Abstract
Incidence of triple-negative breast cancer (TNBC), which is cancer without expression of ER, PgR, and HER2, and nuclear grade (NG) are closely correlated with malignant potential of breast cancer. However, criteria to determine aggressiveness of breast cancer based on these factors have not been elucidated. The aim of this study was to create criteria using these factors to predict tumor recurrence in invasive ductal carcinoma (IDC) of the breast. One hundred and seventy-nine patients with IDC of the breast, which had been treated by surgical resection, were included. One point was added for each factor of the two categories of TNBC and NG 3. The sum of the scores (TGS 0, 1, or 2) was calculated. Significant difference was observed between TGS and the incidence of tumor recurrence (P < 0.0001). Moreover, significant differences were observed regarding relapse-free survival (RFS) between patients with TGS 0 and TGS 1 (P < 0.0001) and patients with TGS 1 and TGS 2 (P = 0.024). TGS might contain a clinical advantage as a useful predictor for tumor recurrence of IDC of the breast and could classify prognosis of the patients with a preferable stratification.
Keywords: Breast, Invasive ductal carcinoma, Triple negative cancer, Nuclear grade, Tumor recurrence
Triple-negative breast cancer (TNBC), which is cancer without expression of ER, PgR, and HER2, has been shown to have tumors with more aggressive potential.1 Additionally, nuclear grade (NG) is a parameter quantifying the cytologic characteristics of cancer cells based on the atypia and mitosis of the tumor nuclei, and it has been demonstrated that it predicts the potential of cellular proliferation of breast cancer.2
While patient populations with breast cancer have been shown to have longer-term survivors when compared with those having other human malignant tumors,3 this may be due to the contribution of long-term continuous chemotherapy, molecular-targeting therapy, or hormone therapy for patients who had tumor recurrence of breast cancers, including TNBC or NG 3 breast cancers, which have more aggressive potential.
In this study, we attempted to create newly established criteria to predict tumor recurrence of invasive ductal carcinoma (IDC) of the breast based on the two factors TNBC and NG 3.
Patients and Methods
Patients
One hundred and seventy-nine patients with IDC of the breast, who had been treated by surgical resection and lymph node dissection in Fukuoka Higashi Medical Center at some point in the period from January 2004 to January 2011, were evaluated.
The ages of the patients ranged from 27 to 91 years, with a mean of 62; there were 31 premenopausal and 148 postmenopausal women.
Immunohistochemical expression
ER, PgR, and HER2 were examined using the usual immunohistochemical methods, and an additional fluorescence in situ hybridization examination was performed to determine HER2 expression for cases with an immunohistochemical expression of HER2 2+.
Pathologic investigation
Pathologic features were presented according to the general rules for clinical and pathologic recording of breast cancer established by the Japanese Breast Cancer Society,4 and the TNM classification of malignant tumors prescribed by the International Union Against Cancer.5
Definition of nuclear grade
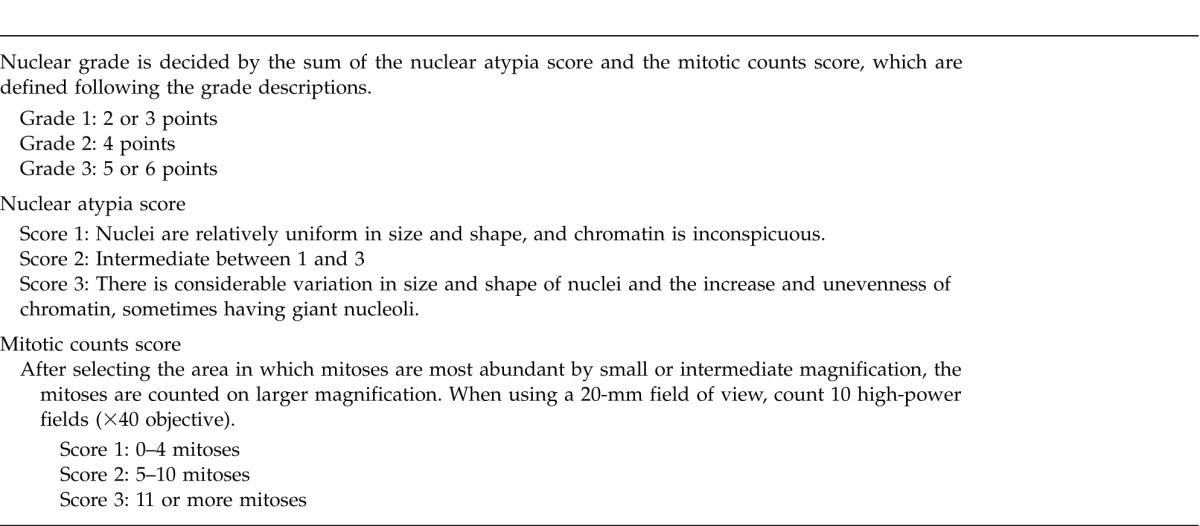
NG was determined as an aggregate of nuclear atypia score and mitotic count score according to the criteria in previous reports6,7 (Table 1).
