Abstract
The human body's response to surgery is correlated with the extent of tissue damage. The aim of the present study was to, over time, map out parameters concerning inflammation, metabolism, nutrition, breathing function, muscle strength, and well-being in elective colorectal surgery. Eighteen patients were prospectively included: colon resection (n = 9) and rectum resection/amputation (n = 9). Postoperative interleukin 10 (IL-10) rose more in the rectum surgery group on day 0 (P = 0.007) and day 3 (P = 0.025). Furthermore, significant differences between groups were detected regarding albumin, prealbumin, and total iron-binding capacity (TIBC). For albumin and TIBC, this difference was seen even on day 7. C-reactive protein, IL-6, IL-8, glucose, cortisol, insulin, pain, fatigue, nausea, grip strength, and forced expiratory volume in 1 second did not show any differences. No correlation was revealed between measured parameters and postoperative complications. Postoperative levels of IL-10, albumin, prealbumin, and TIBC may be used as determinants of surgical stress after colorectal surgery.
Keywords: Surgical stress, Inflammation, Nutrition, Muscle strength
The intestinal tract is very susceptible to surgical stress. At the cellular level, it has been suggested that colorectal resection causes an increase in reactive oxygen species, with subsequent changes in nuclear and mitochondrial DNA.1 Over the years, many strategies have emerged to attenuate the stress response to colorectal surgery, as well as ways to quantify and use it to predict clinical outcome, as delineated below.
Laparoscopic surgery
The laparoscopic approach in colorectal cancer surgery is motivated by the effort to reduce surgical stress by minimizing operative injury. The potential advantages include reduced pain, earlier return of bowel function, and shorter hospital stay.2 It is a common notion that laparoscopic surgery induces a smaller inflammatory response than open surgery. Open colorectal resection may be associated with higher levels of interleukin 6 (IL-6).3,4 Also, cellular immune responses appear to be less affected by laparoscopic colorectal resection compared with open resection as determined by total lymphocyte count, CD4+ and CD8+ T cells, interferon-γ, and human leukocyte antigen–DR.5–8 However, these differences are not consistent at all time intervals, and further studies are needed to fully characterize the importance of temporal changes in these markers. Furthermore, despite potential benefits, laparoscopic surgery still remains technically challenging in rectal cancer and will not be suitable for all patients.2
Fast-track surgery
The fast-track (FT) protocol consists of a set of multimodal, evidence-based care principles. It has been found that FT care may attenuate the surgical stress response and accelerate postoperative recovery.9 The laparoscopic method coupled with FT care could have synergistic effects, as documented by a recent study reporting a reduction in postoperative inflammatory factors and a protective effect on the host immune response.10
Pharmacologic modulation of the stress response
Much research has been directed toward modulating the stress response to colorectal surgery using pharmacologic compounds. Previously, it was reported that intraoperative epidural analgesia in patients undergoing colonic surgery could reduce the postoperative systemic inflammatory response, as well as reduce postoperative complications in comparison with intravenous analgesia.11 Other strategies include the use of immunomodulation. By treating patients with preoperative IL-2 injections subcutaneously, Nespoli et al12 reported that the postoperative immune suppression related to colorectal resection could be counteracted, specifically by attenuating postoperative levels of IL-6, IL-12, vascular endothelial growth factor, total circulating lymphocytes, CD3+ and CD4+ T cells, and dendritic cells. Furthermore, intestinal immunity was more preserved.
Markers of surgical stress
Tabuchi et al13 reported that the blood granulocyte-to-lymphocyte ratio is a simple and clinically relevant parameter for the assessment of perioperative stress in patients undergoing colorectal surgery. Toll-like receptors, α-defensins, high-sensitivity C-reactive protein (CRP), and IL-6 have also been used as early markers to determine the inflammatory response in colorectal resection.14,15
Postoperative complications
Early identification of postoperative complications is crucial for patient management. Lane et al16 reported that postoperative CRP levels could predict the risk of adverse events following colorectal surgery within an FT program. In particular, a CRP value of more than 150 mg/L on day 2 and a rising CRP level on day 3 were found to be significant factors. Using the Glasgow Prognostic Score (GPS), based on CRP and albumin, Ishizuka et al17 found that the systemic inflammatory response predicts perioperative central venous catheter–related bloodstream infection in patients undergoing colorectal surgery who have been administered parenteral nutrition. Furthermore, the metabolic response to surgery may lead to complications. For example, insulin resistance has been shown to be a strong risk factor for the development of postoperative complications in colorectal and other types of surgery.18
Survival
Based on existing literature, systemic inflammation–based scores have prognostic value in colorectal cancer and can help the clinician in outcome prediction and treatment decision making.19–22 Most notably, GPS, modified GPS (mGPS), and the Prognostic Index have been associated with cancer survival.
