Abstract
Preeclampsia (PE) is characterized by widespread endothelial damage with hypertension, proteinuria, glomeruloendotheliosis and elevated soluble Flt-1 (sFlt-1), a natural occurring antagonist of vascular endothelial growth factor (VEGF). Cancer patients receiving anti-VEGF therapy exhibit similar symptoms. We suggested that a decrease in circulating sFlt-1 would alleviate the symptoms associated with PE. Adenoviral (Adv) overexpression of sFlt-1 induced proteinuria, caused glomerular damage and increase in blood pressure in female Balb/c mice. Circulating level of sFlt-1 above 50 ng/ml plasma induced severe vascular damage and glomerular endotheliosis. Albumin concentration in urine was elevated up to 30-fold, compared to control AdvGFP-treated animals. The threshold of kidney damage was in the range of 20–30 ng/ml sFlt-1 in plasma (8–15 ng/ml in urine). Co-administration of AdvsFlt-1 with AdvVEGF to neutralize circulating sFlt-1 resulted in more than a 70% reduction in free sFlt-1 in plasma, more than 80% reduction in urine and rescued the damaging effect of sFlt-1 on the kidneys. This demonstrates that below a critical threshold sFlt-1 fails to elicit damage to the fenestrated endothelium and that co-expression of VEGF is able to rescue effects mediated by sFlt-1 overexpression.
Keywords: preeclampsia, vascular endothelial growth factor, fms-like tyrosine kinase receptor, vascular disease
Introduction
The disruption of endothelial homeostasis and inflammation are fundamental to the initiation and progression of atherosclerosis and preeclampsia (PE) [1–2]. PE is a pregnancy-specific multi-organ syndrome characterized by widespread endothelial damage with a clinical presentation of hypertension and proteinuria after 20 weeks of gestation [3]. It affects 3–8% of all pregnancies, with an incidence of 0.8% before 32 weeks [4]. The WHO reports over 60,000 maternal deaths worldwide per year as a consequence of eclampsia. Women with PE and their offspring are at an increased risk of developing cardiovascular disease later in life [5, 6]. Defective vasculogenesis in early placentation and resultant placental ischemia have been proposed to trigger the release of unknown mediators. Soluble anti-angiogenic factors in the maternal circulation precede the clinical PE and result in systemic maternal endothelial dysfunction [7–14]. We originally proposed that sFlt-1, an alternative mRNA splice variant of the fms-like tyrosin kinase gene (Flt-1) and antagonist of vascular endothelial growth factor (VEGF or VEGF-A), might be a key factor responsible for the clinical manifestation of PE because of a loss of circulating free VEGF [15]. Indeed, cancer patients receiving bevacizumab (Avastin®, Roche; anti-VEGF therapy) exhibit PE-like symptoms (hypertension, proteinuria), suggesting that decreased bioavailability of VEGF causes these symptoms. Given the neurological findings in these patients, this is in line with recent observations that VEGF and transforming growth factor beta (TGFβ) blockage effected choroid plexus integrity and function in adult mice [16].
We proposed earlier, that sFlt-1 may be also involved in several other vascular diseases [17]. Previous studies from our group indicated that the amniotic fluid from PE patients early in pregnancy contains elevated sFlt-1 levels [18]. sFlt-1 is increased in the maternal circulation in PE, even before onset of the clinical disease [8, 10–13]. Despite the multiple genotypes and phenotypes that underlie PE, it appears that serum levels of sFlt-1, placental growth factor (PlGF) and soluble endoglin (sEng) give the highest strength of association with outcome [7–9]. However, based on a recent systematic review, at present the evidence is insufficient to recommend these markers for screening [19].
Direct evidence that excess circulating sFlt-1 plays a role in the pathogenesis of PE was provided by studies in rats where administration of sFlt-1 or a VEGF neutralizing antibody resulted in glomerular endothelial cell damage and proteinuria [20], and adenoviral delivery of sFlt-1 to pregnant animals mimicked the clinical manifestations of PE [21]. The induction of uteroplacental ischemia in a pregnant non-human primate model resulted in the development of clinical symptoms analogous to human PE including a significant elevation of circulating sFlt-1 [22]. However, whether or not a reduction of sFlt-1 by induced complex formation with the corresponding ligand would alleviate hypertension, proteinuria and glomeruloendotheliosis is unknown. Here we report that adenoviral overexpression of sFlt-1 in mice induced proteinuria, caused glomerular damage and increased blood pressure. However, when co-administered with AdvVEGF to neutralize circulating sFlt-1, the damaging effect of sFlt-1 on the kidneys was ameliorated when free sFlt-1 in plasma fell by more than 70%. Thus, reduction in sFlt-1 is a valid surrogate end-point for a clinical outcome measure.
Materials and methods
Reagents and ELISA measurements
Mouse sFlt-1 duo set kits were purchased from R&D Systems (Rüsselsheim, Germany). The minimum detection level was about 0.3–0.6 ng/ml in plasma, urine and in tissue lysates. ELISA for human sFlt-1 and human VEGF were performed as described before [23–24]. ELISA for mouse sFlt-1 was performed according to the manufacturer’s instructions. Briefly, the various samples for ELISA measurements were diluted in diluents as recommended. Liver lysates were diluted 1: 20 or 1: 200 and plasma samples were diluted 1: 10 and urine samples 1: 5. Cell culture supernatants were diluted up to 1: 250. The diluted samples were incubated in 96-well plates precoated wit the capture antibody directed against VEGF, human sFlt-1 and mouse sFlt-1 for 1–2 hrs. The wells were then washed three times in phosphate buffered saline (PBS with 0.1% Tween) and incubated with a biotinylated secondary antibody against the antigens for 1–2 hrs. The plates were than washed again three times and incubated with streptavidin-horseradish peroxidase for 30–60 min. The plates were than washed again and incubated with substrate solution containing H2O2 and tetramethyl benzidine (TMB Plus from KEMENTEC Diagnostics, Denmark). The absorbance was determined at 450 nm. All assays were done in duplicates, and the protein concentrations were calculated using a standard curve derived from known concentrations of recombinant protein standards. Total protein concentrations in all lysates were calculated using the BCA assay (Pierce/Thermo, Rockford, IL, USA). Protein concentrations in lysates were normalized to the total protein concentration and indicated as ng/mg total protein.