Table 1.
Definition of nuclear grade
TNBC and NG 3 score
Patients who had both pathologic characteristics of TNBC and NG 3 were allocated a TGS score of TGS 2. Patients who had one of these characteristics were allocated a TGS 1, and patients who had none were allocated a TGS 0.
Then, correlation of the TGS values with clinicopathologic characteristics, including tumor recurrence and relapse-free survival (RFS) of the patients, was examined.
Neoadjuvant or adjuvant therapy
All of the patients with TGS 1 or TGS 2 and patients who had breast carcinomas with ER−, PgR−, and HER 3+ in the TGS 0 group had been treated with neoadjuvant or adjuvant therapy composed of cyclophosphamide, endoxane, 5-fluorouracil, and docetaxel.
Statistical analysis
All statistical analyses were conducted using StatView (SAS Institute Inc, Cary, North Carolina). Then, a χ2 test was used to compare the difference of proportion values between TGS scores. A Mann-Whitney test was used to compare the mean age of patients. Overall survival (OS) curves and RFS curves were made by the Kaplan-Meier method, and the Mantel-Cox test was used to analyze the equality of the survival curves. P < 0.05 was considered significant.
Results
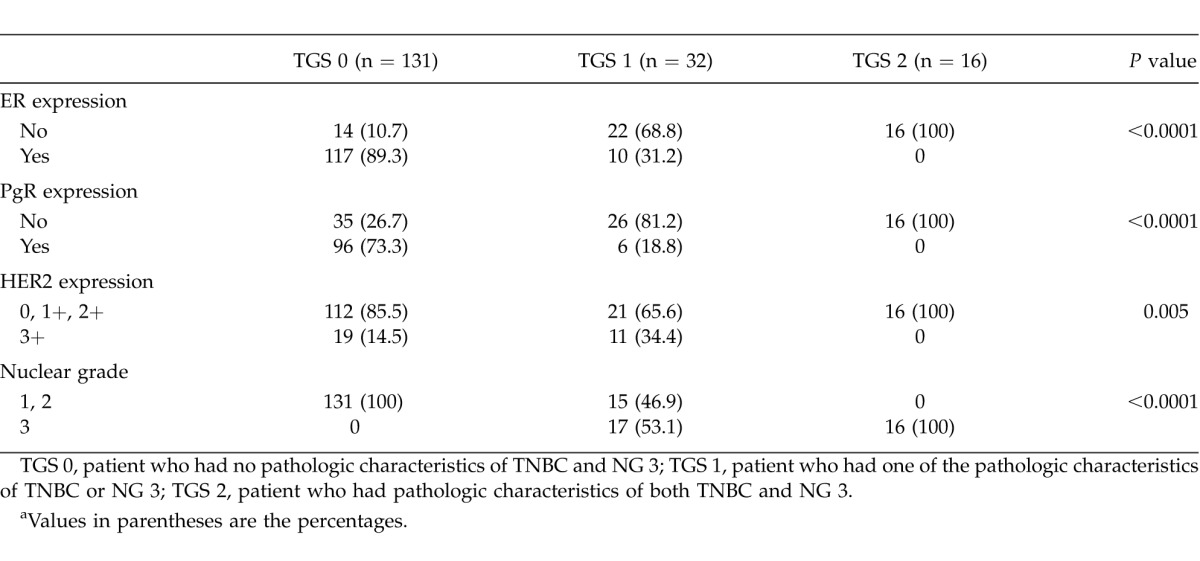
Correlation of TGS with content of hormonal expression and nuclear grade is shown in Table 2. Significant difference was observed regarding all elements.
