Abstract
This report describes a case of port site metastasis after laparoscopic gastrectomy for gastric cancer. A 57-year-old man with clinical cTNM stage II (T2 N0 M0) gastric cancer was admitted to our hospital. After administration of an oral fluoropyrimidine drug (S-1) for 2 weeks, he underwent laparoscopy-assisted distal gastrectomy (LADG). On hematoxylin and eosin staining, the pTNM stage was IA (T1b N0 M0). Eighteen months later, the patient developed a subcutaneous metastasis at the trocar site. A second operation was performed, and the abdominal wall mass was resected. The histological finding confirmed a diagnosis of metastatic gastric carcinoma. Immunohistochemical analysis revealed micrometastasis in fat tissue adjacent to the lymph node near the left gastric artery. Surgeons should be aware that port site metastasis can occur in patients undergoing LADG for gastric cancer with lymphatic micrometastasis, which is undetectable on routine hematoxylin and eosin staining.
Keywords: Laparoscopic gastrectomy, Port site recurrence, Gastric cancer
Laparoscopic-assisted distal gastrectomy (LADG) for early gastric cancer was first performed in 1994,1 and its use in Japan has been steadily increasing since then. The advantages of laparoscopic gastric surgery include better cosmesis, less pain, shortened hospital stay, and early rehabilitation.2 Further, Hwang et al3 reported that the postoperative morbidity rate, the extent of lymphadenectomy, and the survival rate were similar when comparing LADG and open surgery. Studies have also shown that LADG has oncologic outcomes that are comparable to those of open surgery. Port site metastasis can occur following laparoscopic surgery in patients with various cancers,4–6 including after LADG. This report describes a case of a patient who developed port site metastasis 18 months after LADG.
A 57-year-old man was referred for surgical resection of gastric cancer. Examination with upper gastrointestinal endoscopy and computed tomography (CT) revealed a type 3 tumor in the middle body of the stomach without distant metastasis (cTNM stage II; T2 N0 M0). The histologic type of the tumor was moderately differentiated adenocarcinoma. The tumor marker levels were within normal limits. Laparoscopic-assisted distal gastrectomy (LADG) was performed after the administration of an oral fluoropyrimidine drug (S-1) for 2 weeks as neoadjuvant chemotherapy. Port sites at the first surgery were as follows. An Optiview port was inserted in the subumbilical position. A laparoscope was introduced through this port, and 4 other ports were inserted into the upper abdomen. The duodenum was divided distally to the pyloric ring laparoscopically using a stapler device (Endo GIA Ultra 60∼3.5 mm, Covidien, North Haven, Connecticut). A 5-cm transverse incision was made at the left side of the epigastrium and covered with a surgical drape (Alexis, wound protector/retractor S, Applied Medical, Rancho Santa Margarita, California). The distal stomach was delivered through the incision and dissected to an appropriate line of resection using the stapler device. A Roux-en-Y anastomosis was then performed laparoscopically with the stapler device. The final pathologic report revealed pTNM stage IA (T1b N0 M0; the largest diameter was 5 cm). Immunohistochemical analysis revealed a micrometastasis within the fat tissue adjacent to the lymph node near the left gastric artery (Fig. 1). Initial recovery was uneventful. The patient was treated with adjuvant chemotherapy with S-1 (100 mg/d) for 12 months after surgery.
Fig. 1.
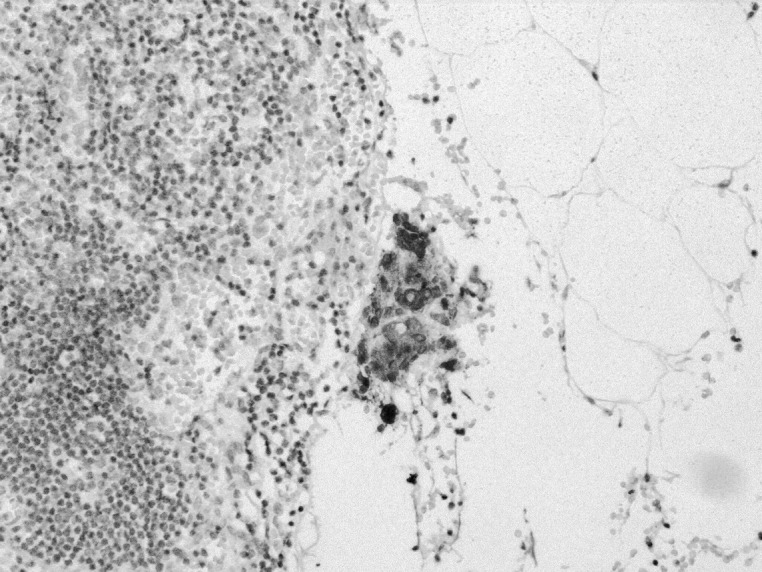
Immunohistochemical staining (×200) for cytokeratin shows a micrometastasis in adipose tissue bordering the lymph node near the left gastric artery.