Immunoprecipitation and Western blotting
Immunoprecipitation (IP) and Western blotting was used for the detection of VEGF-A and VEGFR-2 (Flk-1) in cell lysates, liver lysates or kidney lysates. For IP of VEGF-A from cell lysates and liver lysates after adenovirus treatment 1–6 mg protein was incubated over 2 hr (cell lysate) or 16 hr (liver lysates) with 1 μg anti-VEGF-A antibody (MAB clone 3C5, Reliatech, Wolfenbuettel, Germany). For pull-down lysates were supplemented with 50 μl anti-mouse IgG agarose (Sigma) and incubated over night at 4°C. Beads were washed several times with TBST and pellet was diluted with 4x Laemmli buffer, boiled for 5 min and cooled on ice. Twenty μl of sample were subjected to SDS-PAGE (12.5% gels) and electroblotted onto PVDF membranes (Millipore). The membrane was blocked with 50% low-fat milk in TBS buffer and incubated with an antigen-affinity-purified VEGF-A antibody at 1 μg/ml (#2668, Reliatech) in TBS/10% low-fat milk overnight at 4°C. To visualize the signals, the membrane was incubated with a goat-anti rabbit IgG conjugated with horseradish peroxidase (dil. 1: 5.000 until 1: 10.000, Promega). Signals were developed with Super Signal West (Pierce/Thermo) or ECL system and Hyperfil ECL (GE, Freiburg, Germany) were used for documentation. To detect VEGFR-2 (FLK-1) in cell lysats and kidney lysates a similar procedure was used. The anti-VEGFR-2 antibody diluted 1: 100 (rabbit MAB clone 55B11, Cell Signalling) was used for pull-down and for Western blotting (dil. 1: 1.000; 7.5% gels). For detection of VEGFR-2Tyr1175 the same antibody was used as before for pull-down but for immunoblotting the antibody phospho-VEGFR-2 Tyr1175 (rabbit MAB clone 19A10, Cell Signalling) was employed. The anti-beta actin antibody was from Chemicon (MAB1501).
Animals
All experiments were performed on 10–12 weeks old female Balb/c mice with a mean weight of 22.4 g (range 18.9 and 26.3 g) obtained from Harlan, Germany. The animals were allowed free access to standard chow (0.25% sodium; SNIFF Spezialitaeten, Soest, Germany) and drinking water ad libitum. The protocol was approved by the local council on animal care that corresponds to requirements of the American Physiological Society.
Mouse model
The preparation of the adenoviruses for mouse sFlt-1-Fc (AdvsFlt-1), AdvVEGF165 and adenoviruses for green fluorescence protein (AdveGFP; control) have been described before in detail [25–26]. The extracellular domain of mouse sFlt-1 (amino acid 1–758) was fused with the mouse immunglobulin Fc tail. A size of 130 kD of the gene product was confirmed in cell supernatants. All virus preparations were tested in vitro and gene products were estimated by ELISA or Western Blot as described earlier [26] Mouse sFlt-Fc was also estimated by a Western blot using antibodies against mouse Fc. The virus titre was estimated by an end-point dilution assay and pure virus preparations were desalted and stored in –70°C until used [27]. Approximately 50–100 μl of virus diluted in PBS was used for injections. Both, the pregnant and the non-pregnant Balb/c mice were injected with AdvsFlt-1 (2.5 to 7.5 × 109 pfu) or AdvVEGF165 (1–2 × 108 pfu) or AdveGFP (2–4 × 109 pfu) into the tail vein. Each treatment group existed of 2–3 animals and all experiments were at least three times repeated (n= 6–9). In some experiments pregnant mice were injected at day 4, 8 or 9 of pregnancy and the phenotypes were recorded 10 days later (for details see Table 1). Initially we used non-pregnant and pregnant mice very similar as used in a rat model before [21]. Because we could not detect significant differences in kidney histology, all data presented in the figures are from non-pregnant mice.
Table 1.
Study protocol
| Day | Normal / rescue | Blood pressure | Pregnant |
|---|---|---|---|
| −14 | Implant. of transp. | ||
| −10 | coll. blood | ||
| 0 | inj. AdV | inj. AdV | Mating of mice |
| 4 | d4 of pregn: inj. AdV | ||
| 5 | d5 of pregn: inj. AdV | ||
| 8 | d8 of pregn: inj. AdV | ||
| 10 | End | End | |
| 14/ 15/ 18 | End (d14, d15, d18 of pregn.) |
Conduction of different experiment groups is shown. Blood was collected 10 days before start and at the end of the experiment. Implant. of transp. -implantation of transponder; coll. blood - collection of blood for plasma preparation; inj. AdV - injection of recombinant adenoviruses; d4 of pregn. - day 4 of pregnancy; end- end of the experiment. At the end of every experiment blood (urine if possible) was collected, mice were killed and kidney and liver harvested and stored at –70°C.
Blood samples from untreated animals were collected one week before virus injection. Urine samples were collected in metabolic cages between day 10 and 11 after virus treatment. After euthanasia, on day 10 or day 11 after adenoviral injection, blood samples, kidney, liver, lung and heart were collected and frozen on dry ice or fixed with formaldehyde. Liver weight was also documented as well as the macroscopic appearance of liver and spleen by digital photography. Liver sFlt-1 concentration (endogenous and exogenous concentration) as well as circulating msFlt-1-Fc and urinary sFlt-1-Fc were quantified by ELISA as described above. We measured urinary albumin by a competitive ELISA from Cell Trend GmbH (Berlin, Germany). We used harvested livers and kidneys for histopathology and some kidneys for electron microscopy. Lysates from liver and kidneys were done with RIPA buffer performed with a dispersing device for homogenization. Further lysates from livers and kidneys were also used to determine levels of exogenously induced and endogenous proteins.