Table 2.
Correlation of TGS with hormonal expression and nuclear grade of the tumorsa
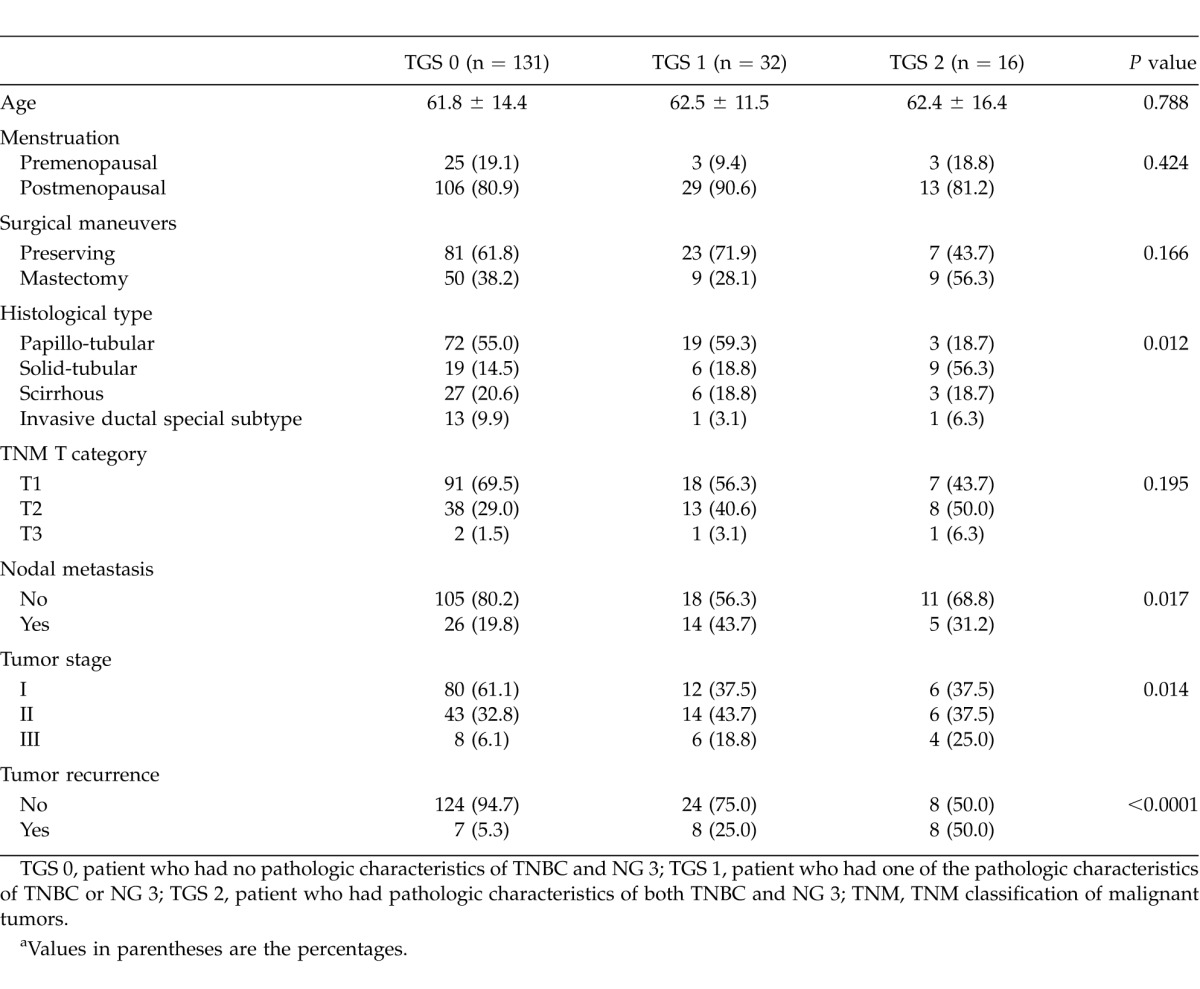
Correlation of TGS with clinicopathologic features is shown in Table 3. Significant differences were observed regarding histologic type (P = 0.012), nodal metastasis (P = 0.017), stage of the tumor (P = 0.014), and proportion of tumor recurrence (P < 0.0001).
