Abstract
Repair of contaminated/infected ventral incisional hernias is marked by high rates of recurrence, complications, and/or explantation of synthetic mesh. Biologic mesh products are recommended for repair to permit reconstruction and reduce complications. A systematic review of PubMed, EMBASE, and Cochrane databases identified English-language articles reporting postoperative outcomes (e.g., hernia recurrence, infection, mesh explantation) in patients undergoing contaminated/infected ventral incisional herniorrhaphy. Eleven studies met inclusion criteria and contained quantitative outcome data. All were retrospective chart reviews of biologic mesh use (mostly human acellular dermal matrix). Hernia recurrence and wound infection rates were highly variable and inconsistently reported across studies. Mesh explantation was rarely reported. Outcome variability is likely owing to heterogenous patient populations, surgical technique variations, and follow-up duration. Biologic mesh use in contaminated/infected herniorrhaphy was marked by low reported rates of secondary surgeries for infected mesh removal. Data from large, well-controlled, prospective trials with biologic mesh products are needed.
Keywords: Ventral incisional herniorrhaphy, Biologic mesh, Hernia recurrence, Postsurgical complication
Ventral incisional hernias frequently occur following abdominal surgery, with a reported cumulative incidence of 11% to 19%.1,2 Techniques for surgical hernia repair have evolved over the past several decades from use of sutures alone for midline closure to the use of a variety of synthetic and biologic mesh products to strengthen repairs.3,4 Primary ventral incisional hernia repair with sutures alone is associated with hernia recurrence rates ranging from 36% to 56%,2,5–7 with 10-year recurrence rates as high as 63%.8 When synthetic mesh is incorporated for reinforcement, reported recurrence rates range from 19% to 32%.5,7,8 Another important advance in ventral incisional hernia repair was the introduction of the components separation technique, in which lateral skin and subcutaneous flap incisions of the external oblique muscles allows midline closure of the rectus muscles.3,9 Other crucial improvements in ventral incisional hernia repair include the development of laparoscopic techniques, which help minimize postoperative complications and infection, and the evolution of polyester-based and biologic mesh products.3,4
According to the Ventral Hernia Working Group (VHWG) recommendations,4 use of synthetic mesh is appropriate for patients who present a low risk of infection or complication, while use of biologics is recommended for higher-risk patients. Nevertheless, there is no widely accepted consensus on appropriate mesh selection for surgical patients who present with elevated risk for contamination or postoperative complications. The present systematic review is intended to assess outcomes in patients undergoing repair of contaminated or infected ventral incisional hernias, with a focus on the use of biologic mesh.
Materials and Methods
Searches of electronic databases (PubMed, EMBASE, and Cochrane) were performed to capture English-language manuscripts published within the past 10 years through January 2, 2012. The following search terms were used: (“clean-contaminated” OR “contaminated” OR “contamination” OR “infected” OR “infection” OR “dirty” OR “burst abdomen” OR “open abdomen” OR “septic dehiscence” OR “septic shock” OR “sepsis” OR “intestinal dehiscence” OR “wound dehiscence” OR “seroma” OR “violation” OR “complication” OR “occurrence”) AND (“hernia” OR “herniorrhaphy” OR “abdominal wall”). A secondary search included the following search terms: (“clean-contaminated” OR “contaminated” OR “contamination” OR “infected” OR “infection” OR “dirty” OR “burst abdomen” OR “open abdomen” OR “septic dehiscence” OR “septic shock” OR “sepsis” OR “intestinal dehiscence” OR “wound dehiscence” OR “seroma” OR “violation” OR “complication” OR “occurrence”) AND (“ventral incisional hernia” OR “ventral herniorrhaphy” OR “abdominal wall”).
