Abstract
Isolated colonic schwannomas are rare gastrointestinal mesenchymal tumors. Only a small number of cases have been reported. Occurrence of these tumors is more common in the stomach than in the large intestine. These spindle cell lesions are distinct from leiomyoma, leiomyosarcoma, and gastrointestinal stromal tumors because the tumor cells have a distinct immunophenotype, with strong diffuse positivity for S-100 and vimentin, as well as corroborative negative staining of CD117 and smooth muscle markers. We present a case of colonic schwannoma in a 70-year-old woman who had no specific symptoms. The patient was diagnosed with a submucosal tumor in the ascending colon on colonoscopy and abdominal computed tomography. Laparoscopic-assisted wedge resection of colon was performed. The very rare pathologic diagnosis of ascending schwannoma was made postoperatively. This case is interesting because schwannomas of the colon and rectum are extremely rare and are treated by laparoscopic-assisted wedge resection.
Keywords: Schwannoma, Neurilemoma, Immunohistochemistry, Colonic neoplasm, Laparoscopic surgery
Schwannoma is a neoplasm originating from the Schwann cell. This tumor can be found throughout the body along the peripheral nerves, but schwannoma of the colon and rectum is extremely rare. This is a case of schwannoma in an elderly woman with a mass in the ascending colon detected on colonoscopy and requiring surgical resection.
Case Report
A 70-year-old female patient was transferred to our hospital because of an endoscopic abnormality on her medical checkup. She had no specific medical history including neurofibromatosis. Colonoscopy revealed a pedunculated mass measuring about 2 cm in the proximal ascending colon (Fig. 1). The tumor was hard, with a short and thick stalk. The surface was slightly nodular and showed a central erosion on the tumor tip, and submucosal tumor was suspected. Abdominal computed tomography (CT) scan revealed a polypoid lesion having homogeneous low attenuation in the ascending colon without adjacent wall thickening (Fig. 2). There were no enlarged pericolic lymph nodes. Laparoscopic exploration was decided without preoperative endoscopic biopsy. Under laparoscopic approach, ascending colon mobilization was performed, and the redundant ascending colon was extracted through a minilaparotomy on the upper midline. The adjacent teniae of localized colon using clipping was incised, and full thickened bowel wall was excised including enough margin around the polyp. The opening of ascending colon was closed via suturing.
Fig. 1.
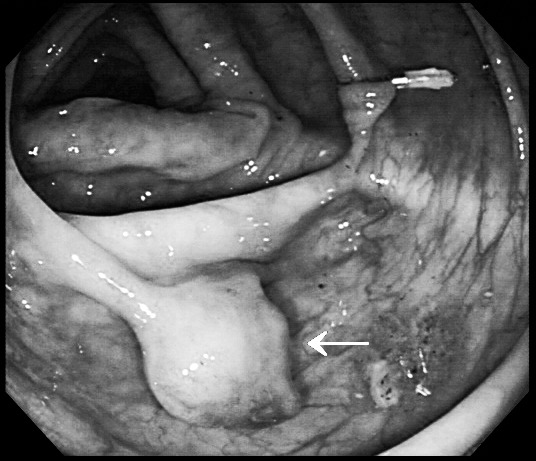
Colonofiberscopic findings. There is a pedunculated mass measuring about 2 cm with a short, thick stalk and central ulceration at the mid ascending colon (arrow). Clipping was done for localization needed at operation.
Fig. 2.
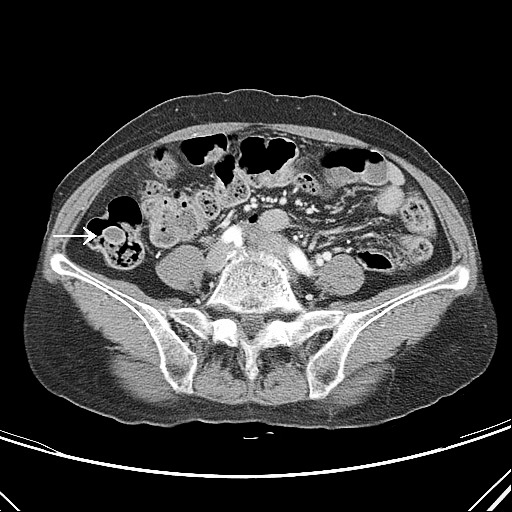
Abdominal CT findings. A round polypoid lesion with homogeneous low attenuation inside was revealed in the ascending colon without adjacent wall thickening (arrow).
