Abstract
The benefits and risks of surgery for splenic hydatid cyst (SHC) remain controversial. We aimed to share our experience about a surgical approach for SHC. Sixteen consecutive patients with SHC disease who underwent open splenectomy at our hospital between January 2006 and July 2012 were retrospectively evaluated. Data on the patients' demographic features, clinical findings, radiological and serological diagnostic methods, and surgical and medicinal treatment options were collected and used to generate descriptive profiles of diagnosis, treatment course, and outcome. The patient population was composed of 6 females and 10 males, with an age range of 18 to 79 years (mean age: 47.0 ± 18.0). Radiological examinations detected hydatid cysts in spleen alone (n = 7) or both spleen and liver (n = 9). Preoperative serological testing identified 13 of the patients as IHA positive. All except 1 patient received a 10- to 21-day preoperative course of albendazole therapy and all patients received vaccination 1 week prior to surgery. Seven patients underwent splenectomy. The remaining patients underwent splenectomy with partial cystectomy and omentopexy (n = 6), partial cystectomy and unroofing (n = 1), pericystectomy (n = 1), or pericystectomy with partial nephrectomy (n = 1). All except one patient received a 10- to 45-day postoperative course of albendazole. No patients developed serious complications or signs of recurrence during the follow-up. The clinical profile of SHC disease at our hospital includes diagnosis by radiological methods, splenectomy treatment by simple or concomitant procedures according to the patient's symptoms, cyst size, number and localization, and compression of adjacent organs, and adjunct vaccination to decrease risk of postoperative septic complications. This profile is associated with low risk of complications and high therapeutic efficacy.
Keywords: Spleen, Splenic hydatid cyst, Splenectomy, Clinical experience, Diagnosis, Outcome
Hydatid disease, also known as echinococcosis, is caused by the Echinococcus parasites belonging to the Taeniidae family of the Cestode class.1–12 While 4 of these parasitic species are known human pathogens, the most commonly diagnosed are E. granulosus, which causes cystic echinococcosis (known as hydatid cysts) and E. alveolaris, which causes alveolar echinococcosis.1 However, humans are considered incidental hosts of the parasites, and infection is established upon oral ingestion of materials contaminated with fecal matter containing Echinococcus eggs from the primary hosts (dogs and other canids) or direct contact with dogs.1,5
Hydatid cyst disease is common among societies where agriculture and stockbreeding are commonplace. Endemic areas currently include the Middle East, Far East, Mediterranean, South America, Australia, and Turkey.1,3,9,13 Epidemiological profiling has shown that the primary organs affected by Echinococcus colonization (and subsequent cyst formation) are the liver (50–77%) and lung (15–47%), with cases of colonization in the kidney (2–4%) being reported rarely.1,2,6,7 Development of splenic hydatid cyst (SHC) is considered especially rare, with some estimates of incidence being as low as 0.5% of all cases.1–4,9,12
SHC cases are classified as either primary (involving the spleen only) or secondary (with multiple organ involvement). In general, hydatid cysts grow very slowly, so that patients remain asymptomatic (and undiagnosed) for years. Even when symptoms occur, often as a result of organ compression, they are often nonspecific.7,8 Once diagnosed, several treatment options are available, each with its own risk and efficacy profile. However, the most efficacious methods involve removal of the infected tissue, either by surgery or the less-invasive puncture-aspiration-injection-reaspiration (PAIR) approach.7,13 The application of these two approaches is usually designed on a patient-by-patient basis, according to the disease stage and individual risk for surviving operation. It is certain that surgery provides the best therapeutic outcome (versus PAIR), but the accumulated evidence has yet to definitively determine whether total splenectomy is more beneficial than the spleen-sparing surgical approaches.5 Therefore, this study was designed as a retrospective review of our hospital's open surgery treatment strategies for SHC disease, to provide a description of the diagnosis, treatment course, and outcome of these patients.
