Abstract
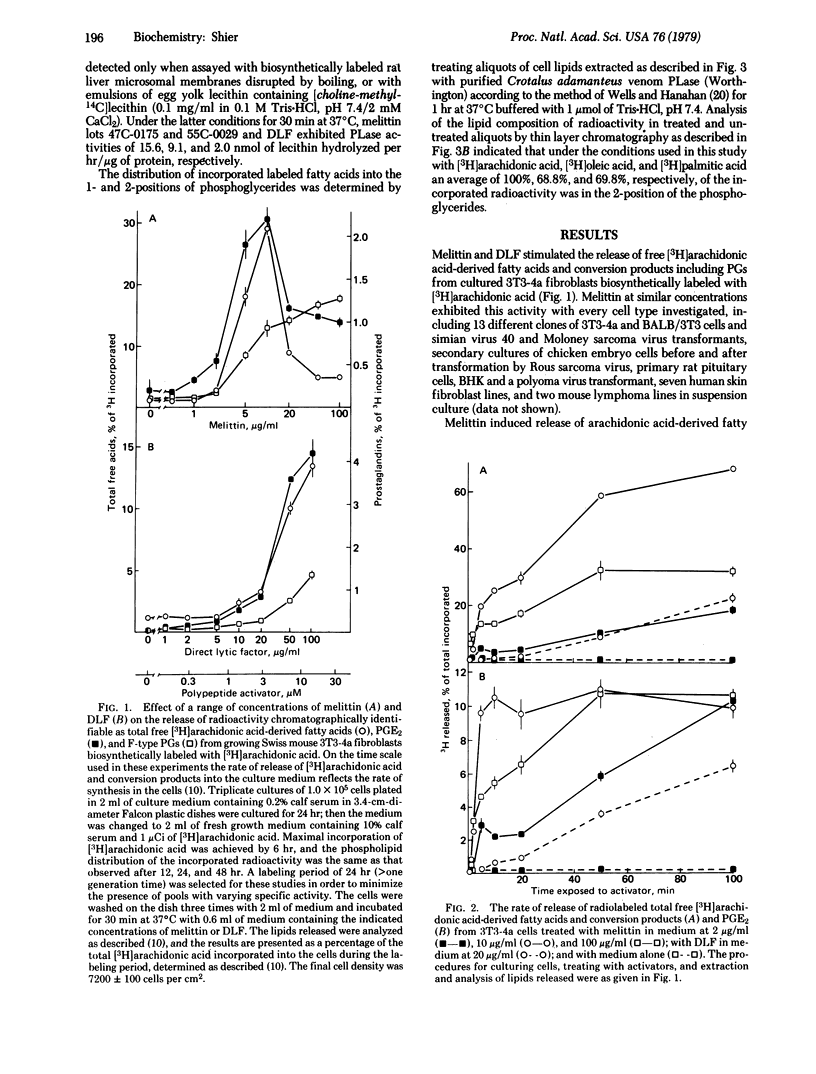
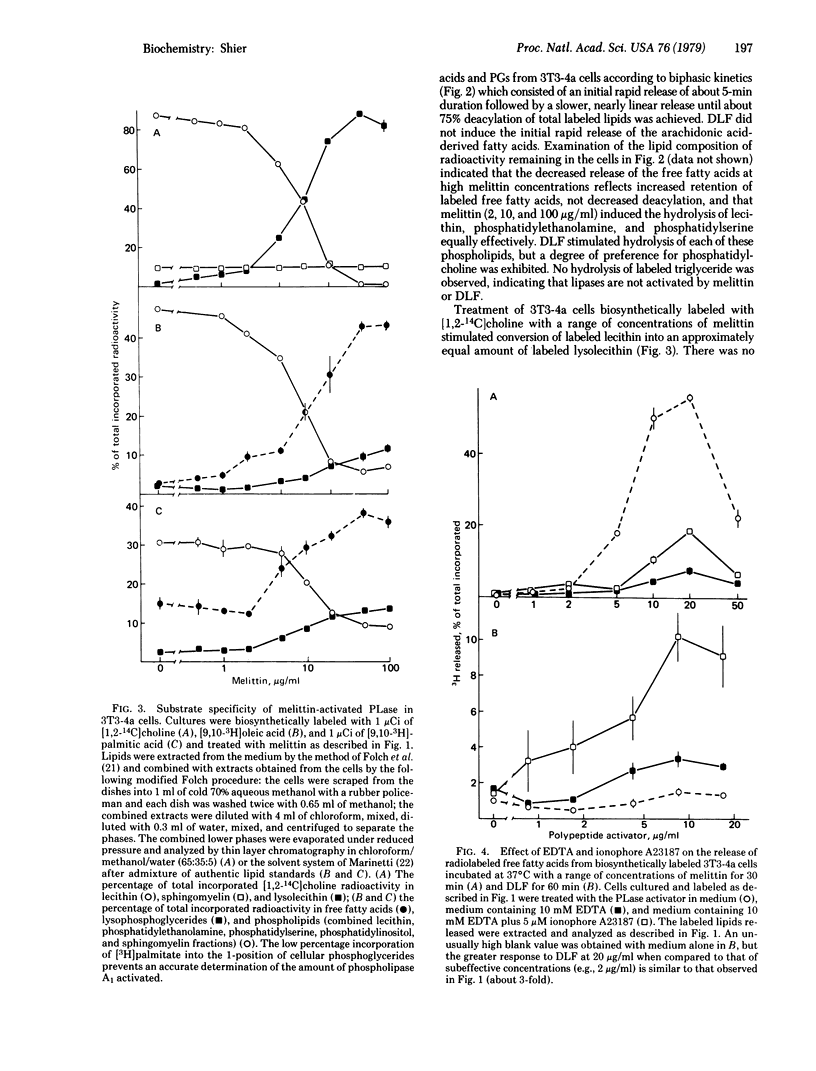
Activatable cellular phospholipase A2 (PLase; phosphatide 2-acyl-hydrolase, EC 3.1.1.4) has been proposed to constitute the first and rate-limiting step in prostaglandin synthesis and to regulate membrane function by altering the levels in the membrane of the detergent lipids lysolecithin and free fatty acids. We have observed that a wide variety of cells in culture contain high levels of endogenous PLase that can be activated by polypeptide toxins, such as melittin purified from bee venom and direct lytic factor purified from the venom of African Ringhals cobra (Hemachatus hemachatus). Activation of PLase by sublytic concentrations of these agents results in the synthesis and release of prostaglandins. Melittin concentrations greater than or equal to 10 microgram/ml activate sufficient PLase in 3T3-4a mouse fibroblasts to hydrolyze 10% of the cellular lecithin in less than 5 min and virtually all of it within 30 min, demonstrating the existence of sufficient activatable PLase to provide the basis for the proposed mechanism of regulation of membrane function by alteration of membrane lipid composition. Lipases, phospholipases B and C, and sphingomyelinases are not activated by melittin. The PLase activated in 3T3-4a cells exhibits little, if any, specificity for individual phosphoglycerides. The PLase activated by direct lytic factor exhibits a Ca2+ dependence characteristic of lysosomal PLase, wherease the Ca2+ dependence of PLase activated by melittin is consistent with the activation of a cell-surface enzyme. The extent of cell death correlates with percent of maximal PLase activation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borle A. B. Kinetic analyses of calcium movements in HeLa cell cultures. I. Calcium influx. J Gen Physiol. 1969 Jan;53(1):43–56. doi: 10.1085/jgp.53.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell A. H., Pressman B. C. Kinetics of transport of divalent cations across sarcoplasmic reticulum vesicles induced by ionophores. Biochem Biophys Res Commun. 1972 Oct 6;49(1):292–298. doi: 10.1016/0006-291x(72)90043-5. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Faulstich H., Zobeley S., Weckauf-Bloching M. Cytolytic properties of phallolysin. Hoppe Seylers Z Physiol Chem. 1974 Dec;355(12):1495–1498. doi: 10.1515/bchm2.1974.355.2.1495. [DOI] [PubMed] [Google Scholar]