Aim
Thus far, most studies have focused on individual aspects of the surgical stress response, and there is a lack of a more systematic mapping, notably regarding the dynamics over time and comparing colon and rectum surgery. In addition, there is much to learn from improving the understanding of how metabolism and nutrition are involved in surgical stress.18 In the present study, we undertook a prospective evaluation to determine the time course of changes in various parameters relevant to surgical stress, including changes in inflammation, metabolism, nutrition, gastrointestinal and respiratory function, muscle strength, and well-being after elective colorectal surgery.
Patients and Methods
A total of 18 patients age ≥18 years who were undergoing elective colonic resection (n = 9; group 1) or rectal resection/amputation (n = 9; group 2) because of cancer were prospectively included. All patients belonged to the primary catchment area of Skåne University Hospital, Lund, Sweden. Exclusion criteria were American Society of Anesthesiologists grade ≥3, emergency surgery, inflammatory bowel disease, any history of immunosuppressive drugs, pregnancy, preoperative evidence of widespread metastatic cancer, dementia/presenility, and living at a nursing home at the time of diagnosis. Written informed consent was obtained from all participating patients. The Human Ethics Committee of Lund University approved the study protocol.
Open colorectal surgery was done through a vertical midline incision or a transrectal incision with minimal length. Prophylactic intravenous cefuroxime and metronidazole, and low–molecular weight heparin were administered to all patients. Bowel anastomosis was performed by an end-to-end, continuous, singe-layer, hand-sewn technique using absorbable sutures 1.4-0 PDS™ (polydioxanone) (Ethicon, Johnson & Johnson, Livingston, UK), except in low anterior resection, where the conventional double-stapling technique was used.
Peripheral blood samples were drawn at admission; the evening after surgery (day 0); and on postoperative days 1, 2, 3, and 7, or until discharge. Plasma CRP levels were measured by an immunoturbidimetric method using the Tina-quant CRP latex high-sensitivity assay (Roche Diagnostics, Basel, Switzerland). Blood cell count was measured using the Sysmex XE-5000/XT-1800i automated hematology analyzer (Sysmex Corporation, Kobe, Japan). IL-6, IL-8, and IL-10 samples were centrifuged for 10 minutes at 2200g and stored at −70°C until analysis by enzyme-linked immunosorbent assay (Quantikine, R&D Systems Europe, Abingdon, United Kingdom). Plasma glucose was measured by the enzymatic hexokinase method, and insulin and cortisol levels were measured using electrochemiluminescence immunoassay (Cobas, Roche Diagnostics).
Body mass index was calculated, and any preoperative weight loss was noted. Albumin was measured by the immunoturbidimetric method (Cobas), and prealbumin and total iron-binding capacity (TIBC) were measured by standard immunonephelometric techniques (Prospec, Siemens Healthcare Diagnostics, Marburg, Germany). Return of normal gut function was defined as the patient's ability to tolerate light hospital meals. Time to tolerance of oral fluids and time to passing flatus and stools were also recorded. Forced expiratory volume in 1 second (FEV1) was recorded using a portable spirometer (Micro Spirometers, Rochester, United Kingdom) and peak expiratory flow with a peak flow meter (Mini Wright, United Kingdom). Hand grip strength is a sensitive measure of skeletal muscle strength and diaphragmatic muscle function, and is affected by surgical trauma.23 Hand grip strength was recorded on both the dominant and nondominant sides using a dynamometer (Jamar Hydraulic Hand Dynamometer, Lafayette Instrument Company, Lounghborough, UK and Mini Wright Peak Flow Meter, KW-MED INC., USA). Visual analogue scale with scores from 1–100 were used to record pain, fatigue and nausea. Pain medication used among patients, including oral drugs, epidural analgesia, and subcutaneous pumps, was recorded. Postoperative complications were registered according to the definition by Dindo et al.24
Continuous variables are presented as medians with 25th and 75th percentiles. Categoric variables are given as frequencies and percentages. Univariate analysis for continuous variables was conducted with the Wilcoxon test. Categoric variables were analyzed by χ2 test. Data were analyzed using Hmisc, Survival, and Design packages of the R software (R Foundation for Statistical Computing, Vienna, Austria), version 2.9.0. The level of significance was set at P < 0.05.
Results
The colon surgery group had a median age of 70 years (range, 65–76 years), and most of the patients were female (n = 6; 67%). There were 4 sigmoidectomies, 4 right-sided hemicolectomies, and 1 ileocecal resection. The duration of surgery was 156 minutes (range, 147–198 minutes). Operative blood loss was 200 mL (range, 100–300 mL). Postoperative complications occurred in 5 patients, ranging from Clavien-Dindo grades I to IIIb. These included wound infection (n = 3), gastric retention (n = 1), blood transfusion (n = 1), and wound dehiscence (n = 1).