Telemetry and blood pressure measurements
The telemetric technique was described in detail elsewhere [28]. Mice were anesthetized with isoflurane (CuraMed Pharma, Karlsruhe, Germany). The pressure-sensing catheter of the TA11PAC20 BP device (Data Sciences International, Saint Paul, MN, USA) was advanced via the carotid artery into the ascending aorta, and the transmitter was placed in a subcutaneous pocket along the right flank. The mice were synchronized to a light-dark schedule of 12: 12 h with lights on at 6: 00 a.m.. All mice were allowed 10–14 days recovery from surgery before baseline blood pressure (BP) and heart rate (HR) values were recorded for 3 days. By this time, the mice had regained their circadian BP and HR rhythm, and the surgery and anesthesia-dependent initial changes in systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MAP) and HR were followed by stable values. Directly after injecting adenoviruses into the tail vein data sampling was continued. All data from TA11PA-C20 device were sampled every 5 min for 10 sec continuously day and night with a sampling rate of 1000 Hz and stored on a hard disk. The BP measurements were conducted over a period of 10 days after virus application. SBP, DBP and HR were recorded using the DATAQUEST software (A.R.T. 2.1; Data Sciences International). HR was computed from the pulse intervals of the BP recordings.
Histology and electron microscopy
The kidneys of all animals used in this study (n > 300) were examined histopathologically. Freshly excised kidneys were immersion-fixed in neutral buffered 10% formalin, paraffin embedded, sectioned at 5 μm thickness and stained with hematoxylin and eosin (HE) as well as by PAS reaction. Glomerular lesions were analysed in at least 10 glomeruli of the inner cortex region per animal and were examined for endothelial swelling, occlusion of capillary loops and protein resorption droplets. For electron microscopy, renal tissues were fixed according to Karnovsky, pre-contrasted with 1% osmium tetroxide, dehydrated in 70% ethanol containing 1% uranyl acetate and 0.5% phosphotungstic acid and embedded in epoxid resin. Sections of 30 to 50 nm thickness were contrasted with lead citrate and examined using a Zeiss 900 transmission electron microscope.
Immunohistochemistry
Immunohistochemistry was performed on serial frozen or formaldehyde-fixed wax-embedded sections as described previously [29]. Briefly, cryostat sections were fixed in acetone at –20°C for 20 min, endogenous peroxidase activity was neutralized with 0.3% (v/v) H2O2 in methanol for 15 min and blocked with 10% normal serum in PBS for 20 min. Sections were incubated with primary antibody for 1 hr at room temperature. Non-immune goat serum was substituted for the primary antibody as a negative control. After washing in PBS, sections were incubated with a 1: 1.000 dilution of the appropriate biotinylated secondary antibodies (Vector Laboratories, Ltd., Peterborough, UK) for 30 min and then streptavidin-peroxidase complex (Vector Laboratories) for 30 min. Staining was visualized using diaminobenzidine (Dako UK, Ltd., Cambridgeshire, UK) and sections were counterstained with haematoxylin.
Albumin measurements and protein silver staining in urine samples
Visualization of total proteins was carried out by protein gel electrophoresis. Therefore, 1.0 μl of urine from treated or untreated mice was placed in 11 μl Laemmli buffer, boiled and loaded on a 10% SDS-PAGE gel. An SDS-PAGE broad-range standard (protein ladder, Fermentas, Germany) was loaded on the first lane on the gel and bands were visualized by silver staining.
Statistics
The mean and standard error were calculated on most of the parameters determined in this study. Data are given as mean ± SEM. ANOVA was used to determine statistical differences. Significant differences are reported when P < 0.05.
Results
Soluble Flt-1 induces histological changes in mouse kidneys
As reported earlier, adenovirus-vector-mediated overexpression of sFlt-1 in pregnant rats led to the formation of renal damage indicated by glomerular endotheliosis, proteinuria and hypertension [21]. To establish a mouse model we started to inject AdsFlt-1 from 1 × 106 pfu until 2 × 1010 pfu into the tail vein.
Most of the doses either had no effect on the kidney histology (≤ 1 × 109 pfu) or were lethal within the 10 day time course (> 7.5 × 109 pfu). However, injection of AdsFlt-1 between 2.5 and 3.5 × 109 pfu-induced moderate histochemical changes in the glomeruli and doses between 5 and 7.5 × 109 pfu-induced severe histological changes including severe endotheliosis and occlusion of capillary lumens (Fig. 1A). No significant changes in morphology were detected in AdveGFP-treated or untreated animals (Fig. 1A). Electron microscopy revealed that endothelial cells and podocytes were both affected in sFlt-1-treated animals (Fig. 1B). Capillary lumens were occluded by microthrombi, endothelial cells were detached from the basal membrane and macrophages were present in thrombotic regions. In addition, macrophages as foam cells could be seen and podocytes showed segmental effacement of foot processes (Fig. 1B). Furthermore, fragmented erythrocytes and microthrombi were seen after Masson Goldner staining as well as the lack of fenestrated endothelium (Fig. 1C). Although the erythrocytes were restricted to capillary loops as observed in untreated animals, fragmented erythrocytes accompanied by erythrocyte dust were widespread in the glomeruli of treated animals. This characteristic glomerular lesion represents a form of glomerular endotheliosis, the thrombotic microangiopathy. Nevertheless, the mice appeared healthy after a 10 days treatment period and showed no unusual behaviour, making cerebral involvement unlikely. As stated before, some single experiments were also made with pregnant mice. Injection early in pregnancy (e.g. day 4 of pregnancy) resulted either in termination of pregnancy or in strong retarded embryos whereas injections late in pregnancy (day 8 or 9) had macroscopically no effect on the appearance of the embryos (not shown). Even if not investigated in a detailed way, early treatment seems to have a strong negative effect on vascular placental development. However, the high heterogeneity of the results prompted us not to investigate changes in more detail.