Materials and Methods
Study design and patient selection
The electronic records of Haydarpasa Numune Training and Research Hospital were searched for patients who underwent splenectomy (elective or urgent) for any reason between January 2006 and July 2012. The query was carried out with the following ICD-10 procedure codes for spleen: D70.0, D70.1, D70.2, D70.3, D70.4, D70.5, D70.6, D70.7, D70.8, and D70.9. Only patients who underwent splenectomy for hydatid cyst disease were selected for study inclusion.
The following data were extracted for each patient: age, sex, clinical presentation, radiologic diagnostic method [ultrasonography (US), computed tomography (CT), or magnetic resonance imaging (MRI)]; cyst number; cyst size (cm); splenic localization of cysts (upper pole, lower pole, or central); primary or secondary disease status; other organs carrying hydatid cysts; results of indirect hemagglutination (IHA) serological test; antihelminthic therapy (albendazole, preoperative and postoperative regimens); operation date; postoperative local or systemic complications (including wound infection and pulmonary infection); disease recurrence; and follow-up duration.
Results
A total of 16 patients with hydatid cyst disease were treated by splenectomy at our hospital during the 6-year study period. The group included 10 males and 6 females, ranging in age from 18 to 79 years (mean age: 47.0 ± 18.0). Eleven of the patients reported having resided in rural areas, at least temporarily, and the remaining 5 reported having consistently lived in urban areas. All patients reported previous physical contact with domestic animals, such as cats and dogs, but the duration of the contact was not recorded. All but 1 of the patients reported a history of having presented to different healthcare centers multiple times with complaint of nonspecific abdominal symptoms prior to diagnosis. For those 15 patients, the symptoms leading to presentation at our hospital that precipitated detailed investigation (and ultimate diagnosis) included general abdominal pain (n = 11), abdominal pain and distention (n = 2), left flank pain (n = 1), and unexplained weight loss with fatigue (n = 1). The single remaining patient had no symptoms and had been diagnosed incidentally during a routine check-up examination.
All patients were diagnosed radiologically, either by US and CT combination (n = 13), US alone (n = 2), or MRI (n = 1) (Figs. 1–3). The findings revealed primary SHC in 7 patients and secondary SHC in 9 patients (all involving liver). The splenic cysts were largely located in the central (n = 7), upper pole (n = 5), or lower pole (n = 3). One patient showed bipolar cyst localization (n = 1). Diameters of the cysts ranged from 3 to 20 cm. All patients underwent preoperative IHA serologic examination and 13 showed IHA positivity of varying titers (range: 1/256–1/2560). Nine of the IHA-positive patients were diagnosed with secondary disease (all involving liver).
Fig. 1.
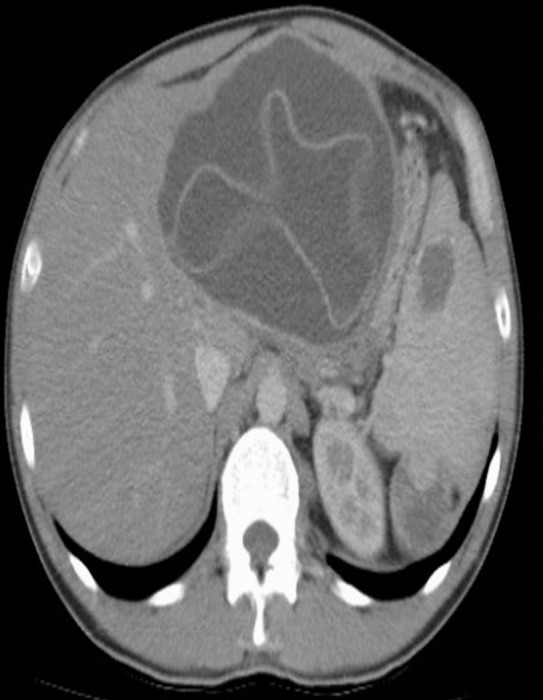
A 39-year-old male patient with splenic hydatid cyst disease. Axial CT sections show lesions consistent with hydatid cysts in the left lobe of the liver and in the spleen. A suspicious lesion of possible hydatid cyst origin is visible in the upper pole of the left kidney.
Fig. 3.