The rectum surgery group had a median age of 67 years (range, 63–80 years), and most patients were male (n = 7; 78%). There were 6 rectal amputations, 2 anterior resections (1 low and 1 high), and 1 low Hartmann procedure. The length of surgery was 390 minutes (range, 315–449 minutes). Operative bleeding was 1000 mL (range, 700-1300 ml). Three patients developed postoperative complications, including urinary tract infection (n = 1), anastomotic insufficiency (n = 1), wound dehiscence (n = 1), and perianal abscess (n = 1), with Clavien-Dindo grades I to IIIb.
There were no differences between groups with regard to preoperative hemoglobin value, and the values were equivalent on postoperative day 1 [colon surgery: 113 g/L (range, 104–123 g/L); rectum surgery: 108 g/L (range, 103–124 g/L); P = 0.77] and day 7 [colon surgery: 112 g/L (range, 106–122 g/L); rectum surgery: 116 g/L (110–121 g/L); P = 0.80].
Inflammatory response
We observed an increase in CRP during the early postoperative period, and peak values were reached on day 2 [colon surgery: 176 mg/L (range, 121–238 mg/L); rectum surgery; 178 mg/L (range, 133–271 mg/L)] that were comparable in both patient groups (P = 0.71). Fig. 1 shows the change in CRP over time.
Fig. 1.
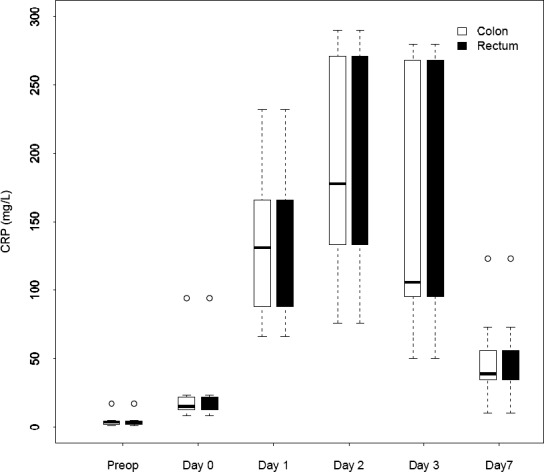
Changes over time in median C-reactive protein for patients who underwent colorectal surgery.
No difference in leukocyte count was seen between the groups during the postoperative course.
We noted a rapid increase in IL-6, IL-8, and IL-10 after colon as well as rectum surgery. The IL-6 level differed preoperatively. The peak value for IL-6 was reached on day 0 in both groups [colon surgery: 157 ng/L (range, 141–189 ng/L); rectum surgery: 380 ng/L (range, 106–407 ng/L); P = 0.061]. The peak value for IL-8 was reached on day 0 [colon surgery: 25 ng/L (range, 19–30 ng/L); rectum surgery: 30 ng/L (range, 25–63 ng/L)] but was not significantly different (P = 0.20). The IL-10 level rose in both groups, and it was highest on day 0 in the rectum surgery group and on day 1 in the colon surgery group [peak values for colon surgery: 27 ng/L (range, 12–41 ng/L); rectum surgery: 85 ng/L (range, 50–118 ng/L]. There was a significant difference in IL-10 levels on day 0 (P = 0.007) and day 3 (P = 0.025). Figures 2 to 4 depict the changes in the IL values over time.
Fig. 2.
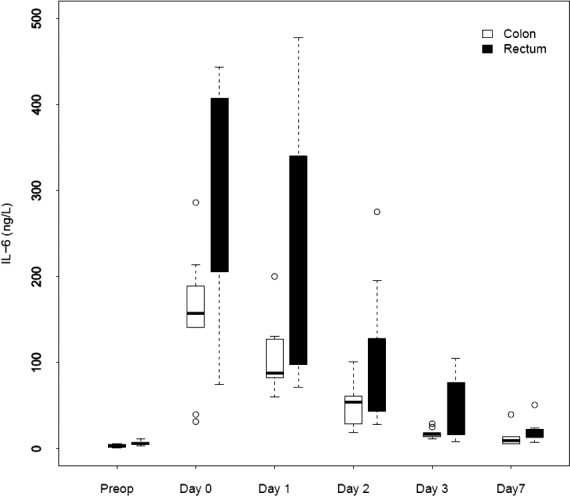
Changes over time in median IL-6 for patients who underwent colorectal surgery.
Fig. 4.