Table 3.
Relationship between TGS and clinicopathologic characteristicsa
OS among enrolled patients in this study was 90.6%. While no patient with TGS 0 is dead and, accordingly, a significant difference was not detected between the OS of patients with TGS 0 and those with TGS 1. OS of patients with TGS 2 was found to be significantly worse compared with that of patients with TGS 1 (P = 0.031; Fig. 1).
Fig. 1.
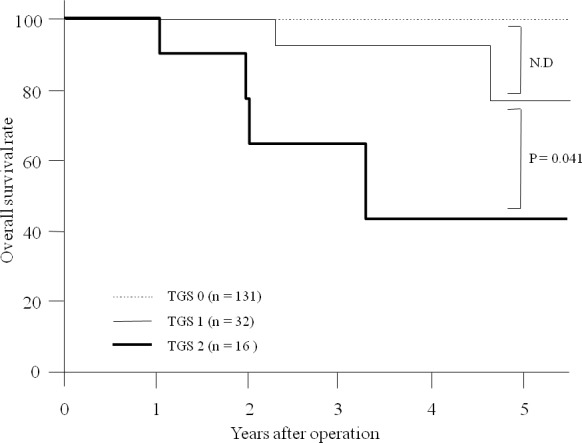
Overall survival. A significant difference was not detected between overall survival (OS) of patients with TGS 0 and TGS 1. OS of patients with TGS 2 was significantly worse than that of patients with TGS 1 (P = 0.031).
On the other hand, significant differences were observed regarding RFS between patients with TGS 0 and TGS 1 (P < 0.0001) and between patients with TGS 1 and TGS 2 (P = 0.024; Fig. 2).
Fig. 2.
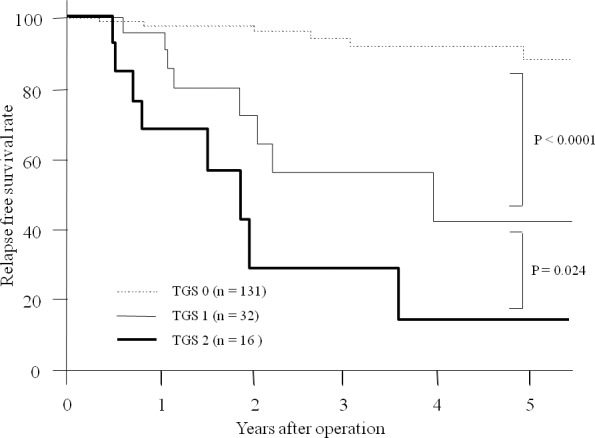
Relapse-free survival. A significant difference was observed regarding relapse-free survival between patients with TGS 0 and TGS1 (P < 0.0001) and between patients with TGS 1 and TGS 2 (P = 0.024).
Discussion
Some histopathologic criteria, such as pathologic grade and nuclear grade, indicate the biological aggressiveness of breast cancer.8–11 It has been reported that the histologic grade could also have a powerful ability to stratify prognosis of patients with breast cancer.11 NG, which has been endorsed in the National Surgical Adjuvant Study of Breast Cancer (NSAS-BC) protocol, has been shown to be more convenient and more reliable regarding reproducibility of the data.6,10 Therefore, this useful and reliable parameter, NG, was used in this study to establish a classification system to predict subsequent tumor recurrence in IDC of the breast.