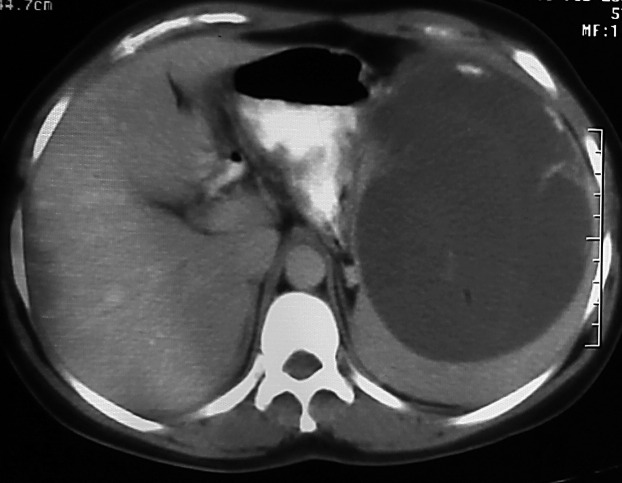
A 32-year-old female patient with splenic hydatid cyst disease. An axial CT section shows a hydatid cyst with near complete filling of the spleen.
The splenectomy was carried out as an elective procedure in all patients (no emergency cases). All but 1 of the patients received preoperative albendazole therapy, with duration varying from 10 to 21 days. The records for the remaining single patient did not indicate whether and why any preoperative medical therapy was or was not administered. All patients received vaccination 1 week prior to surgery. Total splenectomy was performed in 7 patients. The remaining 9 patients underwent splenectomy with partial cystectomy and omentopexy (n = 6), partial cystectomy and unroofing (n = 1), pericystectomy (n = 1), or pericystectomy with partial nephrectomy (n = 1). In the patient who underwent partial pericystectomy with nephrectomy and splenectomy, the preoperative radiological examination had indicated a lesion consistent with hydatid cyst in the upper pole of the left kidney, but perioperative observations revealed the lesion to be a tumor; the conversion to partial nephrectomy was conducted by urologists. Pathological analysis of the resected tissues diagnosed a grade-II clear cell renal cell carcinoma.
Postoperative recovery (in-hospital) ranged from 3 to 10 days (mean: 4 days). Albendazole therapy was administered during this period for 10 to 21 days for 6 patients with primary disease, and for 21 to 45 days for all 9 of the patients with secondary disease. The remaining primary disease patient did not receive any postoperative drug treatment. At follow-up after discharge, 2 patients presented with wound infections, but both were resolved by simple drainage and antibiotherapy. Over the full duration of follow-up, no signs of cyst recurrence were identified for any of the patients.
Discussion
Although medical practitioners have recognized SHC disease since ancient times, the first modern-day report of a case (discovered in autopsy) was published by Berthelot in 1790.1,2 Currently, estimates of the worldwide incidence of this disease range from 0.5 to 8.0%, accounting for 0.5 to 4.0% of all abdominal echinococcosis cases reported.1–3,7,9,12 However, these studies have failed to definitely determine whether primary or secondary cases are more common (with wide ranges of incidence rates being reported; primary: 38.1–80.0%, secondary: 16.0–61.9%),1–3,5,8–10,12 or whether solitary or multiple lesions are more common (solitary: 41.1–94.7%, multiple: 5.3–33.0%).1,2,4,6,8–10 It is possible that these disease characteristics are affected by yet unknown geographic, behavioral, environmental, or other many factors. In our study, the ratios of secondary and solitary SHC were 56.2 and 93.8%, respectively.