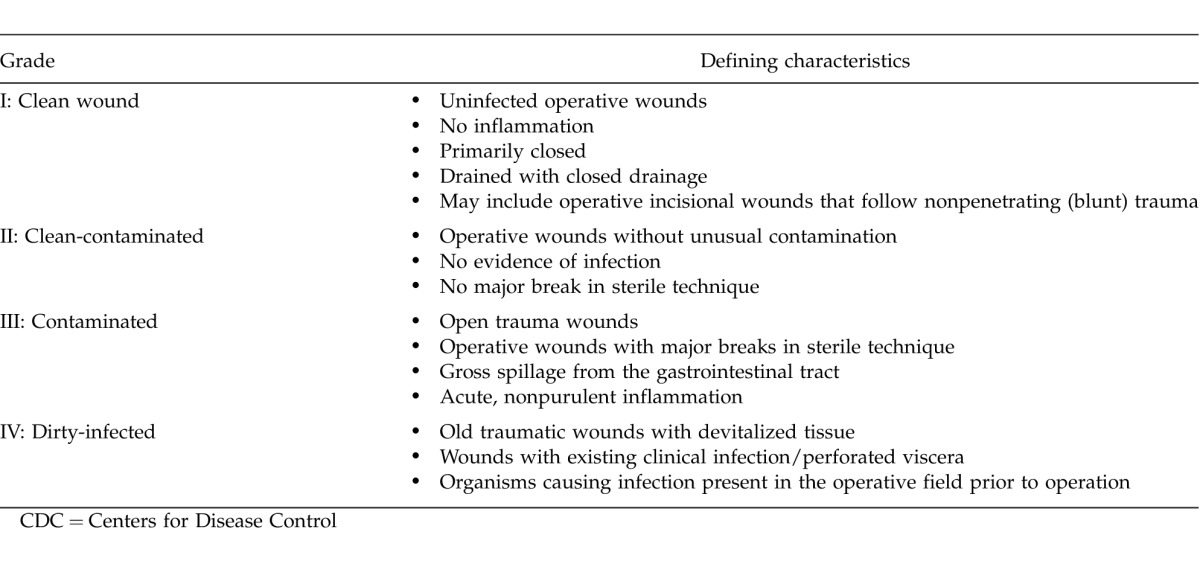
The resulting set of published articles was manually screened to identify randomized controlled clinical trials and retrospective or prospective chart reviews of ventral herniorrhaphy in the setting of contamination, infection, or both. The literature review was conducted using the Population, Intervention, Comparisons, and Outcomes (PICO) approach (Table 1)10,11 and focused on published reports concerning repair techniques and postoperative outcomes in adults undergoing ventral incisional herniorrhaphy for contaminated or infected hernias. Interventions targeted in the search included primary repair, components separation, and/or tissue expansion with suture closure alone (i.e., without mesh) or with use of synthetic or biologic mesh reinforcement. Reports regarding repair without mesh or with use of synthetic mesh were anticipated to be few or absent in the literature but were included in the search to ensure that comparisons of synthetic mesh or no mesh versus biologic mesh were not missed. Surgical sites for study populations in each study were subcategorized as “clean,” “clean-contaminated,” “contaminated,” or “dirty/infected” according to the Centers for Disease Control (CDC) Guidelines for Prevention of Surgical Site Infection (Table 2).12 Outcomes assessed included postoperative rates of hernia recurrence, infection, seroma, hematoma, and additional repair, among others (Table 1). Data related to these outcomes were summarized, and differences in incidences and means were compared as feasible/available. The risk of bias among the included studies was assessed based on the design and methodology details reported for each study and the level of evidence.
Table 1.
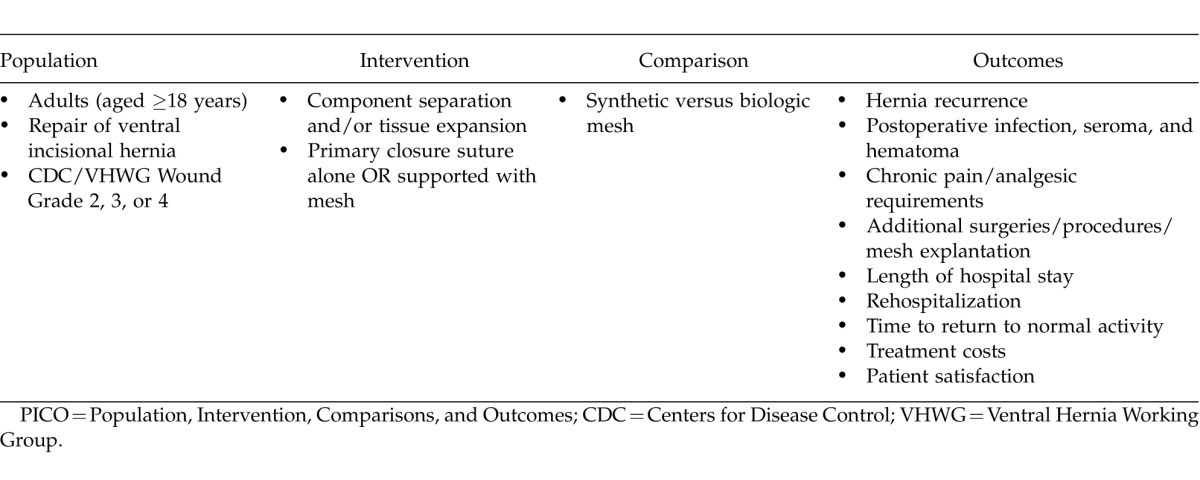
Table 2.