TNBCs are known to have more aggressive biological potential and be more likely to recur than other subtypes of breast cancer12; therefore, clinical attention has been widely paid to this special subtype of breast cancer. Histologic investigation revealed that the majority of cases of TNBC are associated with the high histologic grade of the tumor.13 Also, in the current study, the proportion of cases with higher nuclear grade (NG 3) was found to be significantly higher in TNBC.
The main purpose of this study was to attempt to establish criteria to predict tumor recurrence of breast cancer using the clinicopathologic data that are routinely acquired in every case of breast cancer, so that it would be easy to obtain and clinically useful for all physicians. Then we noted that NG and TNBC account for representative clinicopathologic factors of breast cancer, and are recognized as indicators of the malignant potential of the tumor. Therefore, it would be expected that combined data from these factors would provide more powerful and useful information regarding biological characteristics of the tumors, especially related to the outcome of the patients.
Indeed, the proportion of patients who had subsequent tumor recurrence was significantly higher, and RFS was also significantly more unfavorable, in patients with TGS 2, whose tumors applied to both TNBC and nuclear grade 3, than in those with TGS 0 or TGS 1.
The discrepancy between RFS and OS might demonstrate that anti-cancer agents play a strong role in, and exert a negative influence on, prolonging the survival of patients with IDC of the breast. While administration of anti-cancer drugs has been recommended to treat tumors of high grade, and only some anti-cancer drugs, including anthracyclins and taxanes, are option accepted to treat TNBC, the standard therapeutic options for TNBC have not been defined. Therefore, development and investigation regarding some other therapeutic arms to treat this special and serious breast cancer, including molecular-targeting therapies, are strongly required.14
Although loco-regional recurrence, in addition to occurrence of distant metastasis, is known to be more frequent in patients with TNBC,15,16 the property in application of sentinel lymph node dissection has been discussed insufficiently,17 and a breast-preserving operation has not been considered as a contraindication for TNBC.15,18
Tumor recurrence of TNBC has been reported to rapidly increase within 2 years after treatment and to gradually decrease thereafter, and the risk of recurrence and/or death in TNBC is generally low 5 years after initial diagnosis. Also, in this study the period of tumor recurrence of TNBC after initial treatment was found to be extremely bipolarized. Moreover, on the other hand, tumor recurrence of NG 3 tumors has been known to increase 5 years after the initial surgical treatment. This biological characteristic of NG 3 tumors might be partially explained by the knowledge that high-grade breast cancers, including NG 3 tumors, have shown both a highly sensitive response to and, on the contrary, increasing resistance to cytotoxic anti-cancer agents.19
In conclusion, TGS can be a predictive indicator for tumor recurrence and can classify RFS of the patients with IDC of the breast with a strict stratification. Patients with both tumor characteristics of TNBC and NG 3 would be continuously threatened by the distress and fear of tumor recurrence along with long-term administration and/or intake of anti-cancer drugs. However, this information, which might be unpleasant for these patients, could force the physicians to perform a strict and meticulous follow-up of the patients and select appropriate anti-cancer therapeutic arms.