Fig 1.
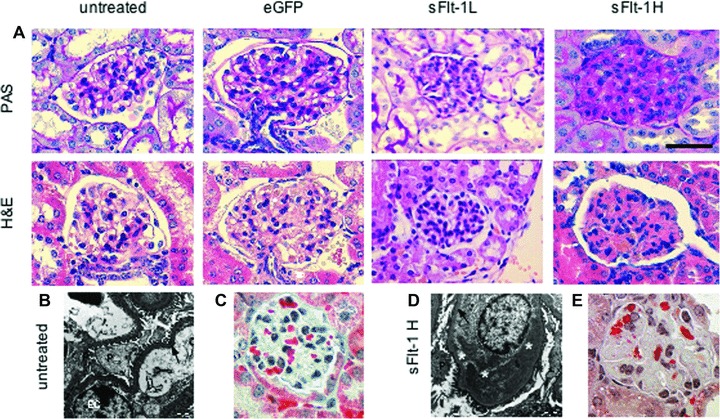
Renal histology in mice either untreated or after treatment with AdvGFP or AdvsFlt-1. (A) Renal histology in mice after GFP (control) and sFlt-1 groups. Untreated and eGFP-treated mice show no glomerular changes. Low-dose soluble Flt-1- (2.5 × 109 pfu = L) injected mice develop moderate swelling of glomerular endothelial cells and mesangial cells with limited occlusion of the glomerular capillary lumen. In contrast, high-dose soluble Flt-1- (7.5 × 109 pfu = H) injected mice show severe occlusion of glomerular capillary lumen. H&E hematoxylin and eosin stain; PAS periodic acid Schiff reaction; scale bar 50 μm (B and D). Transmission EM of the ultra structure in untreated and sFlt-1-treated mice demonstrated regular foot processes of podocytes and missing of endothelial fenestration after treatment. Ten days after treatment (d) podocytes and endothelial cells are affected and microthrombi can be found in the former capillary lumen. Foot processes are partly regular and partly effaced.; P = podocyte, EC = endothelial cell, arrow = basal membrane, * deposit of microthrombi inside of vessel lumen, bar = 1 μm; (C and E) Masson Goldner stain indicates intact erythrocytes (bright red) in the glomerulus of untreated mice (C) and fragmented erythrocytes inside the glomeruli of sFlt-1-treated mice (E).
Effects mediated by sFlt-1 can be rescued by VEGF co-administration
Complex formation between VEGF and sFlt-1 to reduce the bioavailability of VEGF is an important regulatory mechanism to maintain angiogenic balance and is responsible for the avascular cornea in mammals [30].
The pathological effect mediated by sFlt-1 treatment (3.5 × 109 pfu) could be rescued by injection of 1 × 108 VEGF-encoding virus particles at the same time when sFlt-1 was injected (Fig. 2). Higher sFlt-1 doses had not been tested for combinations. Compared to the sFlt-1 virus amount, this dose seems to be very low. However, double that concentration of Adv has been shown before to be lethal in mice and we were able to confirm it exactly [31]. Our observation also indicates that the almost maximum tolerable VEGF amount was induced in the mouse model and that both groups had used a similar bioassay to determine the titre of the virus preparations. Strikingly, the histological analysis of glomeruli of mice administered both viruses was similar to control mice, indicating a significant improvement in capillary patency, a reduction of deposits and endothelial swelling (Fig. 2). Subendothelial accumulation of fibrin and albumin characterizes severe endothelial damage and is associated with rapid loss of renal function. sFlt-1-treated rats showed focal deposition of fibrin within glomeruli [21]. Similarly, AdvsFlt-1-treated mice showed an increase in fibrin and albumin immunostaining in the glomeruli, while AdvVEGF alone treated animals showed no fibrin and albumin (Fig. 2). However, coinfection with AdvsFlt-1 and AdvVEGF produced a marked reduction in expression of albumin and fibrin when compared to AdvsFlt-1-treated mice (Fig. 2).
Fig 2.
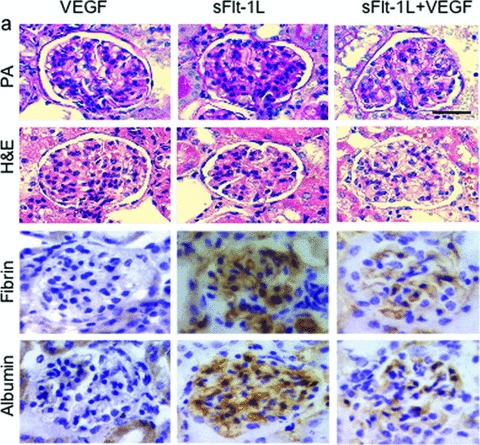
Renal histology in rescue mice. Renal histology in mice after VEGF (1 × 108 pfu) and sFlt-1 (3.5 × 109 pfu) treatment, and in sFlt-1+VEGF groups. VEGF-treated mice show no significant lesions. Soluble Flt-1-injected mice show severe endotheliosis with occlusion of capillary lumens. Soluble Flt-1 + VEGF-treated animals show mild effects of glomerular endotheliosis and much less accumulation of fibrin and albumin seen in sFlt-1-treated animals. HE haematoxylin and eosin stain, PAS periodic acid Schiff reaction; IHC for fibrin and albumin was counterstained with haematoxylin; scale bar 50 μm.