- Fryklund L., Eaker D. Complete amino acid sequence of a nonneurotoxic hemolytic protein from the venom of Haemachatus haemachates (African ringhals cobra). Biochemistry. 1973 Feb;12(4):661–667. doi: 10.1021/bi00728a015. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Hassid A., Levine L. Stimulation of phospholipase activity and prostaglandin biosynthesis by melittin in cell culture and in vivo. Res Commun Chem Pathol Pharmacol. 1977 Nov;18(3):507–517. [PubMed] [Google Scholar]
- Hong S. L., Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc Natl Acad Sci U S A. 1976 May;73(5):1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Levine L. Stimulation of prostaglandin synthesis by bradykinin and thrombin and their mechanisms of action on MC5-5 fibroblasts. J Biol Chem. 1976 Sep 25;251(18):5814–5816. [PubMed] [Google Scholar]
- Kunze H., Vogt W. Significance of phospholipase A for prostaglandin formation. Ann N Y Acad Sci. 1971 Apr 30;180:123–125. doi: 10.1111/j.1749-6632.1971.tb53191.x. [DOI] [PubMed] [Google Scholar]
- Lankisch P. G., Lege L., Oldigs H. D., Vogt W. Binding of phospholipase A to the cirect lytic factor revealed by the interaction of Ca 2+ with the haemolytic effect. Biochim Biophys Acta. 1971 Jul 13;239(2):267–272. doi: 10.1016/0005-2760(71)90172-x. [DOI] [PubMed] [Google Scholar]
- Mollay C., Kreil G., Berger H. Action of phospholipases on the cytoplasmic membrane of Escherichia coli. Stimulation by melittin. Biochim Biophys Acta. 1976 Mar 5;426(2):317–324. doi: 10.1016/0005-2736(76)90340-0. [DOI] [PubMed] [Google Scholar]
- Mollay C., Kreil G. Enhancement of bee venom phospholipase A2 activity by melittin, direct lytic factor from cobra venom and polymyxin B. FEBS Lett. 1974 Sep 15;46(1):141–144. doi: 10.1016/0014-5793(74)80354-6. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Biosynthesis of prostaglandins. Fed Proc. 1972 Sep-Oct;31(5):1442–1450. [PubMed] [Google Scholar]