The etiology and pathogenesis of SHC disease remains unclear, although some hypotheses have been postulated. For example, the parasite may achieve splenic colonization via: (1) an arterial route [after it escapes or passes through first Lehman's filter (hepatic) to the second Lehman's filter (pulmonary)]; (2) a portal vein retrograde extension from the liver to the spleen (particularly in the case of portal hypertension)1,3; (3) rupture of hydatid cysts located in extrasplenic organs located within the peritoneal cavity1; or (4) systemic dissemination. The first two hypotheses fit best with development of primary SHC, while the latter two hypotheses fit best with the etiology of secondary SHC, which have spread to other organs.1,3
SHC have a very slow growth pattern (0.3–2.0 cm/y), which allows patients to remain completely asymptomatic for decades.1–4,7–12 As such, diagnosis is often made incidentally during clinical investigation of other ailments, and especially during radiological tests performed for liver cysts.1,2,4,9,11 However, among the symptomatic cases, the majority of symptoms are nonspecific,4,9 with the most common being abdominal pain and/or bloating, constipation (due to colonic compression), and dyspnea (due to left diaphragmatic compression).1,2 For both asymptomatic and symptomatic cases, the most common findings in physical examination are mass in the left upper quadrant (31.2–71.4% of cases) and splenomegaly (12.5–35.3% of cases).8–11 It has been suggested that the development of symptoms may be triggered by the following cyst-related complications: infection, opening into adjacent hollow organs, rupture into the pleural or peritoneal cavity, hypersplenism, anaphylactic shock, and cysto-pleural fistula formation.1–3,9 However, none of the 16 patients in the current study had signs of any of these complications that may have explained their presenting symptoms.
Diagnosis and differential diagnosis of SHC can be easily accomplished by combined use of radiological and serological testing.1,3,5,9,10 US and CT are the most commonly used radiological tests for hydatid cyst diagnosis, and it is known that the radiological appearance of these cysts depends upon the cyst location, patient age, and presence of complications (rupture or secondary infection).1,3 The 2 tests provide complimentary data for designing clinical management strategies; for example, US (a noninvasive, sensitive, and cost-effective technique) is useful for cyst staging1,3,10 while CT more accurately detects cyst number, size, localization, and presence of cysts in other organs. In addition, CT is preferable for monitoring of postoperative recurrences.1 MRI has only recently been applied as a detection method for cyst hydatid disease, and has proven capable of detecting some cysts missed by US and CT and of providing cystic pathological features.
Serological tests are also commonly used in the diagnosis, screening, and recurrence monitoring of SHC diseases. Besides the common methods of serum immunoelectrophoresis (sensitivity, 90–95%) and IHA (sensitivity, 85–100%), the methods of complement fixation, enzyme-linked immunosorbent assay (sensitivity, 80–100%), and immunofluorescence assay have been successfully employed.1,2,4,8,9 However, it has been noted that the rate of seropositivity is generally higher for hydatid cyst cases involving the liver (80–94%) than for those involving other organs (65%).1,3,5 In the current study population with SHC disease, the rate of IHA positivity was 57% in primary cases and 100% in secondary cases.
The differential diagnosis of SHC should include other parasitic cysts of the spleen, nonparasitic cysts, and neoplastic cystic lesions. The biggest challenge to differential diagnosis, however, is the similar radiologic appearance of most types of splenic cysts.1 By combining the results from various radiological methods (such as US, CT, and MRI) along with those from serological tests aids in determining the differential diagnosis.12 In addition, physicians, especially in echinococcosis-endemic regions are aware of the fact that hydatid cysts account for the majority of parasitic cysts of the spleen.1–3,11
Three modalities of treatment exist for SHC: open or laparoscopic surgeries to carry out total splenectomy or spleen-sparing procedures, minimally invasive approaches (such as PAIR), and medicinal approaches. Selecting the appropriate therapy for a patient requires consideration of the patient's age; overall health status; severity of symptoms; complications; experience of the surgeon or radiologist; and the number, size, stage, and location of cysts. The main aim of the surgical approach is to prevent complications, eliminate the local disease, and minimize rates of mortality, morbidity, and postoperative recurrences1,5; both the total splenectomy and spleen-sparing surgery can achieve these outcomes, but the latter is less traumatic for the patient.
In total splenectomy, the parasitic elements are completely excised, thereby minimizing or eliminating complications related to a cyst cavity.9 However, post-splenectomy sepsis-associated mortality and morbidity is high1,4,8,9 and physicians are applying this procedure with much more caution; for example, the benefit of total splenectomy may outweigh the risk in cases of multiple cysts, where >75% of the spleen is involved, or when cysts are located centrally or intraparenchymally.4,9 In addition, adult patients tend to tolerate the total splenectomy much better than pediatric patients, and the risk of post-splenectomy sepsis can be significantly minimized by preoperative vaccination.