Quantitative measurements of sFlt-1 in plasma indicated that the circulating concentration was lowered from 32 ± 15,9 ng/ml to about 10.6 ± 3,4 ng/ml (Fig. 3A) in rescued mice. A similar decrease was found in 24-h urine of treated animals. Here, the detectable concentration was 8 ± 0,6 ng/ml and declined to 1.3 ± 1,8 ng/ml in co-treated animals. Injection of high-dose sFlt-1 led to concentrations of 79 ± 29,6 ng/ml in plasma and 36.9 ± 25,6 ng/ml in urine when measured by sandwich ELISA (Fig. 3B). However, the highest sFlt-1 concentrations were found in liver tissue, which ranged between 200 and 1000 ng/mg total protein (data not shown). Interestingly, in AdvsFlt-1-treated mice, the concentration of total sFlt-1 protein in the kidney (endogenous and exogenous) was more than tripled, compared to untreated animals (87,7 ± 4,5 ng/mg total protein versus 26.1 ± 16,3 ng/mg total protein, data not shown). The concentrations detected in plasma were similar to recent rat and mouse models where AdV have been used for sFlt-1 overexpression [21, 32–33]. However, the concentrations in our mouse model were about 10-fold higher than the concentrations found in patients with severe PE but taken into account, that the concentrations were measured with different assays for human and mouse sFlt-1 [8, 21].
Fig 3.
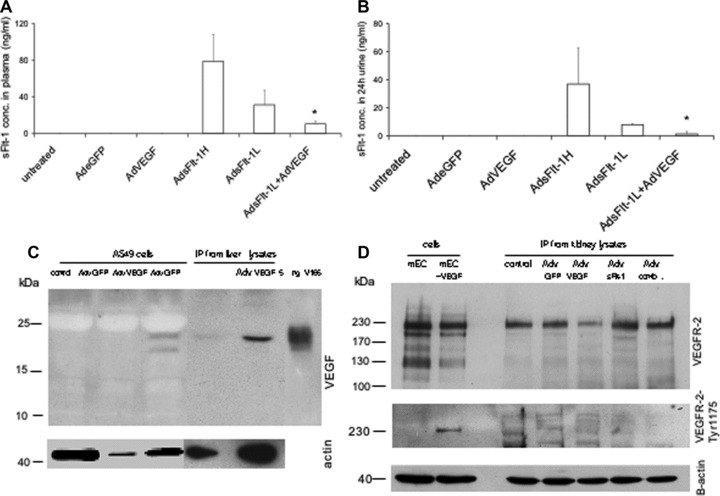
Quantitative measurement of mouse sFlt-1 by ELISA and detection of VEGF-A and VEGF-R in mice. In mice either untreated (n= 4) or treated with Adv for eGFP (n= 4) or AdvVEGF (n= 6) endogenous sFlt-1 concentrations are under the detection level in plasma (A) and in urine (B). Mice treated with high concentrations of Adv for sFlt-1 (5–7,5 × 109 pfu, n= 10) show extremly high concentrations and mice treated with lower levels, which were also used for rescue experiments (2,5–3,5 × 109 pfu, n = 10) have a reduced level for sFlt-1 in plasma and urine. Rescue experiments with AdsFlt-1 and AdVEGF (1 × 108, n = 4) have significant reduced levels in plasma und urine. Data are mean (±SEM) of several mice used for each experiment. *P < 0.05 as compared with low-dose sFlt-1- (AdsFlt-1- L) treated mice. Representative detection of VEGF-A (C) and VEGFR-2/FLK-1 (D) in adenovirus-treated cells and mice by reducing Western blot. To detect VEGF-A 1 mg cell lysates was used for IP. For IP from kidney lysates 5 mg total protein was immunoprecipitated with an anti-VEGF-A monoclonal antibody. As positive control 5 ng rh VEGF-A (isoform 165 from insect cells) was used directly. For sample loading beta-actin was detected by immunoblotting for the same blot with an appropiate antibody. To detect the VEGFR-2/Flt-1 total protein or the phosphorylated protein, 20 μg cell lysates from mouse endothelial cells either unstimulated or stimulated with VEGF-A (50 ng/ml over 10 min) was loaded directly on the gel. For IP from kidney lysates 2 mg of total protein was immunoprecipitaed with an anti-mouse VEGFR-2 antibody and immunoblotted with the same antibody. For detection of phosphorylated mouse VEGFR-2 400 μg cell lysates and 4 mg kidney lysates were used for IP followed by immunoblotting with an antibody for phospho-VEGFR-2-Tyr1175. For sample loading beta actin was detected by using 40 ug lysates from each samples for immunoblotting with an appropiate antibody.
Verifying VEGF-A overexpression in mice and changes of VEGFR-2 levels in kidneys
To verify the overexpression of VEGF-A by adenovirus treatment we used infected cell lysates and liver lysates from Adv VEGF-treated animals together with immunprecipitation and immunoblotting. In infected cells, two bands between 22 and 24 kD corresponding to unglycosylated and glycosylated VEGF-A were found (Fig. 3C). Liver lysates from Adv VEGF-A-treated mice show a significant higher amount of exogenous VEGF-A and only the full glycosylated protein can be detected. Endogenous VEGF-A was detected in minor amounts in the liver (Fig. 3C). Based on the structural changes of the capillaries observed in the glomerulus, we were further interested to find out, if the structural changes influenced the amount of VEGFR-2/Flk1 in the whole kidney or if the phosphorylation status of the receptor molecule is changed in this organ after treatment with antagonists. The detection of VEGFR-2 by IP and immunoblotting was straight forward in mouse endothelial cells. Two bands of about 210 and 230 kD can be found where the larger bands represent the full glycosylated molecule. Stimulation of these cells after starvation with VEGF-A over 10 min leads to phosphorylation of the 230 kD receptor protein, verified by an antibody specific for phospho Tyr1175 of VEGFR-2. No significant changes of the receptor protein amounts were observed after different adenoviral treatment of mice. However, mice treated with Adv VEGF-A show a down-regulation of VEGFR-2 as a result of VEGF-A overexpression in the animal. No receptor down-regulation was observed in mice co-treated with Adv sFlt-1 and VEGF-A. Further, we were not able to detect phosphorylated VEGFR-2 in kidney lysates with our antibodies. Confirmation of phosphorylated VEGFR-2 in kidney lsates resulted merely in unspecific bands although immediately shock-frozen tissue was used. Probably the used antibodies were not able to detect phosphorylated VEGFR-2 in our kidney lysates.