Eighteen months after surgery, a mass was palpated at the left lower quadrant trocar site. Laboratory data, including tumor markers, were normal. Abdominal CT showed the mass to be localized above the fascia in the abdominal wall (Fig. 2), and no distant metastasis, such as peritoneal dissemination, lymph node swelling, or liver metastasis, was seen. A core needle biopsy revealed metastatic adenocarcinoma. A diagnosis of the port site recurrence of gastric cancer was made, and the abdominal wall tumor was resected with a leaf skin incision with a 1.5-cm margin from the tumor. The tumor was removed along with the abdominal rectal muscle. The defect in the abdominal wall was closed by flap of the tensor fasciae latae muscle.
Fig. 2.
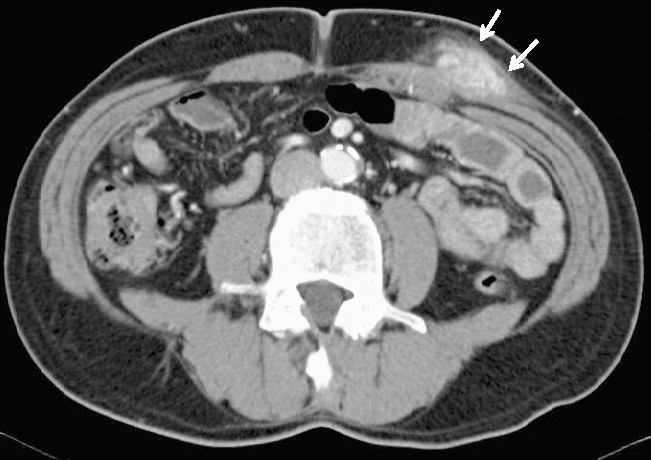
Abdominal computed tomography showing a tumor extending from the subcutaneous layer to the muscle layer in the left lower abdomen.
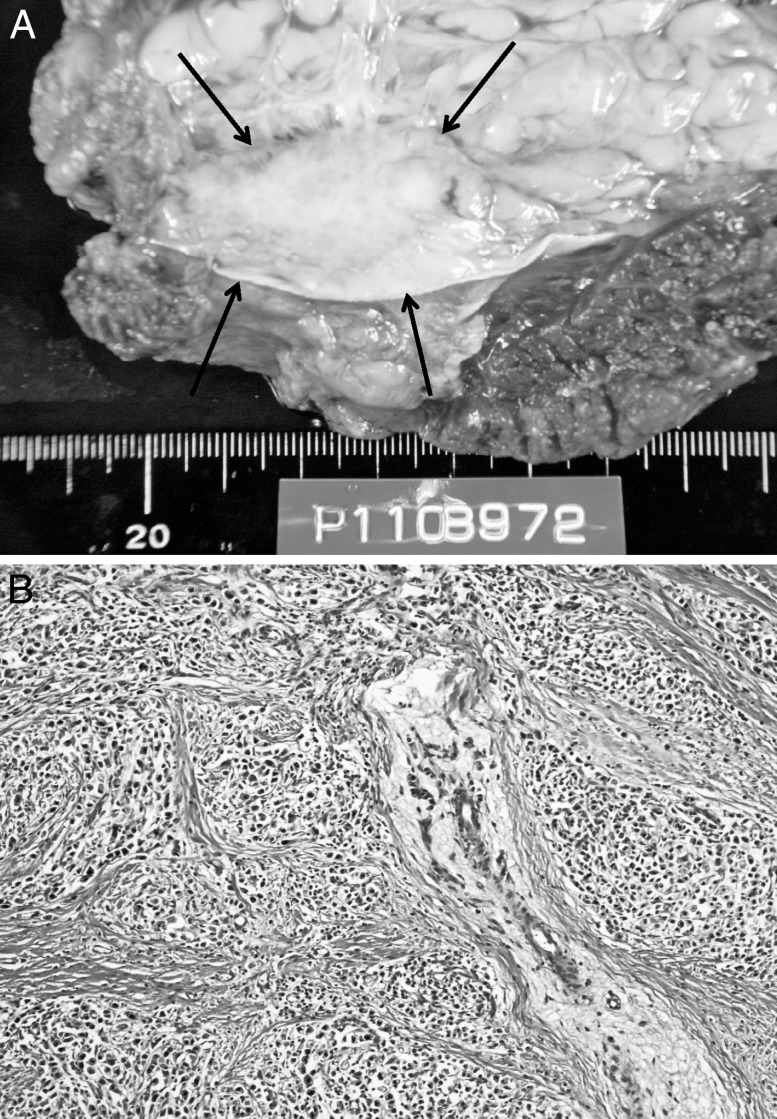
Inspection of resected specimen revealed a whitish tumor measuring 4.5 × 2.5 cm (Fig. 3A). The pathologic diagnosis was metastatic adenocarcinoma (Fig. 3B). The postoperative course was uneventful, and the patient was well. However, abdominal CT performed at the 1-year follow-up after the second operation revealed peritoneal metastasis and ascites. The patient died 2 months after the recurrence, despite additional chemotherapy.
Fig. 3.
(A) The specimen resected from the left lower quadrant sites appears as a whitish tumor measuring 20 × 50 mm. (B) Pathologic examination shows metastatic adenocarcinoma that is consistent with the primary gastric carcinoma. Hematoxylin-eosin (×200).
Discussion
Port site metastasis (PSM) after laparoscopic surgery was initially reported in 1985 in patients with disseminated disease at the time of surgery.7 However, the exact incidence of port site recurrence after laparoscopic surgery for gastric cancer is unknown.
