There are corrections concerning the figures and figure legends of an article published in a previous issue of the Journal of Cellular and Molecular Medicine[1]. Figures 2 and 7 were missing asterisks denoting statistical significance while Figs. 3, 4, 5, 6, 7 and 8 had mismatched legends. The corrected figures and their legends are shown below:
Fig 2.
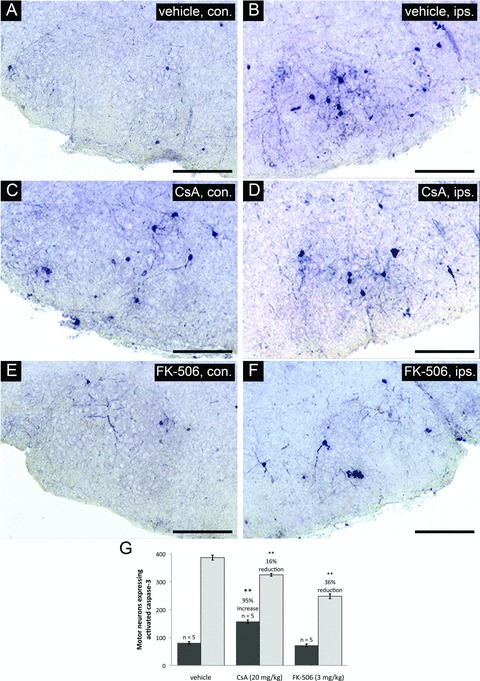
Coronal cross-sections through the facial nucleus were examined for activated caspase-3 at 20 hours following axotomy for drug treatment groups and vehicle-treated controls. Panels (A), (C), and (E) show sections through uninjured facial nuclei (contralateral – con.). Panels (B), (D), and (F) show sections through injured facial nuclei (ipsilateral –ips.). Scale bars represent a distance of 250 μm. (G) Stereologic counts of activated caspase-3 positive neurons through the facial nucleus show that both CsA and FK-506 treatments significantly reduced the number of motor neurons positive for activated caspase-3 compared to vehicle treatment (**indicates statistical significance at p < 0.01 between treatment groups and vehicle controls). Notably, CsA treatment was observed to increase the total number of motor neurons with activated caspase-3 in the uninjured facial nuclei compared to vehicle controls (**indicates statistical significance at p < 0.01 between CsA and vehicle treatments) while FK-506 did not appear to have any effects on uninjured facial motor neurons.
Fig 7.
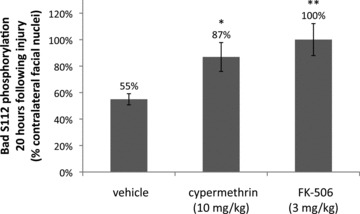
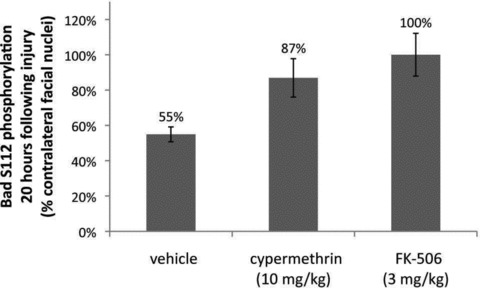
Inhibition of calcineurin-mediated Bad S112 dephosphorylation by cypermethrin and FK-506. Levels of Bad S112 phosphorylation were examined y immunofluorescence in coronal sections of injured and uninjured facial nuclei for vehicle, cypermethrin and FK-506-treated animals at 20 hrs following facial nerve axotomy. Bad S112 phosphorylation in the injured facial nuclei was significantly reduced following axotomy to 55 ± 4% compared to the uninjured facial nuclei (P < 0.01). Both cypermethrin and FK-506 treatments inhibited Bad S112 dephosphorylation by calcineurin and restored phosphorylation levels in the injured facial nuclei to 87 ± 11% (* indicates statistical significance at P < 0.05 between vehicle and cypermethrin treatment) and 100 ± 12% (** indicates statistical significance at P < 0.01 between vehicle and FK-506 treatment) of the uninjured facial nuclei, respectively.