Glomerular function can be changed by sFlt-1 and sFlt-1/VEGF treatment
Mice treated with high and low doses sFlt-1 had heavy albuminuria (Fig. 4A), but not control mice. Co-treatment with AdvVEGF and AdvFlt-1 inhibited proteinuria, as indicated by polyacrylamide gel electrophoresis and silver staining of 24-h urine. In these rescued mice, the albumin concentration was reduced from 15.6 ± 3,0 mg/ml to 0.13 ± 0,05 mg/ml (Fig. 4B), a reduction to the range of control animals. In addition, the albumin creatinine ratio determined in control animals, sFlt-1-treated and VEGF/sFlt-1-treated animals. Low dose sFlt-1-treated animals had a urine albumin/creatinine ratio of about 49,2 ± 3,3 mg/mg. However, VEGF-co-treated showed a ratio of only 0,27 ± 0,04 mg/mg, which was very similar to untreated animals that were in the range of 0,15 mg/ml (data not shown).
Fig 4.
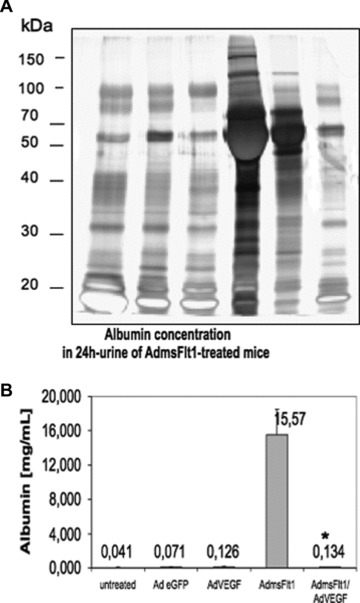
Functionality of glomerular filtration of sFlt-1-treated mice after a 10 days time period. (A) SDS-PAGE analysis was performed with 1 μl of representative mouse urine samples. Lane 1 contains urine from untreated mice, lane 2 urine from eGFP mice (2 × 109 pfu) and lane 3 shows urine from mice treated with Adv for VEGF (1 × 108 pfu). Lane 4 and 5 contain urine from mice treated with AdvsFlt-1 (7,5 × 109pfu (H) and 3.5 × 109 pfu (L), respectively) and lane 6 urine from a rescue experiment. The presence of large amounts of albumin around 67 kD is identified in the AdvsFlt-1-treated mouse and demonstrated damage of the kidney filter. (B) Mouse albumin was measured by ELISA in 24-h urine from untreated (n= 6), GFP (2 × 109 pfu, n= 2), VEGF (1 × 108pfu, n = 3) and msFlt-1- (3,5 × 109 pfu, n= 3) treated animals. For rescue mouse were co-treated with msFlt-1 and VEGF (n= 2). Data are mean (±SEM) of several mice used for each experiment. *P < 0.01 as compared with sFlt-1- (AdsFlt-1) treated mice.
Effects of sFlt-1 overexpression in the absence and presence of VEGF on blood pressure
Hemodynamic effects following AdvsFlt-1 injection or after co-treatment with AdvVEGF and AdvsFlt-1 was monitored using telemetry. Injection of adenovirus for eGFP (2 × 109 pfu) had almost no effect on the heart rate but led to a decrease in SBP (–5 mm Hg) and DBP (–16 mm Hg) that normalized after several days (Fig. 5). Overexpression of sFlt-1 resulted in significant increases in SBP (149 versus 130 mm Hg) and DBP (119 versus 100 mm Hg), compared to the eGFP-treated animals before virus injection. Co-administration of AdvsFlt-1 and AdvVEGF led to an initial sharp decrease in heart rate and reduction of blood pressure (Fig. 5) for the first 2 days and returned thereafter to baseline values (SBP 122 versus 125; DBP 93 versus 101). The sharp decrease in blood pressure was probably because of the effect of VEGF, which is also a potent hypotensive factor [34–35]. There probably was an initial excess of free VEGF in the circulation, which was later complexed with the corresponding receptor and led to normal heart rate and blood pressure. These results are in agreement with the histology and proteinuria data we analysed from rescue experiments.
Fig 5.
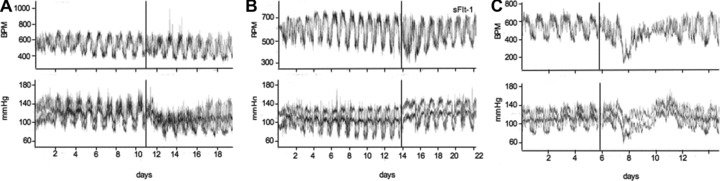
Representative recordings of heart rate and systolic/diastolic blood pressure. The injection time-point of adenoviruses is indicated by a vertical line on the time axis. Recording of data was estimated for 8–10 days after treatment. Side a shows the treatment with AdveGFP (2 × 109 pfu), side b with AdvsFlt-1 (3 × 109 pfu) and side c shows the co-treatment with AdvsFlt-1 (3 × 109 pfu) and AdvVEGF (1 × 108 pfu) for rescue experiments. Indicated is the heart rate (HR) in beats per min (BPM, upper panel) and the systolic (SBP, lower panel, upper graph) and diastolic (DBP lower panel, lower graph), blood pressure in mm Hg.
In vitro complex formation between VEGF165 and sFlt-1
Measurements to quantify free VEGF either by our own ELISA or with a commercial ELISA (R&D Systems) in the serum of VEGF-treated mice were not successful probably because of the low half life during circulation and the low amount of free VEGF (data not shown). However, complex formation by the decrease of detectable sFlt-1 by VEGF co-expression could be easily shown in vitro with A549 cells. Soluble Flt-1 concentrations in the supernatant which could be measured by ELISA decreased between 70% and 80% when VEGF was co-expressed in low amounts (Fig. 6). Furthermore, sFlt-1 measurements of complementary cell lysates verified observations made in the supernatant of co-infected cells (not shown). These results indicated, that the lower amounts of sFlt-1 detected in Adv co-treated animals were because of the inability of the ELISA’s to detect complexed (liganded) sFlt-1.