CDC wound grading system12
Articles were excluded if they (1) described research unrelated to ventral incisional hernia, (2) did not involve evaluation of surgical ventral hernia repair (e.g., diagnostic methodology articles, registries), or (3) reported studies including fewer than 5 patients with contaminated or infected ventral incisional hernias. The quality of the studies was rated based on the levels of evidence grading system recommended by the American Society of Plastic Surgeons (Table 3).13
Table 3.
Levels of evidencea

Results
Study selection and synthesis of results
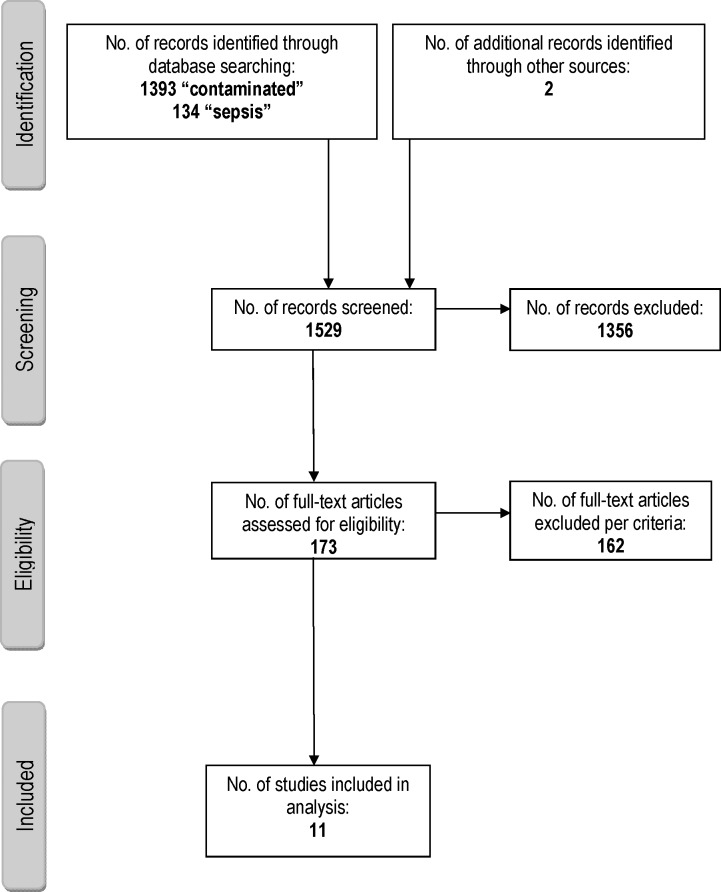
The electronic database searches, as well as manual searches of article reference lists, yielded 1529 records, which were screened for inclusion eligibility (Fig. 1). From these eligible articles were found to have partial or complete data available for the population of interest and were used for the synthesis of results (Table 4).14–24
Fig. 1.
Article selection flow diagram.
