces apoptosis in colon cancer cells and associates with the nucleus in response to tumour necrosis factor alpha signalling. Exp Cell Res. 2007;313:437–49. doi: 10.1016/j.yexcr.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura K, Murozumi K, Shirahata A, et al. Independent roles of eIF5A and polyamines in cell proliferation. Biochem J. 2005;385:779–85. doi: 10.1042/BJ20041477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–7. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 19.Ausubel FM, Brent R, Kingston RE, et al. Current protocols in molecular biology. John Wiley & Sons. Inc; 2007. , USA; [Google Scholar]
- 20.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–78. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 21.Pirinen E, Kuulasmaa T, Pietilä M, et al. Enhanced polyamine catabolism alters homeostatic control of white adipose tissue mass, energy expenditure, and glucose metabolism. Mol Cell Biol. 2007;27:4953–67. doi: 10.1128/MCB.02034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jell J, Merali S, Hensen ML, et al. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J Biol Chem. 2007;282:8404–13. doi: 10.1074/jbc.M610265200. [DOI] [PubMed] [Google Scholar]
- 23.Tjabringa GS, Vezeridis PS, Zandieh-Doulabi B, et al. Polyamines modulate nitric oxide production and Cox-2 gene expression in response to mechanical loading in human adipose tissue-derived mesenchymal stem cells. Stem Cells. 2006;24:2262–9. doi: 10.1634/stemcells.2005-0625. [DOI] [PubMed] [Google Scholar]
- 24.Tjabringa GS, Zandieh-Doulabi B, Helder MN, et al. The polyamine spermine regulates osteogenic differentiation in adipose stem cells. J Cell Mol Med. 2008;12:1710–7. doi: 10.1111/j.1582-4934.2008.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