- Seeger R., Burkhardt M., Haupt M., Feulner L. The haemolytic effect of phallolysin. Naunyn Schmiedebergs Arch Pharmacol. 1976 May;293(2):163–170. doi: 10.1007/BF00499222. [DOI] [PubMed] [Google Scholar]
- Shier W. T., Baldwin J. H., Nilsen-Hamilton M., Hamilton R. T., Thanassi N. M. Regulation of guanylate and adenylate cyclase activities by lysolecithin. Proc Natl Acad Sci U S A. 1976 May;73(5):1586–1590. doi: 10.1073/pnas.73.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T. Inhibition of acyl coenzyme A:lysolecithin acyltransferases by local anesthetics, detergents and inhibitors of cyclic nucleotide phosphodiesterases. Biochem Biophys Res Commun. 1977 Mar 7;75(1):186–193. doi: 10.1016/0006-291x(77)91307-9. [DOI] [PubMed] [Google Scholar]
- Shier W. T. Inhibition of prostaglandin synthesis by lysolecithin. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1168–1174. doi: 10.1016/0006-291x(77)91416-4. [DOI] [PubMed] [Google Scholar]
- Shier W. T., Trotter J. T., 3rd Stimulation of liver microsomal sialyltransferase activity by lysolecithin. FEBS Lett. 1976 Feb 15;62(2):165–168. doi: 10.1016/0014-5793(76)80044-0. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Zorgniotti F. Calcium content and distribution as a function of growth and transformation in the mouse 3T3 cell. J Cell Biol. 1977 Oct;75(1):12–22. doi: 10.1083/jcb.75.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M., Scherphof G. L., Boshouwers F. M., van Deenen L. L. Differentiation of phospholipases A in mitochondria and lysosomes of rat liver. J Lipid Res. 1969 Jul;10(4):411–420. [PubMed] [Google Scholar]
- Wells M. A., Hanahan D. J. Studies on phospholipase A. I. Isolation and characterization of two enzymes from Crotalus adamanteus venom. Biochemistry. 1969 Jan;8(1):414–424. doi: 10.1021/bi00829a057. [DOI] [PubMed] [Google Scholar]
- Yunes R., Goldhammer A. R., Garner W. K., Cordes E. H. Phospholipases: melittin facilitation of bee venom phospholipase A2-catalyzed hydrolysis of unsonicated lecithin liposomes. Arch Biochem Biophys. 1977 Sep;183(1):105–112. doi: 10.1016/0003-9861(77)90424-6. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]