Fig 3.
CsA and FK-506 do not suppress levels of activated microglia following facial axotomy. Cryostat sections through facial nuclei were labeled with tomato lectin to examine numbers of activated microglia at 4 days following motor neuron injury. (A), (D) and (G) show sections of unoperated (contralateral – con.) facial nuclei. (B), (E) and (H) show sections through operated (ipsilateral – ips.) facial nuclei. Scale bars indicate a distance of 500 μm. (C), (F) and (I) show higher magnification views of that shown in (B), (E) and (H), respectively. Scale bars represent a distance of 250 μm. Note that similar levels of microglial activation were observed between vehicle and CsA/FK-506 treatment groups.
Fig 4.
CsA and FK-506 do not suppress levels of reactive gliosis following facial axotomy. Sections were labeled with GFAP to examine numbers of reactive astrocytes at 4 days following facial axotomy. (A), (D) and (G) show sections through unoperated facial nuclei (contralateral – con.). (B), (E) and (H) show sections through operated facial nuclei (ipsilateral – ips.). Scale bars represent 500 μm. (C), (E) and (I) show injured facial nuclei at higher magnification. Scale bars represent 250 μm. Note that similar levels of reactive gliosis were observed between vehicle and CsA/FK-506 treatment groups.
Fig 5.
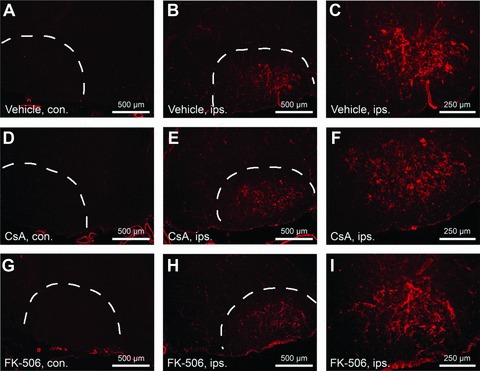
17-AAG and rapamycin treatments do not enhance facial motor neuron survival following injury. (A) Histogram of stereological counts of facial motor neurons from mice treated with 17-AAG (10 mg/kg) or vehicle performed at 4 days after axotomy. Similar to rapamycin, treatment with 17-AAG did not enhance levels of motor neuron survival compared to controls (22 ± 1% versus 25 ± 2% for 17-AAG and vehicle-treated groups, respectively), indicating these respective immunophilin-related pathways are not involved in neuroprotection. (B) depicts a typical njured facial nucleus from an animal treated daily with 17-AAG following axotomy. (C) Histogram of facial motor neuron survival following rapamycin treatment. Mice receiving daily rapamycin administration (3 mg/kg) following injury until time of killing showed no significant difference in motor neuron survival compared to vehicle-treated controls (17 ± 2% versus 13 ± 1%, respectively). Shown in (D) is a typical injured facial nucleus from rapamycin-treated animals.
Fig 6.
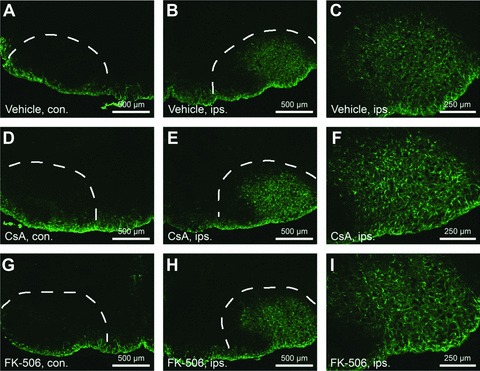
Direct calcineurin inhibition results in enhanced facial motor neuron survival following axotomy, but calcineurin A alpha (CNAα) plays a dispensable role in regulating neuronal survival. (A) Daily administration of cypermethrin (10 mg/kg) demonstrated enhanced facial motor neuron survival from 12 ± 1% in vehicle-treated animals to 32 ± 3% in cypermethrintreated mice, thus suggesting a prominent role for calcineurin inhibition in promoting neuronal survival (** indicates statistical significance between cypermethrin treatment and controls at p < 0.01). (B) shows an increased number of motor neurons in whole facial nuclei of cypermethrin-treated animals compared to controls. To further examine the role of calcineurin inhibition in enhancing neuronal survival following injury, axotomyinduced injury was performed in Ppp3ca null mice and heterozygous controls. (C) Histogram of facial motor neuron survival in Ppp3ca null mice. Mice homozygous for a targeted deletion of the dominant isoform of CNA in the CNS did not exhibit enhanced motor neuron survival compared to heterozygous littermates (19 ± 1% versus 21 ± 3%, respectively). (D) A typical injured facial nucleus from Ppp3ca null animals following axotomy, with no observable enhancement in motor neuron survival compared to heterozygous controls.