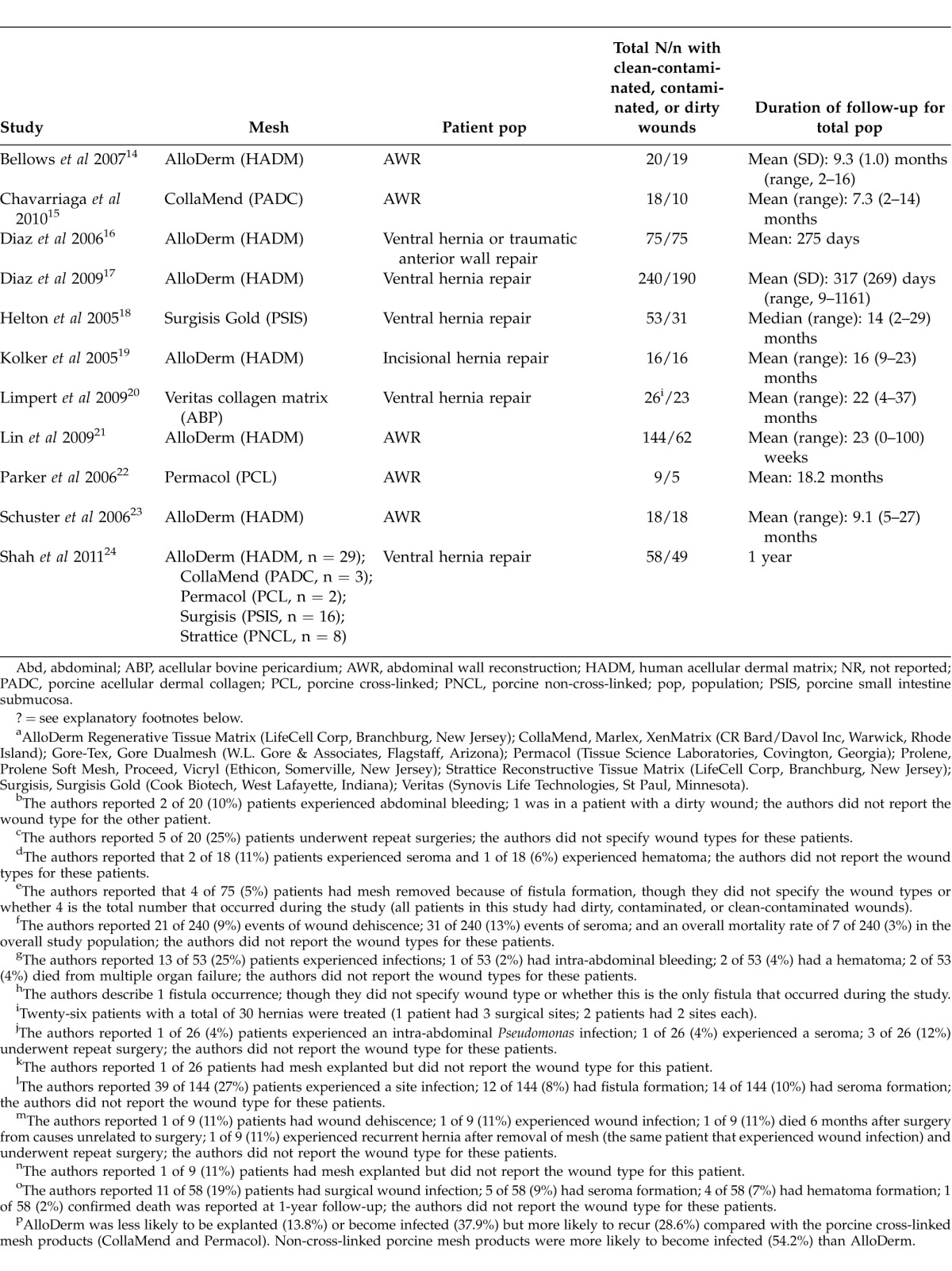
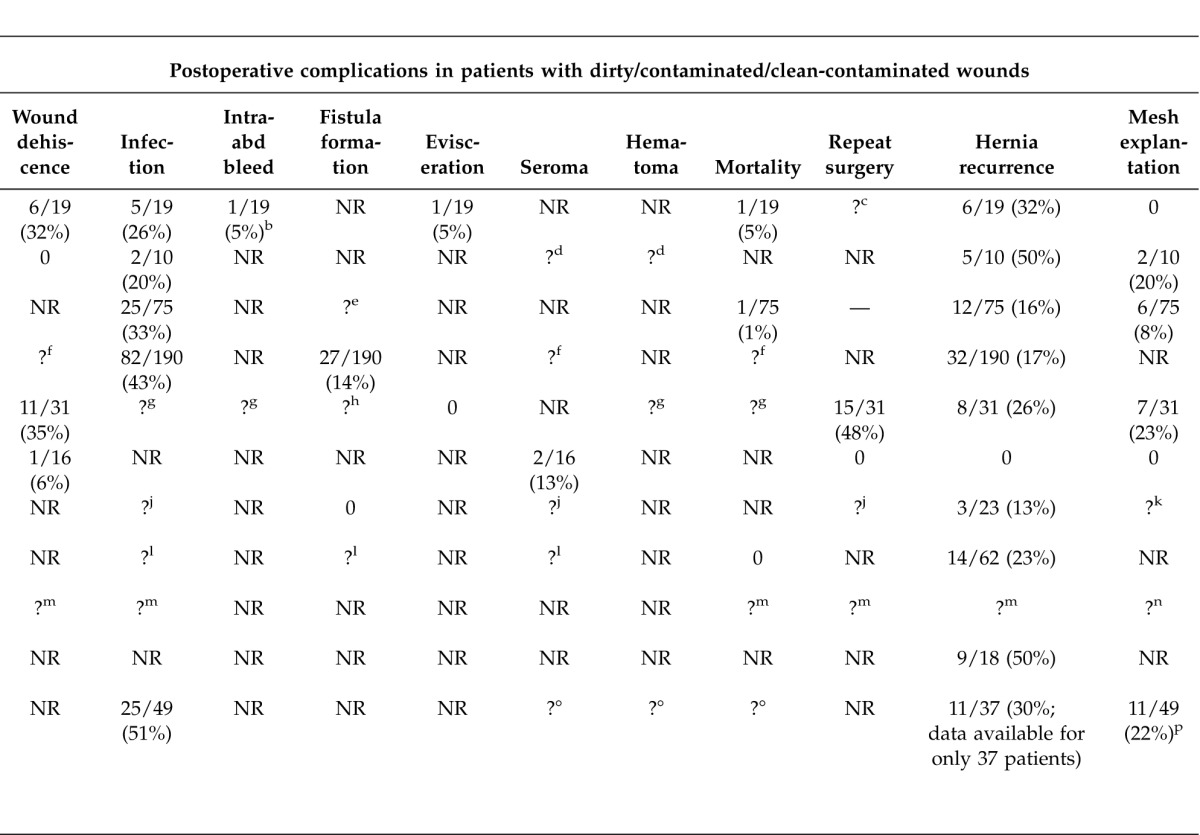
Table 4.
Summary of studies with data specific to contaminated or infected casesa
These articles provided data regarding the following biologic mesh products: AlloDerm (n = 7); Surgisis (n = 2); CollaMend (n = 2); Permacol (n = 2); Strattice (n = 1); and Veritas (n = 1) (see Table 4 footnotes for manufacturers). None provided data on surgical repair procedures conducted using synthetic mesh or no mesh.
All 11 articles reported results from retrospective chart reviews involving patient populations ranging in size from 9 to 240 (Table 4). Overall, 677 patients were included across the studies. The 3 largest studies examined outcomes among patients (N = 459) with a contaminated surgical field who underwent repair of ventral hernia (primary or recurrent) or abdominal wall repair for other indications using acellular human dermal matrix (AlloDerm).16,17,21 Two other larger studies (N = 5318 and N ¼ 5824) retrospectively reviewed outcomes following ventral hernia repair with a porcine submucosa-derived mesh [Surgisis Gold (SIS)]18 or a variety of biologic mesh products24 in the setting of a contaminated field. Across the 11 studies, 84 repairs were performed using components separation, and 17 involved laparoscopic repair.
Outcomes of interest
Outcomes that were reported varied widely across the 11 studies. The most commonly reported complications were hernia recurrence (range, 0%–50%), wound infection (20%–75%), wound dehiscence (0%–35%), mesh explantation (0%–23%), seroma (11%–13%), and fistula formation (0%–14%). Rarely, incidence rates for complications such as hematoma/bleeding and death were reported (Table 4). Postoperative timing of complications was rarely specified. The frequency of postoperative complications observed with individual types of biologic mesh is discussed in the following sections.