Additional reports of PSM were reported in the 1990s in patients with various malignant diseases, including those with cancers of the colon, gallbladder, and pancreas.4–6 PSM after LADG for early gastric cancer is a very rare type of recurrence. Previous reports indicate that the incidence of PSM after laparoscopic resection of gastric cancer is 0.4% to 11%.3,8,9 Most cases of PSMs occur in patients with advanced cancer, including those with peritoneal dissemination and invasion to serosa. Schaeff et al8 summarized 164 cases of PSMs after videoscopic procedures, including 6 cases with gastric cancer as the primary tumor. A total of 5 of these 6 cases were patients who underwent diagnostic laparoscopy with liver metastasis or dissemination, and in the remaining case PSM occurred after therapeutic laparoscopy. In contrast, Lee et al10 reported that PSM did not occur among 600 patients who underwent LADG in their hospital. The difference in incidence is possibly due to the fact that the indication for LADG in Japan is limited to those with early cancer.
Several animal and clinical studies have been conducted to clarify the mechanisms responsible for PSM. The etiology of PSM is probably multifactorial and may involve direct wound implantation, contamination of instruments, aerosolization of tumor cells, chimney effect, surgical technique, excessive manipulation of tumor, pneumoperitoneum, and hematogenous spread.6,11,12 Multiple studies have documented a higher incidence of PSM when surgery is conducted by surgeons with less experience in laparoscopic technique.11,13,14 Handling of tumor is more common when surgeons are at an earlier stage in the laparoscopic learning curve. However, the issue with most significant relevance to PSM is implantation of cancer cells secondary to surgical technique.
The present case was a patient with pT1b N0 tumor, which was unlikely to be associated with dissemination from the primary tumor. Therefore, we hypothesized that cancer cells in the fat tissue close by the lymphoid tissue of a lymph node might have disseminated to the port site when the lymph node was dissected during the operation. Immunohistochemical analysis using cytokeratin antibody was performed to detect micrometastasis in the perinodal fat tissue that is otherwise undetectable on routine hematoxylin and eosin staining. This showed micrometastasis in the fat tissue adjacent to the lymph node near the left gastric artery, indicating that some cancer cells might have spilled during surgery, leading to formation of PSM.