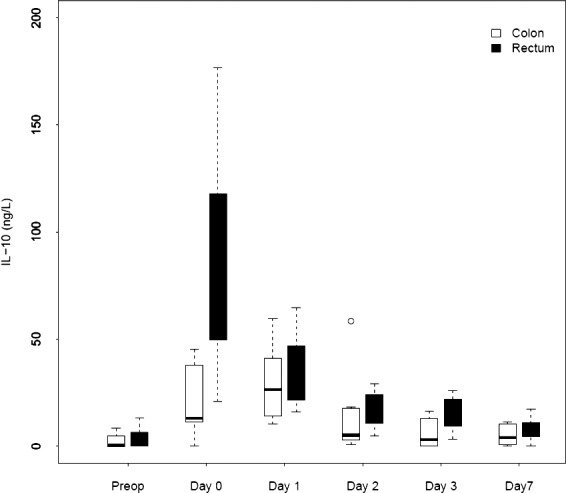
Changes over time in median IL-10 for patients who underwent colorectal surgery.
Metabolic response
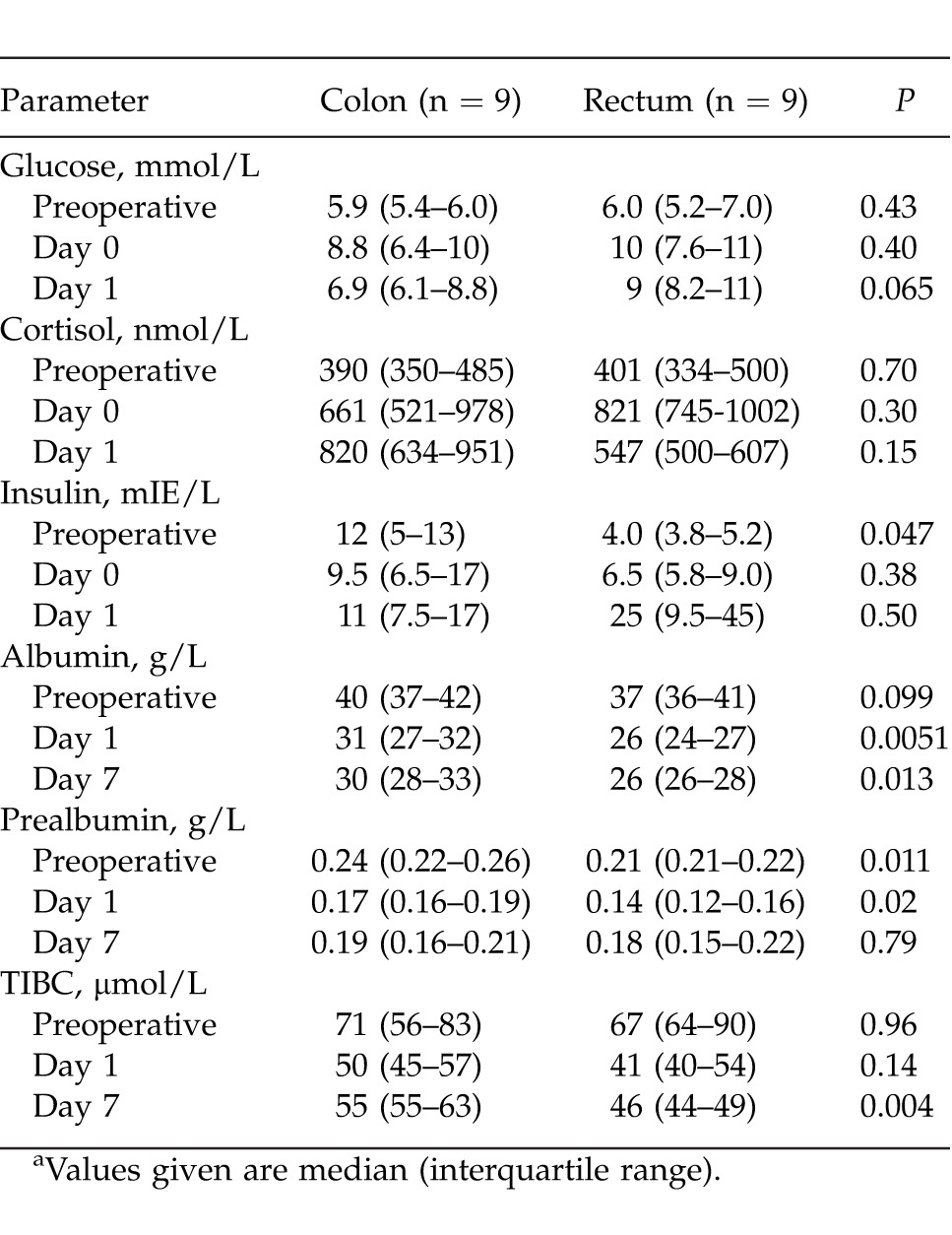
The glucose, cortisol, and insulin levels were comparable in the colon and rectum surgery groups at all evaluated time points (Table 1).