Fig 6.
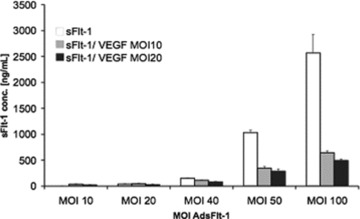
ELISA quantification of in vitro complex formation between sFlt-1-Fc and VEGF in cell supernatants. Five hundred fifty-nine cells were infected with increasing amounts of Ad-sFlt-1in the absence (white bars) or in the presence of Ad-VEGF with a MOI of 10 (grey bars) or with MOI of 20 (black bars). The amounts of sFlt-1 in the supernatant 72 hrs after infection were diluted and measured by an ELISA for mouse Flt-1. Indicated are the mean values from duplicates (mean ± SEM).
Discussion
This study demonstrates that infection of non-pregnant and pregnant mice with adenovirus carrying mouse sFlt-1 leads to an increase in circulating levels of sFlt-1 followed by albuminuria and hypertension. We further show that co-administration of AdvsFlt-1 with AdvVEGF neutralizes circulating free sFlt-1 and the damaging effects of sFlt-1 on the kidneys are ameliorated and blood pressure normalized. Previous reports in human beings suggested that perturbation in free VEGF and PlGF plasma levels weeks before the clinical onset and manifestation of PE, point to a systemic endothelial dysfunction and a possible mechanism [7–8]. However, according to our working hypothesis, blocking of VEGF and PlGF bioavailability at local sites of paracrine and autocrine loops are the primary cause of endothelial dysfunction. The circulating levels of VEGF and PlGF were significantly lower than the KD of VEGFR-1 and -2, and therefore should not have had the observed biological effects [36–37]. For example, free VEGF declined from about 0.3 pM to 0.14 pM in women with clinical PE, wheras the KD of VEGFR-2 was calculated with 760 pM [8, 36]. However, our results in combination with previous reports in rat and mouse by Maynard et al.[21] and Lu et al. [33] strongly support a causative role for sFlt-1 in endothelial dysfunction, either primary or intermediary.
We used classical adenovirus vectors that express sFlt-1 and VEGF, either separately or in combination. Because of vector tropism, such vectors will be sequestered primarily in the liver when injected [38]. This fact was nicely demonstrated by our ELISA measurements of liver samples, where up to 0.1% of the liver total protein was sFlt-1. However, the high serum and urine level of sFlt-1 (Fig. 1C) suggested that the delivered adenoviruses effectively transduced murine liver cells that subsequently secreted sFlt-1 and VEGF into the systemic circulation. As a matter of fact, the amount of total Flt-1 (endogenous and exogenous) almost tripled in the kidney after 10 days of treatment. In another model of endothelial dysfunction and crescentic glomerulonephritis, rats were treated with antibodies for antiglomerular basement membranes and then transfected with plasmid DNA encoding the murine sFlt-1 gene into the femoral musculature [39]. Here, sFlt-1 accelerated the progression of glomerular sclerosis and interstitial fibrosis during a 56-day time course. The effects observed by the additional sFlt-1 transfection were mild compared to our model. Also sFlt-1 concentrations in the urine after 6-days of treatment from about 0.2 ng/ml were 50–100-fold lower than our concentrations [39]. However, over a longer time course, VEGF inhibition in this model accelerated glomerular and peritubular endothelial loss.
Ideally, conditional expression of sFlt-1 in placental trophoblasts in pregnant mice induced in the last trimester of the pregnancy would be the best experimental model. Therefore, overexpression of sFlt-1 in the liver does not mimic the development of PE. In our mouse model, complex formation of VEGF with its corresponding soluble receptor takes place in liver cells or in the circulation. This situation is different from human PE, where sFlt-1 is produced by cytotrophoblasts and enters the maternal circulation and tissue compartments [21]. This site is probably the place where dimerisation takes place. Complex formation between VEGF and excess sFlt-1 and blocking the loss of endogenous VEGF can be also initiated by administration of recombinant VEGF121. This fact was shown very recently in a rat model with adenoviral overexpression of sFlt-1 [40]. Human recombinant VEGF (up to 400 μg/kg) was injected once or twice daily subcutaneously over a period of several hours. Treatment with exogenous VEGF121 also reduced symptoms characteristic for PE, such as increased blood pressure, albuminuria and kidney damage. Both studies showed that the administration of exogenous VEGF in a mouse-and-rat model for PE can alleviate the symptoms caused by high systemic concentrations of sFlt-1 when applied over 8–10 days.
Extrapolating the data to human PE is difficult, because in human beings circulating sFlt-1 concentrations are between 2 and 10 ng/ml in plasma over a 3–4-month time period, namely about 3–10 fold higher than in normal pregnancies. Reducing the sFlt-1 levels to below a given threshold by induction of complex formation with the corresponding ligand may be one therapeutic approach. However, treatment with blocking antibodies for Flt-1/VEGFR-1 is also a possibility and may have fewer side effects than delivery of VEGF in patients. More important, the paracrine and autocrine VEGF signalling required for normal vascular homeostasis will not be affected by this approach [41].
More recently, a second soluble receptor protein known as sEng or CD105 having an activity as a TGF-β co-receptor was reported to be involved in the pathogenesis of PE [42]. Placental-derived sEng is elevated in the sera of preeclamptic woman and may correlate with disease severity. Co-administration with AdvsFlt-1 and AdvsEng led to PE including the hemolysis, elevated liver enzyme and low platelet (HELLP) syndrome and restricted fetal growth in pregnant rats. Very recently, it was shown that statins (HMG-CoA reductase inhibitors), a class of lipid-lowering drugs, inhibit cytokine-mediated release of sFlt-1 and sEng [43]. Based on these findings, statins offer a logical potential therapy and our study demonstrates that a reduction in circulating sFlt-1 alleviates PE-like signs thus sFlt-1 appears to be a not only a valid marker for a clinical outcome measure but directly linked to signs of PE.