Hernia recurrence
Reported rates of hernia recurrence varied widely, and only 3 of the 11 reports included statistical information on defect size.14,17,21 The average defect size across these 3 studies ranged from 111 to 210 cm2; Lin et al reported that more than 90% of the defects repaired in their study were ≥25 cm2 but did not provide further description.21 Human acellular dermal matrix (AlloDerm) was the most commonly used mesh product and was applied in the largest overall number of patients in the studies reviewed. Among patients who underwent repair with AlloDerm, hernia recurrence rates ranged from 0% to 50% (Table 4). The highest recurrence rate (50%) was reported by Schuster et al.23 Further examination of the Schuster article revealed that when primary repair was achieved, 33% of patients (4 of 12) experienced hernia recurrence, but when primary repair could not be achieved and mesh was used to bridge the gap, the recurrence rate was 83% (5 of 6; P = 0.03). In the largest studies of complex hernia repair using AlloDerm, Diaz et al (2006)16 and Diaz et al (2009)17 reported much lower rates of hernia recurrence [16% (12 of 75) and 17.1% (32 of 190), respectively]. In a small case series reported by Kolker et al, in which all patients underwent components separation, no hernia recurrences with AlloDerm were observed in 16 patients followed for an average of 16 months.19
There was also broad variability in hernia recurrence rates reported in smaller retrospective chart reviews of other biologic mesh products in patients undergoing complex abdominal wall reconstruction in the setting of a contaminated or infected surgical field. Chavarriaga et al reported a hernia recurrence rate of 50% (5 of 10) in patients who received a cross-linked porcine acellular dermal matrix product (CollaMend) who were followed for a mean of 7.3 months.15 Shah et al reported a 25% (1 of 4) rate of recurrence with CollaMend or another porcine cross-linked acellular dermal matrix, Permacol.24 In the study from Parker et al,22 it was not possible to determine the rate of hernia recurrence in the target population, although only 1 patient experienced hernia recurrence during an average of 18.2 months of follow-up. With Surgisis, Helton et al18 observed a 26% (8 of 31) rate of recurrence, while Shah et al24 reported recurrence in 33% (4 of 12) of patients who had clean/contaminated or contaminated wounds repaired with porcine non-cross-linked products (Surgisis or Strattice). Limpert et al20 reported a 13% (3 of 23) rate of hernia recurrence with application of acellular bovine pericardium (Veritas).
Postoperative infection (and related complications)
Reported rates of postoperative surgical site infection in studies of AlloDerm also varied widely. The lowest rate (26%, 5 of 19) was observed in the relatively small review from Bellows et al14; all 5 observed infections occurred in patients with open wounds managed with vacuum-assisted closure. In the two relatively large-scale chart reviews from Diaz et al,16,17 infection rates were 33% (25 of 75) and 43% (82 of 190), respectively. In the study by Shah et al,24 which included 5 types of biologic mesh, the lowest infection rates were observed in patients treated with AlloDerm (39.3% versus 75% with cross-linked porcine Permacol/CollaMend and 64.7% with non-cross-linked porcine Surgisis/Strattice). Similarly, explantation rates were lowest with AlloDerm: 17.8% versus 50% (Permacol/CollaMend) and 23.5% (Surgisis/Strattice).
Surgical site infection rates in patients with contaminated or dirty surgical fields were not reported in most of the identified retrospective reviews that investigated less widely used mesh products. In the report from Chavarriaga et al,15 which examined outcomes with CollaMend, 20% (2 of 10) of patients experienced postoperative infection.
Wound dehiscence
Rates of wound dehiscence were not uniformly described across the available published chart reviews. In the studies of AlloDerm, wound dehiscence rates in contaminated/dirty fields are provided only in the small-scale reports from Bellows et al14 (31.5%, 6 of 19) and Kolker et al19 (6%, 1 of 16). The 2009 large-scale study reported by Diaz et al17 did not report wound dehiscence rates by specific wound types but showed a wound dehiscence rate of 8.8% (21 of 240) in the entire patient population (including 50 patients with clean surgical fields); all patients in this study received AlloDerm. With other types of biologic mesh, reported wound dehiscence rates varied from 0% (0 of 10) with CollaMend15 to 35.5% (11 of 31) with Surgisis.18
Other complications
Occurrences of seroma, hematoma, bleeding, and fistula formation were not uniformly reported for the relevant population across the 11 studies (Table 4). With AlloDerm, Diaz et al17 reported fistula formation in the population of interest at a rate of 14% (27 of 190); for the overall patient population (including those with clean wounds), a seroma rate of 12.9% (31 of 240) was observed. Kolker et al19 reported a seroma rate of 13% (2 of 16) in the population of interest—the entire study population comprised patients with dirty or contaminated wounds. Bellows et al14 reported that following AlloDerm repair, 2 patients experienced intra-abdominal bleeding. The authors specifically mention that 1 of these occurred in a dirty wound (population of interest) but did not report the wound type for the other bleeding occurrence.