Table 1.
Metabolic and nutritional response in connection to colorectal surgerya
Nutrition
Baseline measures of albumin and TIBC did not differ preoperatively, but prealbumin was lower in group 3. Postoperatively, levels were reduced for all 3 parameters, with significantly lower values after rectum surgery. The difference was seen even on day 7 between groups (Table 1).
Pulmonary function
No difference between the groups was noted for peak expiratory flow and FEV1, but for both groups a decrease was registered, with a reduction between the preoperative value and the results on days 1, 3, and 7. This was most pronounced on postoperative day 1 for both FEV1 (colon surgery, −29%; rectum surgery, −42%) and peak expiratory flow (colon surgery, −31%; rectum surgery, −44%). The change over time is presented in Figure 5.
Fig. 5.
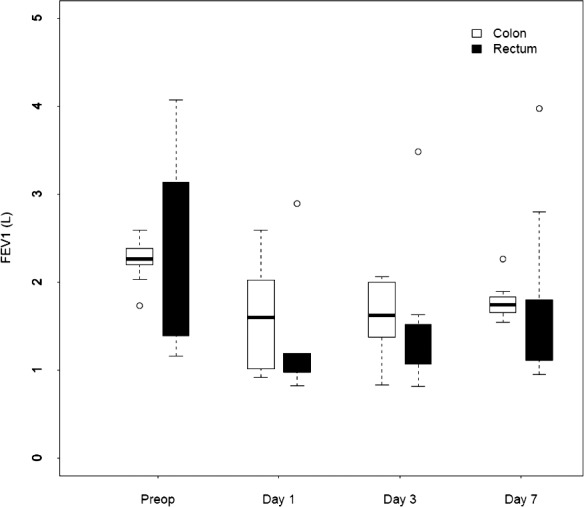
Changes over time in median FEV1 for patients who underwent colorectal surgery.
Hand grip strength
Grip strength, both in the dominant and nondominant hands, showed no difference between the groups. After colon and rectal surgery, the grip strength in the dominant hand showed a reduction compared with preoperative values on days 1, 3, and 7 (colon surgery: day 1, −8%; day 3, −7%; day 7, −5%; rectum surgery: day 1, −27%; day 3, −18%; day 7, −14%). The reduction in grip strength was not that prominent in the nondominant hand.
Pain, fatigue, and nausea
Visual analogue scale for pain, fatigue, and nausea did not differ between the groups during hospital stay.
Gastrointestinal function
Return to normal gastrointestinal function was registered for all patients, with no change between groups. The times to normal feeding for the colon and rectum surgery groups were 4 days (range, 2–6 days) and 5 days (range, 4–7 days), respectively. The time until the patients could pass flatus was 4 days (range, 3–5 days) for both groups, and the time to passing stools was 5 days (range, 4–6 days) and 5 days (range, 3–6 days), respectively.
Complications versus no complications
There was no significant correlation between measured parameters and postoperative complications (data not shown).
Follow-up
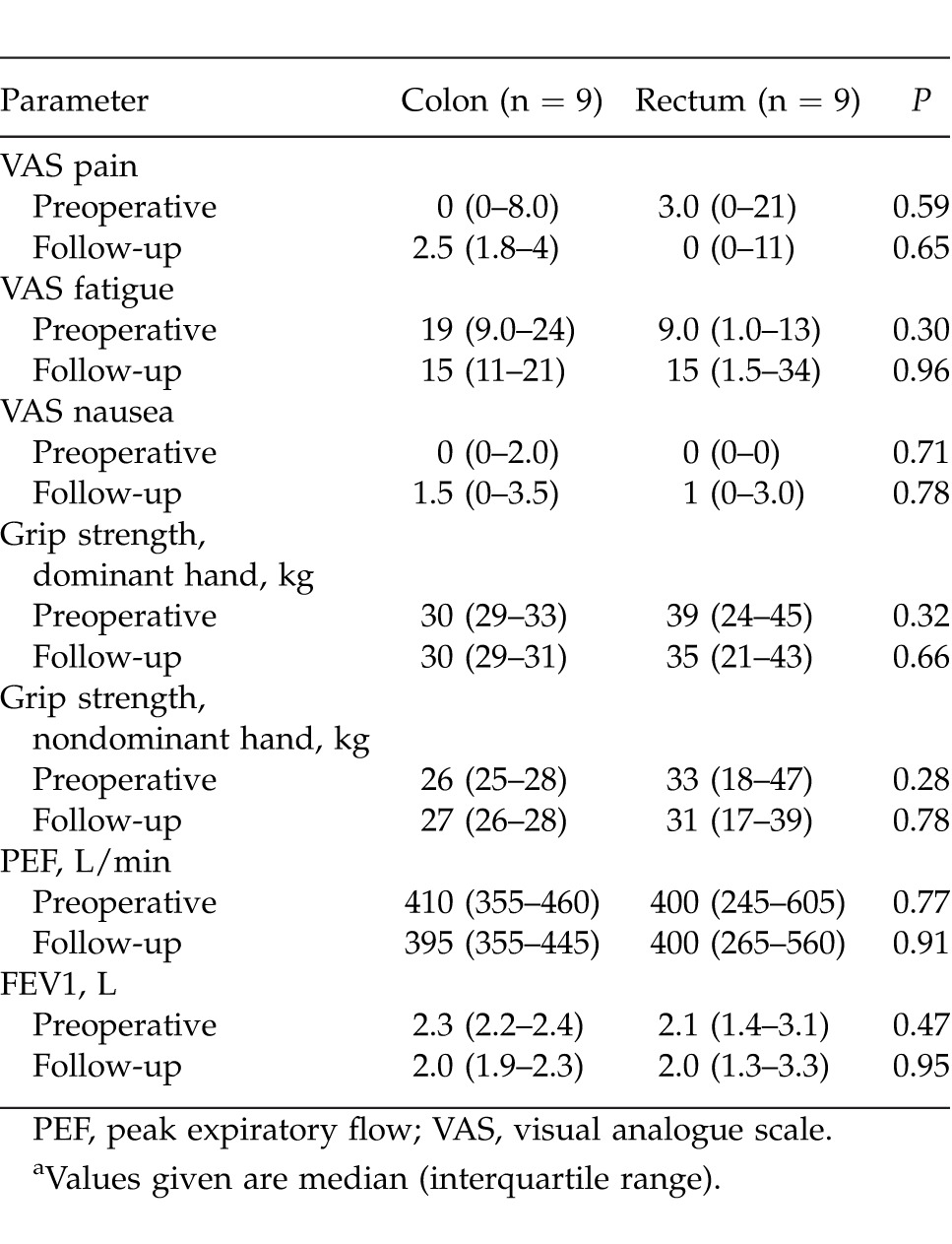
By the time for follow-up there was a comparable weight change between the groups [colon surgery: −4.1 kg (range, −5.1 to −2.4 kg); rectum surgery: −5.6 kg (range, −7.6 to −4.1 kg); P = 0.15]. No patient subjectively had problems with nutrition. Two patients in the colon surgery group had experienced nausea, and one had been vomiting after discharge. All patients had normal stool. Three patients in each group used painkillers. Four patients in the colon surgery group and 7 patients in the rectum surgery group experienced some kind of complication in the postoperative course. Other parameters at follow-up are presented in Table 2.