It is more probable that micrometastasis was present either within lymph nodes or outside of them at the time of the first surgery, leading to the cancer cells' dissemination to the port site. Because gastric serosa was not invaded by carcinoma in the present case, the cancer cells could not spill from the serosal surface to the port site. The present case suggests that use of LADG may be associated with a risk of PSM even if gastric serosa is not invaded by carcinoma and routine hematoxylin-eosin staining reveals no metastasis in lymph nodes.
In conclusion, standardization of laparoscopic procedures, minimal tumor handling, isolation of the specimen within a bag, and wound protection during tumor extraction are necessary to prevent dissemination of cancer cells. Surgeons should be aware of the risk of PSM even if routine hematoxylin and eosin staining reveals no metastases in lymph nodes.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review from the Editor-in-Chief of this journal.
Footnotes
Copyright statement: This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non-commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode.
References
- 1.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4(2):146–148. [PubMed] [Google Scholar]
- 2.Adachi Y, Suematsu T, Shiraishi N, Katsuta T, Morimoto A, Kitano S, et al. Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg. 1999;229(1):49–54. doi: 10.1097/00000658-199901000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang SI, Kim HO, Yoo CH, Shin JH, Son BH. Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc. 2009;23(6):1252–1258. doi: 10.1007/s00464-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 4.Barsoum GH, Windsor CW. Parietal seeding of carcinoma of the gallbladder after laparoscopic cholecystectomy. Br J Surg. 1992;79(8):846. [PubMed] [Google Scholar]
- 5.Jorgensen JO, McCall JL, Morris DL. Port site seeding after laparoscopic ultrasonographic staging of pancreatic carcinoma. Surgery. 1995;117(1):118–119. doi: 10.1016/s0039-6060(05)80243-0. [DOI] [PubMed] [Google Scholar]
- 6.Nduka CC, Monson JR, Menzies-Gow N, Darzi A. Abdominal wall metastases following laparoscopy. Br J Surg. 1994;81(5):648–652. doi: 10.1002/bjs.1800810506. [DOI] [PubMed] [Google Scholar]
- 7.Stockdale AD, Pocock TJ. Abdominal wall metastasis following laparoscopy: a case report. Eur J Surg Oncol. 1985;11(4):373–375. [PubMed] [Google Scholar]
- 8.Schaeff B, Paolucci V, Thomopoulos J. Port site recurrences after laparoscopic surgery: a review. Dig Surg. 1998;15(2):124–134. doi: 10.1159/000018605. [DOI] [PubMed] [Google Scholar]
- 9.Jeong SH, Lee YJ, Park ST, Choi SK, Hong SC, Jung EJ, et al. Risk of recurrence after laparoscopy-assisted radical gastrectomy for gastric cancer performed by a single surgeon. Surg Endosc. 2011;25(3):872–878. doi: 10.1007/s00464-010-1286-5. [DOI] [PubMed] [Google Scholar]
- 10.Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg. 2010;211(1):33–40. doi: 10.1016/j.jamcollsurg.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Ziprin P, Ridgway PF, Peck DH, Darzi AW. The theories and realities of port-site metastases: a critical appraisal. J Am Coll Surg. 2002;195(3):395–408. doi: 10.1016/s1072-7515(02)01249-8. [DOI] [PubMed] [Google Scholar]
- 12.Zmora O, Gervaz P, Wexner SD. Trocar site recurrence in laparoscopic surgery for colorectal cancer. Surg Endosc. 2001;15(8):788–793. doi: 10.1007/s004640080151. [DOI] [PubMed] [Google Scholar]
- 13.Lee SW, Southall J, Allendorf J, Bessler M, Whelan RL. Traumatic handling of the tumor independent of pneumoperitoneum increases port site implantation rate of colon cancer in a murine model. Surg Endosc. 1998;12(6):828–834. doi: 10.1007/s004649900723. [DOI] [PubMed] [Google Scholar]
- 14.Lee SW, Gleason NR, Bessler M, Whelan RL. Port site tumor recurrence rates in a murine model of laparoscopic splenectomy decreased with increased experience. Surg Endosc. 2000;14(9):805–811. doi: 10.1007/s004640000231. [DOI] [PubMed] [Google Scholar]