References
- 1.de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC. Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncol. 2011;137(2):183–192. doi: 10.1007/s00432-010-0957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Q, Mori I, Sakurai T, Yoshimura G, Suzuma T, Nakamura Y, et al. Correlation between nuclear grade and biological prognostic variables in invasive breast cancer. Breast Cancer. 2001;8(2):105–110. doi: 10.1007/BF02967488. [DOI] [PubMed] [Google Scholar]
- 3.Valdivieso M, Kujawa AM, Jones T, Baker LH. Cancer survivors in the United States: a review of the literature and a call to action. Int J Med Sci. 2012;9(2):163–173. doi: 10.7150/ijms.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Japanese Breast Cancer Society. General rules for clinical and pathological recording of breast cancer. Breast Cancer. 2005;12(suppl):S12–S14. [PubMed] [Google Scholar]
- 5.Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. New York, NY: Wiley-Blackwell; 2009. pp. 73–77. [Google Scholar]
- 6.Tsuda H, Akiyama F, Kurosumi M, Sakamoto G, Watanabe T. Establishment of histological criteria for high-risk node-negative breast carcinoma for a multi-institutional randomized clinical trial of adjuvant therapy. Japan National Surgical Adjuvant Study of Breast Cancer (NSAS-BC) Pathology Section. Jpn J Clin Oncol. 1998;28(8):486–491. doi: 10.1093/jjco/28.8.486. [DOI] [PubMed] [Google Scholar]
- 7.The Japanese Breast Cancer Society. General rules for clinical and pathological recording of breast cancer 2005. Breast Cancer. 2005;12(suppl):S8–S9. [PubMed] [Google Scholar]
- 8.Freedman LS, Edwards DN, McConnell EM, Downham DY. Histological grade and other prognostic factors in relation to survival of patients with breast cancer. Br J Cancer. 1979;40(1):44–55. doi: 10.1038/bjc.1979.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a larger study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda H, Akiyama F, Kurosumi M, Sakamoto G, Watanabe T. Monitoring of interobserver agreement in nuclear atypia scoring of node-negative breast carcinomas judged at individual collaborating hospitals in the National Surgical Adjuvant Study of Breast Cancer (NSAS-BC) protocol. Jpn J Clin Oncol. 1999;29(9):413–420. doi: 10.1093/jjco/29.9.413. [DOI] [PubMed] [Google Scholar]
- 11.Otsuki Y, Shimizu S, Suwa K, Yoshida M, Kanzaki M, Kobayashi H. Which is the better pathological prognostic factor, the Nottingham histological grade or the Japanese nuclear grade? A large scale study with a long-term follow-up. Jpn J Clin Oncol. 2007;37(4):266–274. doi: 10.1093/jjco/hym026. [DOI] [PubMed] [Google Scholar]
- 12.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn IIK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 13.Bulut N, Aksoy S, Dizdar O, Dede DS, Arslan C, Dogan M, et al. Demographic and clinico-pathological characteristics in patients with triple-negative and non-triple-negative breast cancer. Med Oncol. 2011;28(suppl 1):S75–S79. doi: 10.1007/s12032-010-9715-9. [DOI] [PubMed] [Google Scholar]
- 14.Vaklavas C, Forero-Torres A. How do I treat “triple-negative” disease. Curr Treat Options Oncol. 2011;12(4):369–388. doi: 10.1007/s11864-011-0168-y. [DOI] [PubMed] [Google Scholar]
- 15.Freedman GM, Anderson PR, Li T, Nicolaou N. Locoregional recurrence of triple-negative breast cancer after breast-conserving surgery and radiation. Cancer. 2009;115(5):946–951. doi: 10.1002/cncr.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol. 2010;33(6):637–645. doi: 10.1097/COC.0b013e3181b8afcf. [DOI] [PubMed] [Google Scholar]
- 17.Yagata H, Kajiura Y, Yamauchi H. Current strategy for triple-negative breast cancer: appropriate combination of surgery, radiation, and chemotherapy. Breast Cancer. 2011;18(3):165–173. doi: 10.1007/s12282-011-0254-9. [DOI] [PubMed] [Google Scholar]
- 18.Parker CC, Ampil F, Burton G, Li BD, Chu QD. Is breast conservation therapy a viable option for patients with triple-receptor negative breast cancer? Surgery. 2010;148(2):386–391. doi: 10.1016/j.surg.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Osako T, Horii R, Matsuura M, Ogiya A, Domoto K, Miyagi , et al. Common and discriminative clinicopathological features between breast cancers with pathological complete response or progressive disease in response to neoadjuvant chemotherapy. J Cancer Res Clin Oncol. 2010;136(2):233–241. doi: 10.1007/s00432-009-0654-9. [DOI] [PubMed] [Google Scholar]