On section, there was a relatively well-circumscribed mass measuring 1.3 × 1.1 cm. The color was yellowish gray, and the consistency was firm and fibrotic. Microscopically, the tumor attached to the muscularis mucosa and was mainly found in the lamina propria with pushing of the mucosa (Fig. 3a). It was composed of a proliferation of spindle cells arranged in nodular, fascicular, or vague palisading patterns in loose edematous stroma. Tumor cells had eosinophilic, fibrillary cytoplasm and elongated, sometimes pointed nuclei (Fig. 3b). There was no cellular atypism or atypical mitotic figures. Immunohistochemical study showed diffuse cytoplasmic and nuclear stain for S-100 protein and CD34, but was negative for CD117 (c-KIT) and desmin (Fig. 4). Ki-67 labeling was demonstrated in fewer than 5% of tumor cells. Based on the immunohistochemical results, schwannoma was diagnosed. The patient's postoperative progress was uneventful and she was discharged from the hospital on the third postoperative day.
Fig. 3.
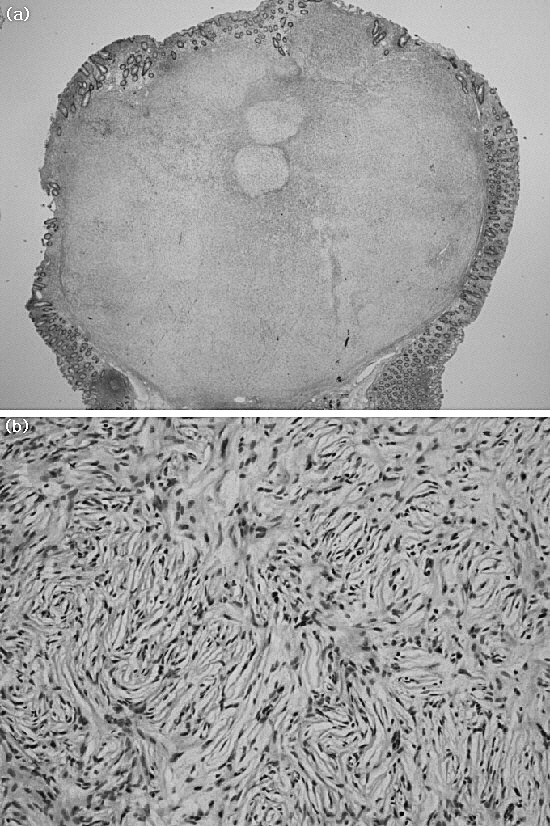
The tumor locates in the lamina propria and attaches to the muscularis propria. The tumor cells show nodular and fascicular arrangement of spindle cells in edematous stroma (a). The tumor cells show elongated and pointed nuclei (b).
Fig. 4.
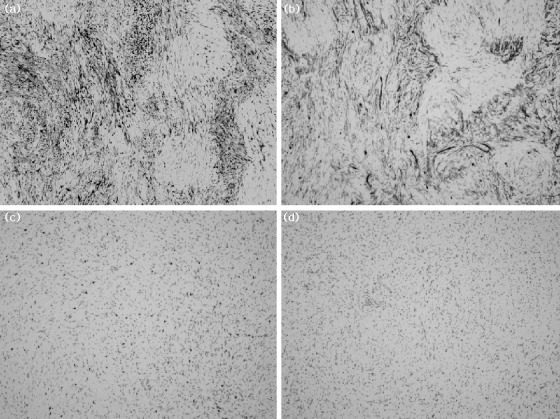
The tumor cells are positive for S-100 protein (a) and CD34 (b), but they are negative for CD117 (c) and desmin (d; all ×10).