Table 2.
Preoperative values and at follow-up after colorectal surgery
Discussion
The present study shows that colorectal resection leads to a pronounced inflammatory and anti-inflammatory response. We noted that the IL-10 level showed an early difference between colon and rectum surgery. Parameters correlating with nutrition also were significantly changed during a later postoperative time, and with a clear difference between groups.
In major abdominal surgery, the anti-inflammatory cytokine IL-10 may be of great importance. IL-10 inhibits the synthesis of proinflammatory cytokines and shifts the immune response from Th1 type to Th2 type.25 In colorectal cancer, elevated serum levels of this cytokine have been reported to be a negative prognostic factor for disease-free and overall survival, and responsiveness to treatment.26 One study indicated that the tumor necrosis factor-α/IL-10 quote predicted the occurrence of postoperative complications.27 Ugras et al28 investigated the role of peritoneal cytokine levels in developing anastomotic leakage after colorectal surgery. It was found that the peritoneal cytokine levels of IL-6, IL-10, and tumor necrosis factor-α can be an additional diagnostic tool that can support the early prediction of anastomotic leakage (AL) in colorectal surgery. The results presented in our study could not verify any relationship between postoperative morbidity and concentrations of IL-10.
In the present study, significant decreases in albumin, prealbumin, and TIBC levels were found, findings that persisted to day 7 in the colonic and rectal surgery groups. Of these biochemical parameters, albumin is the most evaluated in colorectal surgery. By combining CRP and albumin, the inflammation-based GPS has been developed. The GPS/mGPS is the most extensively validated of the systemic inflammation–based prognostic scores and has been associated with increased weight loss, poor performance status, increased comorbidity, increased proinflammatory and angiogenic cytokines, and complications with treatment as well as survival among patients with colorectal cancer and other cancer types.29 In the present study, albumin and TIBC were affected up to 7 days after surgery, and weight loss persisted at the follow-up.