Fig 8.
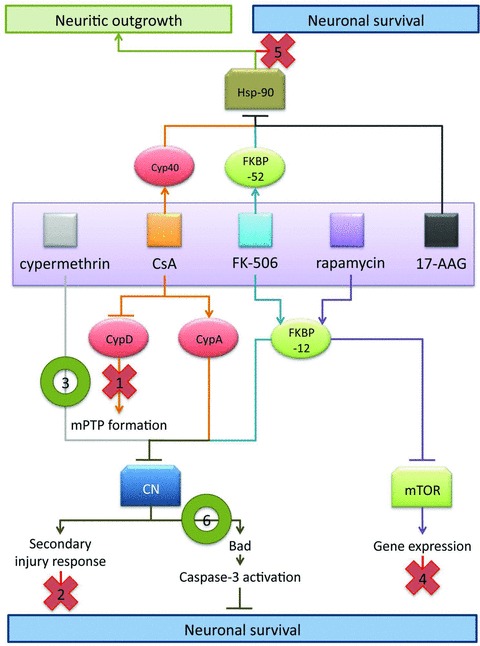
Mechanistic model of neuroprotection mediated by CsA and FK-506. Based upon results in facial motor neurons, the following model is proposed to explain the effects observed using CsA and FK-506. As indicated in this model, data from the present study suggest that the observed survival promoting and neurotrophic effects of these immunophilin ligands are mediated through distinct sets of molecular interactions. Evidence for exclusion of specific pathway is as indicated below. (1) CsA-mediated MPTP blockade is likely not the principle route of CsA-mediated neural rescue given that CsA and FK-506 treatment exhibit several similar mechanistic features, and co-administration of CsA and FK-506 did not enhance motor neuron survival over FK-506 treatment alone. (2) Administration of CsA or FK-506 did not act to reduce levels of reactive gliosis or infiltration of activated microglia; thus the enhanced survival of facial motor neurons was not due to repression of secondary injury responses. (3) Cypermethrin, an immunophilin-independent inhibitor of calcineurin, significantly enhanced motor neuron survival, hence highlighting calcineurin inhibition as a principal mechanism of regulating neuronal survival mediated by immunophilin ligands. (4) FK-506 and rapamycin both interact with FKBP-12, but only FK-506/FKBP-12 complexes inhibit calcineurin signalling. The failure of rapamycin to enhance motor neuron survival, following injury implicates calcineurin in the signaling pathway of motor neuron injury, and rules out mTOR-mediated effects. (5) Application of 17-AAG (a pharmacologic inhibitor of Hsp-90) failed to exhibit enhance motor neuron survival following injury, indicating that FK-506- and CsA-mediated enhancement in neuronal survival is distinct from the documented disruption in steroid receptor complex formation involved in enhancing neuritic outgrowth. (6) Inhibition of calcineurin-mediated Bad S112 dephosphorylation was observed at 20 hrs following axotomy for treatments with a single dose of either cypermethrin or FK-506, demonstrating a common mechanism by which cypermethrin, FK-506 and possibly CsA can enhance neuronal survival following injury.
We apologise for these errors.
References
- [1].Hui KKW, Liadis N, Robertson J, et al. Calcineurin inhibition enhances motor neuron survival following injury. J Cell Mol Med. 2010:671–86. doi: 10.1111/j.1582-4934.2009.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