Length of hospital stay and rehospitalization
Length of hospital stay in the population of interest was not consistently examined. Diaz et al16 reported an average hospital stay of 19.8 days in patients with some degree of surgical wound contamination who received AlloDerm compared with 17 days in the overall patient population. None of the other articles reported length of hospital stay for the targeted population following repair procedures.
Subsequent surgeries
Postoperative surgical interventions were not consistently described among the 11 articles. Among patients who underwent ventral hernia repair in the setting of a compromised surgical field, the most commonly reported reasons for a secondary surgical procedure included repair of recurrent hernia, mesh removal, drainage of seroma, and drainage of surgical site abscess.16,18,22,24 Some authors highlighted the management of surgical site infection with antibiotics and wound care without mesh removal as a benefit of using biologic mesh.14,16
Interpretative challenges
Owing to the retrospective nature of the investigations included, there is clear potential for bias in the outcomes data presented here; most authors of these reports were the treating surgeons. Interpretation of the outcomes presented in this systematic review was also complicated by the many different biologic mesh products used in the repair procedures described, the broad variation in the patient populations included, differences in surgical techniques (often highly individualized), and variability in the duration of follow-up. The paucity of reported data regarding several of the stated postoperative complications that were focused upon (e.g., seroma, hematoma, fistula formation, patient satisfaction, time to return to normal activities, patient satisfaction, cost, chronic pain) prevented the ability to make conclusions regarding these predetermined outcome measures.
Discussion
The results of this systematic review highlight a need for high-quality evidence upon which to make unbiased, evidence-based clinical decisions regarding the optimal use of mesh in patients with ventral incisional hernia and a potentially contaminated surgical field. The publications that met inclusion criteria for this review presented level II or III evidence that included quantitative data specific to postsurgical outcomes in a total of 677 patients with various types of abdominal wall defects. Based on the literature search, no randomized controlled clinical trials of mesh products for the treatment of contaminated or infected ventral incisional hernias were found. Of the 11 included articles, most described outcomes following repair with AlloDerm; results with other biologic mesh products, such as Surgisis and CollaMend, were described in a few relatively small retrospective studies.
Repair of ventral incisional hernia in the context of a contaminated or infected operative field presents a considerable surgical challenge.4 Closure of ventral incisional hernia with sutures alone yields suboptimal results; and according to current recommendations from the VHWG, synthetic mesh prostheses are contraindicated in such patients because of the potential for chronic postoperative infection of the mesh, as well as their known association with fistula and adhesion formation.4 Postoperative infection at the surgical site, and complications such as wound dehiscence, frank infection, bleeding, hernia recurrence, and the need for mesh removal are seen more frequently in patients with contaminated or infected surgical fields than in patients with clean surgical fields.4,17,24 Ideally, the mesh used within a potentially contaminated field should be strong, become vascularized, and be incorporated into the abdominal wall without triggering foreign body reactions and inflammation; it should also be associated with a low rate of short- and long-term complications or recurrences. Currently, biologic mesh materials are generally recommended for use in such patients, although evidence from high-quality controlled trials is lacking.4 While there are a multitude of different types of synthetic and biologic mesh products available, few published prospective investigations, randomized controlled trials, or direct head-to-head comparisons of different types of mesh exist.4
The rationale for use of biologic mesh rather than synthetic mesh for ventral incisional hernia repair in the setting of contamination or infection hinges on evidence demonstrating that biologic mesh supports tissue regeneration, marked by revascularization and cell repopulation.25 Better assimilation and revascularization may in turn lead to improved wound healing and better clearance of bacteria/infection. Moreover, as has been demonstrated in preclinical investigations of biologic mesh, the hernia repair site may be more likely to exhibit improved abdominal wall integrity, marked by tensile strength that meets or exceeds that of abdominal fascia.25 This may help to reduce hernia recurrence rates and the need for mesh removal in cases of infection.
Specific outcomes were quantified based on the data specific to the population of interest (i.e., patients undergoing repair of contaminated or infected ventral incisional hernias). Of note, no data were found regarding the use of synthetic mesh products in the relevant population. This finding most likely reflects the increasingly common preference of biologic over synthetic mesh in contaminated or infected settings. The reported outcomes varied widely across the studies, regardless of the type of mesh product applied for repair. In 2 of the largest studies analyzed (Diaz et al, 2006 and 2009),16,17 which examined outcomes using AlloDerm, hernia recurrence rates were 16% and 17%, respectively. Recurrences with CollaMend,15 Surgisis,18 and Veritas20 in much smaller studies were 50%, 26%, and 13%, respectively.