We conducted this prospective clinical study to evaluate the potential differences in surgical stress after different degrees of colorectal resection. This study was limited by its small sample size. Nevertheless, the findings that postoperative levels of IL-10, albumin, prealbumin and TIBC significantly differed after colon surgery compared with rectum surgery indicate a potentially greater systemic stress response. These easily available biochemical markers may be useful as surrogates of surgical stress and may be used when comparing biologic responses after different degrees of operative trauma, especially concerning newer developments of perioperative care.
Fig. 3.
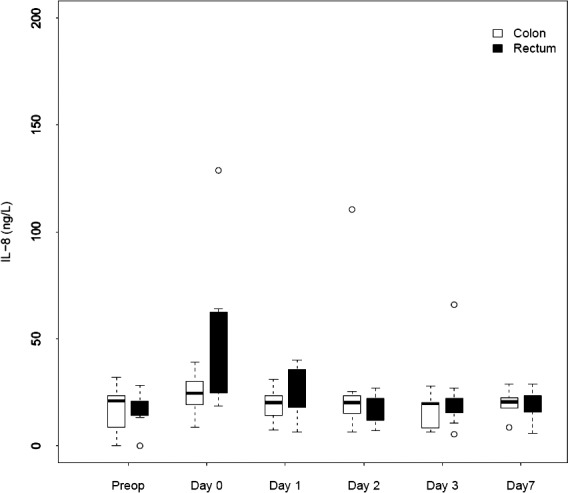
Changes over time in median IL-8 for patients who underwent colorectal surgery.
References
- 1.Potenza L, Calcabrini C, Bellis RD, Mancini U, Polidori E, Zeppa S, et al. Effect of surgical stress on nuclear and mitochondrial DNA from healthy sections of colon and rectum of patients with colorectal cancer. J Biosci. 2011;36(2):243–251. doi: 10.1007/s12038-011-9064-7. [DOI] [PubMed] [Google Scholar]
- 2.Jones OM, Lindsey I, Cunningham C. Laparoscopic colorectal surgery. BMJ. 2011;343:d8029. doi: 10.1136/bmj.d8029. [DOI] [PubMed] [Google Scholar]
- 3.Pascual M, Alonso S, Pares D, Courtier R, Gil MJ, Grande L, et al. Randomized clinical trial comparing inflammatory and angiogenic response after open versus laparoscopic curative resection for colonic cancer. Br J Surg. 2011;98(1):50–59. doi: 10.1002/bjs.7258. [DOI] [PubMed] [Google Scholar]
- 4.Sammour T, Kahokehr A, Chan S, Booth RJ, Hill AG. The humoral response after laparoscopic versus open colorectal surgery: a meta-analysis. J Surg Res. 2010;164(1):28–37. doi: 10.1016/j.jss.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Huang R, Jiang T, Huang K, Cao J, Qiu Z. Laparoscopic and open resection for colorectal cancer: an evaluation of cellular immunity. BMC Gastroenterol. 2010;10:127. doi: 10.1186/1471-230X-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsamis D, Theodoropoulos G, Stamopoulos P, Siakavellas S, Delistathi T, Michalopoulos NV, et al. Systemic inflammatory response after laparoscopic and conventional colectomy for cancer: a matched case-control study. Surg Endosc. 2012;26(5):1436–1443. doi: 10.1007/s00464-011-2052-z. [DOI] [PubMed] [Google Scholar]
- 7.Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM. vd Pas MH, Meijerink WJ, et al. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis. 2011;26(1):53–59. doi: 10.1007/s00384-010-1056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veenhof AA, Vlug MS, van der Pas MH, Sietses C, van der Peet DL. de Lange-de Klerk ES, et al. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255(2):216–221. doi: 10.1097/SLA.0b013e31824336e2. [DOI] [PubMed] [Google Scholar]
- 9.Ren L, Zhu D, Wei Y, Pan X, Liang L, Xu J, et al. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg. 2012;36(2):407–414. doi: 10.1007/s00268-011-1348-4. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Jiang Z, Zhao K, Li G, Liu F, Pan H, et al. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg. 2012;16(7):1379–1388. doi: 10.1007/s11605-012-1880-z. [DOI] [PubMed] [Google Scholar]
- 11.Moselli NM, Baricocchi E, Ribero D, Sottile A, Suita L, Debernardi F. Intraoperative epidural analgesia prevents the early proinflammatory response to surgical trauma. Results from a prospective randomized clinical trial of intraoperative epidural versus general analgesia. Ann Surg Oncol. 2011;18(10):2722–2731. doi: 10.1245/s10434-011-1700-9. [DOI] [PubMed] [Google Scholar]
- 12.Nespoli L, Uggeri F, Romano F, Nespoli A, Brivo F, Fumagalli L, et al. Modulation of systemic and intestinal immune response by interleukin-2 therapy in gastrointestinal surgical oncology. Personal experience in the context of current knowledge and future perspectives. Anticancer Res. 2012;32(3):989–996. [PubMed] [Google Scholar]