Conservative surgical techniques, such as partial splenectomy, cyst enucleation, deroofing with omentoplasty, internal drainage with cystojejunal anastomosis, and external drainage, are primarily applied to cases with cysts that are inactive, superficial, for which a sufficient amount of splenic parenchyma will remain after surgery, located at the upper or lower poles of spleen, or involving severe adhesions.2,10–12 Children are especially good candidates for conservative therapy since they are at higher risk of splenectomy-related sepsis and more frequently show severe adhesions with adjacent organs. Both total splenectomy and spleen-sparing therapy can be carried out with laparoscopic (conventional or robotic) instruments, but this type of procedure is limited by associated risk of cyst rupture and peritoneal spillage,5,9 as well as the requirements for complicated equipment and highly skilled operator experience.
One of the most recently developed alternative to surgery is PAIR, a percutaneous drainage technique. Using PAIR to access the cyst cavity by passing through the organ parenchyma under US and CT guidance, the risk of anaphylaxis and peritoneal seeding is eliminated.1,13 According to the literature, the PAIR technique appears to be a safe method for treating Type I or II SHC, cysts with diameter <50 mm, and for patients who refuse surgical therapy or have an unacceptably high risk for anesthesia.1
The final treatment modality, medicinal therapy, is insufficient to replace surgical therapy, as antihelminthic agents administered via an oral route have very poor gastrointestinal absorption and usually cannot reach sufficient concentrations in the cyst cavity to kill the entire parasite population at all life stages.1 As a result, medicinal therapy is primarily applied as adjunct therapy to cases with multiple cysts involving more than 1 organ, with multiple recurrences, and with severe peritoneal hydatidosis.1 Recent studies have provided promising results for antihelminthic agents used in combination with surgical therapy or PAIR,1,9 when the anthelminthic drug prophylaxis is administered prior to the physical procedure to sterilize hydatid cysts, reduce cyst pressure, and decrease the risk of anaphylactic reactions as a result of cyst manipulation.1,2 In the case of splenectomy (total or partial) the preoperative course is 2 to 4 weeks, and for PAIR it is 1 week. Postoperative antihelminthic therapy is not necessary in cases of isolated SHC or when a successful total splenectomy is achieved. However, cases receiving spleen-sparing surgery, experiencing recurrence, and with extra-splenic cysts have shown benefit from postoperative antihelminthic therapy; splenectomy surgeries use 2- to 6-week courses, and PAIR uses 3- to 8-week courses. The most frequently used antihelminthic agents are albendazole, mebendazole, and praziquantel, all of which have good tolerance and safety profiles.
In summarizing our 6-year clinical experience with surgical treatment of SHCs, we were reminded of the importance of including this disease in differential diagnoses of patients with unexplained abdominal symptoms, especially those living in or traveling to echinococcosis-endemic regions. The most useful and simple imaging modality for diagnosing and monitoring this disease is ultrasonography. In cases with large-size or multiple splenic cysts, surgical therapy is best as it eliminates the high risk of rupture and its associated complications. Finally, while prophylactic antihelminthic therapy is able to reduce recurrence rates, preoperative or early postoperative vaccination is important to minimize post-splenectomy sepsis risk.
Acknowledgments
The authors declare that they have no conflicts of interest.
Eris C, Yildiz MK, Abuoglu H, Odabasi M, Ozkan E, Atalay S, and Gunay E performed surgical procedure; Akbulut S and Eris C contributed to writing the article and reviewing the literature in a comprehensive literature search; Akbulut S designed and prepared the manuscript.
Fig. 2.