Discussion
Schwannomas were first described by Verocay in 1910. Although there has been an increase in reports of mesenchymal tumors in recent years with the advent of newer immunohistochemical staining, primary schwannomas of the colon and rectum, unassociated with systemic neurofibromatosis (von Recklinghausen disease), are extremely rare.1,2 Because of the small number of such cases, the incidence and the characteristics of this tumor are not fully established. Schwannoma is a benign neoplasm of ectodermic origin that has slow growth and has the possibility of malignant degeneration if not removed.1–3 Schwannomas affect men and women with equal frequency and can appear at any age, but they are most commonly found after the sixth decade of life. Although they usually evolve asymptomatically, they may in some cases present with bleeding, rectal pain, and tenesmus.2,4,5
Schwannomas tend to share the gross morphologic features with other submucosal tumors and may be hard, solid, ulcerated, or calcified in rare cases.6 Immunohistochemical study of the tumor cells remains the most important diagnostic tool.7 Typically, schwannoma cells are immunoreactive to S-100 protein and vimentin, but are negative for smooth muscle actin, CD117 (KIT), cytokeratin, and desmin.8 Smooth muscle tumors typically have desmin and smooth muscle actin reactivity, whereas gastrointestinal stromal tumor shows immunopositivity to CD117 and CD34 but not S-100 protein.9 Because the tumor showed proliferation of spindle cells in loose edematous stroma and eosinophilic infiltration, inflammatory fibroid polyp should be excluded, which is usually immunoreactive to CD34 but not S-100 protein.
Many parameters have been studied in relation to tumor behavior, and so far no single parameter alone allows sufficient prediction of the degree of malignity. A mitotic activity rate of more than 5 mitoses per field at high magnification and a tumor size greater than 5 cm tend to be associated with a high risk of metastasis or recurrence. The labeling index of Ki-67 antibodies (MIB-1) with highly assessable reproducibility is recommended as an indicator of malignancy, with more than 10% considered to be malignant.
The best therapeutic option is complete surgical removal with margins free from neoplastic involvement.10 Because the risk of malignant transformation is small, broad lymph node resections are not recommended. For more aggressive tumors, the same surgical criteria should be used, but greater rigor in postoperative follow-up is recommended. The surgical approach depends on the size and location of the tumor and on its histopathologic pattern. The use of radiotherapy or adjuvant chemotherapy has conflicting results and is not recommended for routine use.
References
- 1.Daimaru Y, Kido H, Hashimoto H, Enjoji M. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol. 1988;19(3):257–264. doi: 10.1016/s0046-8177(88)80518-5. [DOI] [PubMed] [Google Scholar]
- 2.Nonose R, Lahan AY. Santos Valenciano J, Martinez CA. Schwannoma of the colon. Case Rep Gastroenterol. 2009;3(3):293–299. doi: 10.1159/000237736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauwers GY, Erlandson RA, Casper ES, Brennan MF, Woodruff JM. Gastrointestinal autonomic nerve tumors: a clinicopathological, immunohistochemical, and ultrastructural study of 12 cases. Am J Surg Pathol. 1993;17(9):887–897. doi: 10.1097/00000478-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Tsunoda C, Kato H, Sakamoto T, Yamada R, Mitsumaru A, Yokomizo H, et al. A case of benign schwannoma of the transverse colon with granulation tissue. Case Rep Gastroenterol. 2009;3(1):116–120. doi: 10.1159/000214837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanneganti K, Patel H, Niazi M, Kumbum K, Balar B. Cecal schwannoma: a rare cause of gastrointestinal bleeding in a young woman with review of literature. Gastroenterol Res Pract. 2011;2011:142781. doi: 10.1155/2011/142781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomozawa S, Masaki T, Matsuda K, Yokoyama T, Ishida T, Muto T. A schwannoma of the cecum: case report and review of japanese schwannomas in the large intestine. J Gastroenterol. 1998;33(6):872–875. doi: 10.1007/s005350050191. [DOI] [PubMed] [Google Scholar]
- 7.Arai T, Sugimura H, Suzuki M, Iwase T, Sakuramachi S, Kimura T, et al. Benign schwannoma of the esophagus: report of two cases with immunohistochemical and ultrastructural studies. Pathol Int. 1994;44(6):460–465. doi: 10.1111/j.1440-1827.1994.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 8.Das Gupta TK, Brasfield RD. Tumors of peripheral nerve origin: benign and malignant solitary schwannomas. CA Cancer J Clin. 1970;20(4):228–233. doi: 10.3322/canjclin.20.4.228. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002;10(2):81–89. doi: 10.1177/106689690201000201. [DOI] [PubMed] [Google Scholar]
- 10.Maciejewski A, Lange D, Wloch J. Case report of schwannoma of the rectum–clinical and pathological contribution. Med Sci Monit. 2000;6(4):779–782. [PubMed] [Google Scholar]