- 13.Tabuchi T, Shimazaki J, Satani T, Nakachi T, Watanabe Y. The perioperative granulocyte/lymphocyte ratio is a clinically relevant marker of surgical stress in patients with colorectal cancer. Cytokine. 2011;53(2):243–248. doi: 10.1016/j.cyto.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Tsimogiannis KE, Tellis CC, Tselepis AD, Pappas-Gogos GK, Tsimoyiannis EC, Basdanis G. Toll-like receptors in the inflammatory response during open and laparoscopic colectomy for colorectal cancer. Surg Endosc. 2012;26(2):330–336. doi: 10.1007/s00464-011-1871-2. [DOI] [PubMed] [Google Scholar]
- 15.Tsimogiannis KE, Telis K, Tselepis A, Pappas-Gogos GK, Tsimoyiannis EC, Basdanis G. Alpha-defensin expression of inflammatory response in open and laparoscopic colectomy for colorectal cancer. World J Surg. 2011;35(8):1911–1917. doi: 10.1007/s00268-011-1140-5. [DOI] [PubMed] [Google Scholar]
- 16.Lane JC, Wright S, Burch J, Kennedy RH, Jenkins JT. Early prediction of adverse events in enhanced recovery based upon the host systemic inflammatory response. Colorectal Dis. 2013;15(2):224–230. doi: 10.1111/j.1463-1318.2012.03125.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Systemic inflammatory response predicts perioperative central venous catheter-related bloodstream infection in patients undergoing colorectal cancer surgery with administration of parenteral nutrition. Anticancer Res. 2012;32(9):4045–4050. [PubMed] [Google Scholar]
- 18.Ljungqvist O. Insulin resistance and outcomes in surgery. J Clin Endocrinol Metab. 2010;95(9):4217–4219. doi: 10.1210/jc.2010-1525. [DOI] [PubMed] [Google Scholar]
- 19.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 20.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Inoue Y, Iwata T, Okugawa Y, Kawamoto A, Hiro J, Toiyama Y, et al. Prognostic significance of a systemic inflammatory response in patients undergoing multimodality therapy for advanced colorectal cancer. Oncology. 2013;84(2):100–107. doi: 10.1159/000343822. [DOI] [PubMed] [Google Scholar]
- 22.Richards CH, Leitch FE, Horgan PG, McMillan DC. A systematic review of POSSUM and its related models as predictors of post-operative mortality and morbidity in patients undergoing surgery for colorectal cancer. J Gastrointest Surg. 2010;14(10):1511–1520. doi: 10.1007/s11605-010-1333-5. [DOI] [PubMed] [Google Scholar]
- 23.Da Costa ML, Qureshi MA, Brindley NM, Burke PE, Grace PA, Bouchier-Hayes D. Normal inspiratory muscle strength is restored more rapidly after laparoscopic cholecystectomy. Ann R Coll Surg Engl. 1995;77(4):252–255. [PMC free article] [PubMed] [Google Scholar]
- 24.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kvarnstrom AL, Sarbinowski RT, Bengtson JP, Jacobsson LM, Bengtsson AL. Complement activation and interleukin response in major abdominal surgery. Scand J Immunol. 2012;75(5):510–516. doi: 10.1111/j.1365-3083.2012.02672.x. [DOI] [PubMed] [Google Scholar]
- 26.Toiyama Y, Miki C, Inoue Y, Minobe S, Urano H, Kusunoki M. Loss of tissue expression of interleukin-10 promotes the disease progression of colorectal carcinoma. Surg Today. 2010;40(1):46–53. doi: 10.1007/s00595-009-4016-7. [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulou I, Armaganidis A, Douka E, Mavrou I, Augustatou C, Kopterides P, et al. Tumour necrosis factor-alpha (TNFalpha) and interleukin-10 are crucial mediators in post-operative systemic inflammatory response and determine the occurrence of complications after major abdominal surgery. Cytokine. 2007;37(1):55–61. doi: 10.1016/j.cyto.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Ugras B, Giris M, Erbil Y, Gokpinar M, Citlak G, Issever H, et al. Early prediction of anastomotic leakage after colorectal surgery by measuring peritoneal cytokines: prospective study. Int J Surg. 2008;6(1):28–35. doi: 10.1016/j.ijsu.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 29.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev. 2012 doi: 10.1016/j.ctrv.2012.08.003. Sept 17 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