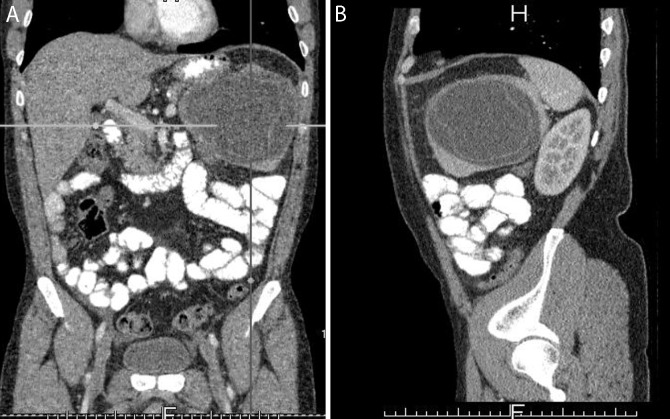
A 42-year-old male patient with splenic hydatid cyst disease. Coronal (A) and sagittal (B) CT sections showing a 13 × 9 cm lesion, consistent with hydatid cyst in the central portion of the spleen.
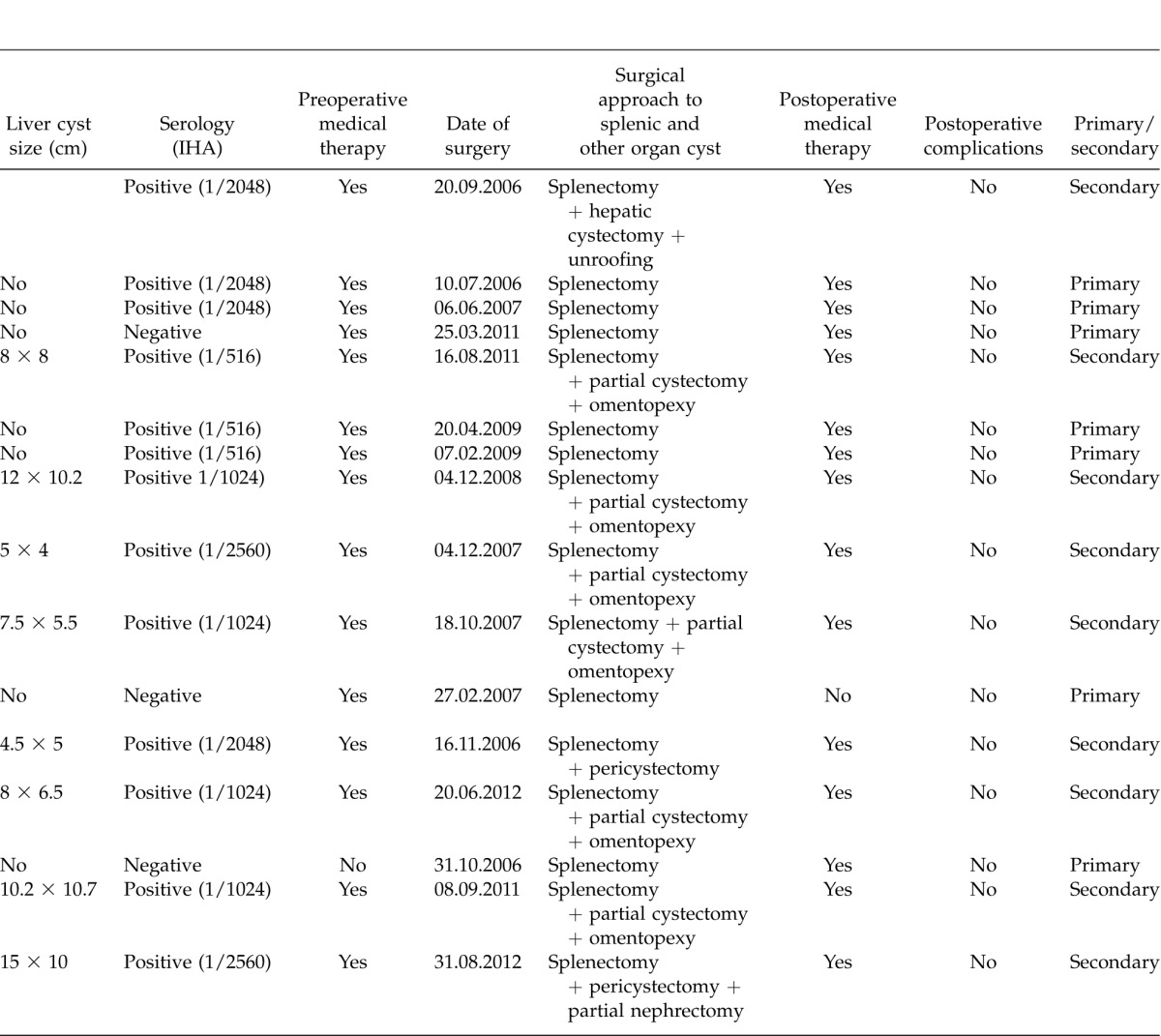
Table 1.