Acknowledgments
We would like to thank Brigitte Pawletta and Ilona Kamer for technical assistance and the staff from the HZI animal unit (TEE) for help with the mouse work. We gratefully acknowledge support from Friedrich C. Luft, Florian Herse, Ralf Dechend and Norbert Gretz.
The study was funded by grants from the German Research Council (DFG grant We1211/5–3 + 7–1) to Dr. Herbert A. Weich and from the Medical Research Council (G0601295 and GO0700288) to Professor Asif Ahmed.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–94. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 3.Sibai B, Dekker G, Kupferminc M. Preeclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 4.Espinoza J, Romero R, Nien JK, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gyneco. 2007;196(326):e321–e13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening. BMJ. 2002;325:57–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 8.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 10.Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 11.Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–51. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 12.Hertig A, Berkane N, Lefevre G, et al. Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin Chem. 2004;50:1702–03. doi: 10.1373/clinchem.2004.036715. [DOI] [PubMed] [Google Scholar]
- 13.Crispi F, Dominguez C, Llurba E, et al. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–7. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Yuan HT, Haig D, Karumanchi AS. Angiogenic factors in the pathogenesis of preeclampsia. Curr Top Dev Biol. 2005;71:297–312. doi: 10.1016/S0070-2153(05)71009-7. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed A. Heparin-binding angiogenic growth factors in pregnancy. Trophoblast Res. 1997;10:215–58. [Google Scholar]
- 16.Maharaj AS, Walshe TE, Saint-Geniez M, et al. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205:491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornig C, Weich HA. Soluble VEGF receptors. Angiogenesis. 1999;3:33–9. doi: 10.1023/a:1009033017809. [DOI] [PubMed] [Google Scholar]
- 18.Vuorela P, Helski S, Hornig C, et al. Amniotic fluid-soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol. 2000;95:353–7. doi: 10.1016/s0029-7844(99)00565-7. [DOI] [PubMed] [Google Scholar]
- 19.Widmer M, Villar J, Benigni A, et al. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol. 2007;109:168–80. doi: 10.1097/01.AOG.0000249609.04831.7c. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto H, Hamano Y, Charytan D, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278:12605–8. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 21.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makris A, Thornton C, Thompson J, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFlt-1. Kidney International. 2007;71:977–84. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 23.Hornig C, Barleon B, Ahmad S, et al. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest. 2000;80:443–54. doi: 10.1038/labinvest.3780050. [DOI] [PubMed] [Google Scholar]
- 24.Bando H, Brokelmann M, Toi M, et al. Immunodetection and quantification of vascular endothelial growth factor receptor-3 in human malignant tumour tissues. Int J Cancer. 2004;111:184–91. doi: 10.1002/ijc.20211. [DOI] [PubMed] [Google Scholar]
- 25.Compagni A, Wilgenbus P, Impagnatiello MA, et al. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Research. 2000;60:7163–69. [PubMed] [Google Scholar]
- 26.Mayer H, Bertram H, Lindenmaier W, et al. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005;95:827–39. doi: 10.1002/jcb.20462. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton NA, Russo RC, Thurston RV. Trimmed Spearman-Kärber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol. 1977;11:714–19. [Google Scholar]
- 28.Gross V, Tank J, Obst M, et al. Autonomic nervous system and blood pressure regulation in RGS-2-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1134–R42. doi: 10.1152/ajpregu.00246.2004. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–91. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 30.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGFR-1. Nature. 2006;443:993–7. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nature Med. 2000;6:460–63. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 32.Mahasreshti PJ, Kataram M, Wang MH, et al. Intravenous delivery of adenovirus-mediated soluble Flt-1 results in liver toxicity. Clinical Cancer Res. 2003;9:2701–10. [PubMed] [Google Scholar]
- 33.Lu F, Long M, Tamayo E, et al. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196:396e1–396e7. doi: 10.1016/j.ajog.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Komori Y, Sugihara H. Purification and physiological study of a hypotensive factor from the venom of Vipera aspis (Aspic viper) Toxicon. 1990;28:359–69. doi: 10.1016/0041-0101(90)90073-g. [DOI] [PubMed] [Google Scholar]
- 35.Yang R, Bunting S, Ko A, et al. Substantially attenuated hemodynamic responses to Escherichia coli-derived vascular endothelial growth factor given by intravenous infusion compared with bolus injection. J Pharmacol Exp Ther. 1998;284:103–10. [PubMed] [Google Scholar]
- 36.Waltenberger J, Claesson-Welsh L, Siegbahn A, et al. Different signal transduction properties of KDR and Flt-1 two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–95. [PubMed] [Google Scholar]
- 37.Olander JV, Connolly DT, DeLarco JE. Specific binding of vascular permeability factor to endothelial cells. Biochem Biophys Res Commun. 1991;175:68–75. doi: 10.1016/s0006-291x(05)81201-x. [DOI] [PubMed] [Google Scholar]
- 38.Everts M, Curiel DT. Transduction targeting of adenoviral cancer gene therapy. Curr Gene Ther. 2004;4:337–46. doi: 10.2174/1566523043346372. [DOI] [PubMed] [Google Scholar]
- 39.Hara A, Wada T, Furuichi K, et al. Blockage of VEGF accelerates proteinuria, via decrease in nephrin expression in rat screscentic glomerulonephritis. Kidney International. 2006;69:1986–95. doi: 10.1038/sj.ki.5000439. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Zhang Y, Ying M, et al. Recombinant vascular endothelial growth factor 121 attenuates Hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–92. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkateshda S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nature Med. 2006;12:642–6. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 43.Cudmore M, Ahmad S, Al-Ani B, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–97. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]