Demographic, clinic and surgical characteristics of 16 patients with primary or secondary splenic hydatid cyst
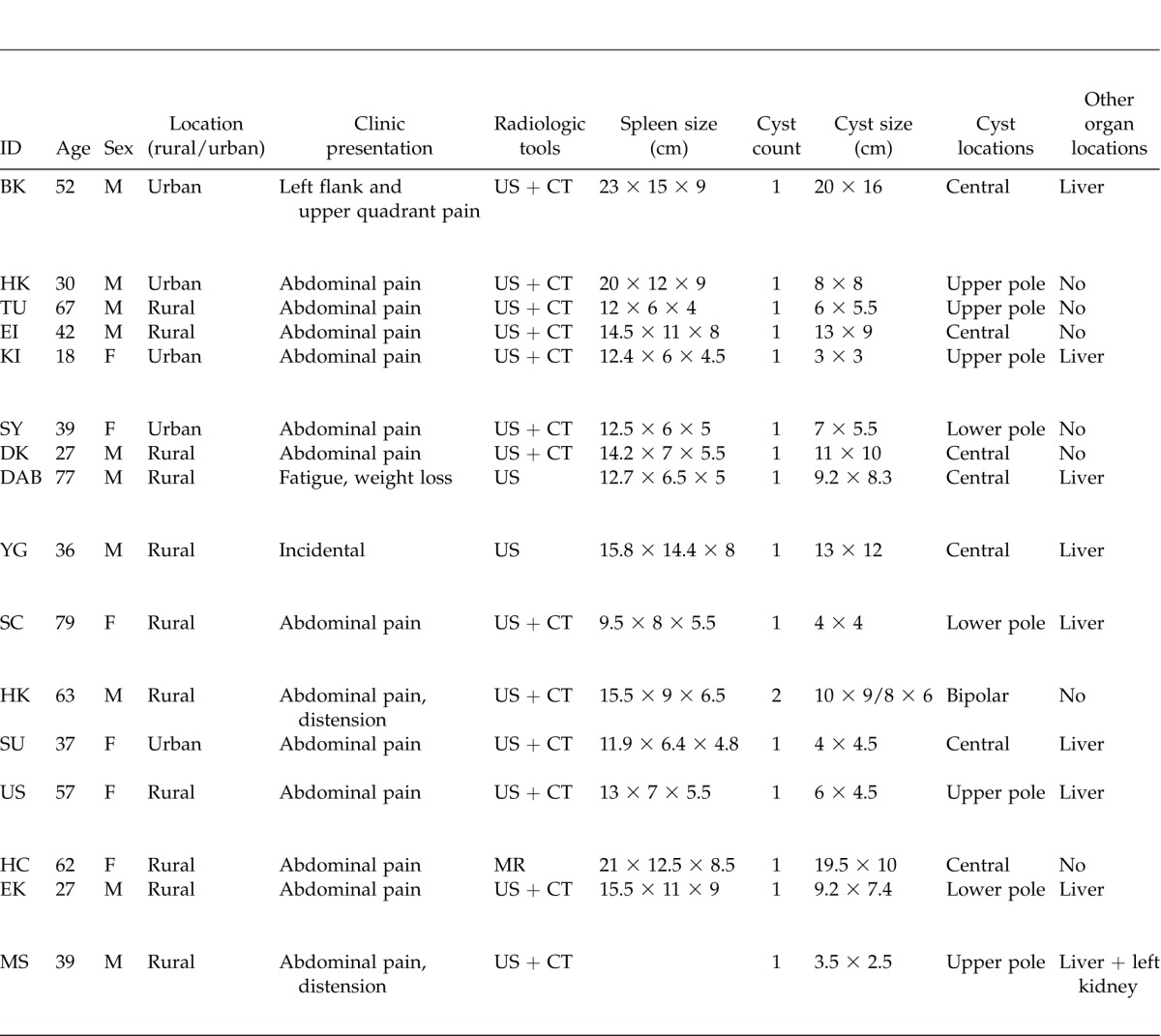
Table 1.
Extended
References
- 1.Rasheed K, Zargar SA, Telwani AA. Hydatid cyst of spleen: a diagnostic challenge. N Am J Med Sci. 2013;5(1):10–20. doi: 10.4103/1947-2714.106184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik AA. ul Bari S, Younis M, Wani KA, Rather AA. Primary splenic hydatidosis. Indian J Gastroenterol. 2011;30(4):175–177. doi: 10.1007/s12664-011-0104-x. [DOI] [PubMed] [Google Scholar]
- 3.Ramia-Angel JM, Gasz A, de la Plaza-Llamas R, Quinones-Sampedro J, Sancho E, Garcia Parreno J. Hidatidosis of the spleen. Pol Przegl Chir. 2011;83(5):271–275. doi: 10.2478/v10035-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 4.Culafic DM, Kerkez MD, Mijac DD, Lekić NS, Ranković VI, Lekić DD, et al. Spleen cystic echinococcosis: clinical manifestations and treatment. Scand J Gastroenterol. 2010;45(2):186–190. doi: 10.3109/00365520903428598. [DOI] [PubMed] [Google Scholar]
- 5.Vasilescu C, Tudor S, Popa M, Tiron A, Lupescu I. Robotic partial splenectomy for hydatid cyst of the spleen. Langenbecks Arch Surg. 2010;395(8):1169–1174. doi: 10.1007/s00423-010-0647-9. [DOI] [PubMed] [Google Scholar]
- 6.Meimarakis G, Grigolia G, Loehe F, Jauch KW, Schauer RJ. Surgical management of splenic echinococcal disease. Eur J Med Res. 2009;14(4):165–170. doi: 10.1186/2047-783X-14-4-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhan O, Koroglu M. Hydatid disease of the spleen. Semin Ultrasound CT MR. 2007;28(1):28–34. doi: 10.1053/j.sult.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Atmatzidis K, Papaziogas B, Mirelis C, Pavlidis T, Papaziogas T. Splenectomy versus spleen-preserving surgery for splenic echinococcosis. Dig Surg. 2003;20(6):527–531. doi: 10.1159/000073689. [DOI] [PubMed] [Google Scholar]
- 9.Ameur HB, Affes N, Abdelhedi C, Kchaou A, Boujelbene S, Beyrouti MI. Hydatid cyst of the spleen: Tunisian series of 21 cases. Indian J Surg. doi: 10.1007/s12262-013-0905-5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozogul B, Kisaoglu A, Atamanalp SS, Ozturk G, Aydinli B, Yildirgan MI, et al. Splenic hydatid cysts: 17 cases. Indian J Surg. doi: 10.1007/s12262-012-0788-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durgun V, Kapan S, Kapan M, Karabiçak I, Aydogan F, Goksoy E. Primary splenic hydatidosis. Dig Surg. 2003;20(1):38–41. doi: 10.1159/000068864. [DOI] [PubMed] [Google Scholar]
- 12.Dar MA, Shah OJ, Wani NA, Khan FA, Shah P. Surgical management of splenic hydatidosis. Surg Today. 2002;32(3):224–229. doi: 10.1007/s005950200025. [DOI] [PubMed] [Google Scholar]
- 13.Zerem E, Nuhanovic A, Caluk J. Modified pair technique for treatment of hydatid cysts in the spleen. Bosn J Basic Med Sci. 2005;5(3):74–78. doi: 10.17305/bjbms.2005.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]