In line with expectations based on the preoperative complexity of the patients and the presence of contamination or infection, reported postoperative infection rates were often high. Nevertheless, where reported, the need for mesh removal was often low, even in cases of postoperative infection. Diaz et al17 reported a wound dehiscence rate of 8.8% (21 of 240) with AlloDerm in the overall population, though they did not provide rates for the specific population of interest. Helton et al18 reported a rate of 35.5% (11 of 31) among patients with dirty or clean-contaminated wounds who received Surgisis. Few data were available for comparison with regard to hematoma/bleeding, chronic pain, length of hospital stay, rehospitalizations, subsequent surgeries, overall costs, time to return to normal activities, and patient satisfaction.
There was a limited number of articles that provided quantitative outcome data specific to the population that was the focus of this review. Owing to the retrospective nature of the included reports, most of which were authored by the operating surgeons, there is the potential for bias related to patient selection and outcome reporting. Moreover, patient populations included in these reports were heterogenous, marked by mostly small samples (<50 patients) and a wide variety of presenting indications for abdominal wall repair; several studies included patients with abdominal wall defects resulting from cancer and/or emergent trauma rather than incisional hernia specifically. The patient populations also varied widely across studies with respect to level of preoperative wound contamination. No prospective, head-to-head comparisons of synthetic versus biologic mesh options were identified.
Three new investigations relevant to the current review appeared in the literature after the systematic literature search was conducted.26–28 In a retrospective chart review of outcomes in 35 high-risk ventral herniorrhaphy patients, Janfaza et al found that rates of postoperative infection and hernia recurrence at 1 year were lower in patients who received a neonatal bovine mesh implant (SurgiMend, TEI Biosciences, Boston, Massachusetts) (17% and 5%, respectively) compared with patients who received a human-derived mesh (Flex HD, Ethicon Inc, Somerville, New Jersey) (50% and 33%, respectively).26 In a large prospective chart analysis of >33,000 patients from the US National Surgical Quality Improvement Program (NSQIP) who underwent ventral hernia repair, Choi et al found that patients with some level of operative field contamination were three- to fivefold more likely to experience postoperative complications than those with clean surgical fields.27 However, it is not possible to decipher whether the type of mesh applied (synthetic versus biologic) influenced such outcomes in Choi's analysis. Itani et al recently reported results of a prospective, single-arm, observational study [Repair of Infected or Contaminated Ventral Incisional Hernias (RICH)] of non-cross-linked porcine acellular dermal matrix (Strattice) for the repair of contaminated ventral hernia in 80 patients.28 Observed complication rates at 24 months of follow-up were infection, 35%; hernia recurrence, 28%; and recurrence requiring reoperation, 9%. No patient required mesh explantation. The emergence of these reports signals that the research community is beginning to respond to the need for higher-quality and more rigorous investigation of mesh products for hernia repair.
Conclusions
This systematic review identified 11 articles reporting data specific to patients undergoing repair of contaminated or infected ventral incisional hernias. Although most outcomes of interest were inconsistently reported across studies, the use of biologic mesh in this setting was marked by low reported rates of the need for secondary surgical intervention for infected mesh removal. Most of the studies were small retrospective chart reviews and more than half involved human acellular dermal matrix as the only mesh applied to the study population. Based on these findings, additional studies are needed, including large, well-controlled prospective investigations of biologic mesh products and direct comparisons of synthetic versus biologic and biologic versus biologic mesh products. Additional data from prospective, multicenter trials may help address this need. In addition, outcomes of interest should be expanded to include economic variables and patient-centered variables, such as functional status, quality of life, and patient satisfaction.
Acknowledgments
Editorial support for this article was provided by Peloton Advantage, LLC, Parsippany, New Jersey, and funded by LifeCell, Branchburg, New Jersey. The opinions expressed in this article are those of the author. The author received no honoraria/fee for service or other form of financial support related to the development of this article.
Table 4.
Extended
References
- 1.Israelsson LA, Jonsson T. Incisional hernia after midline laparotomy: a prospective study. Eur J Surg. 1996;162(2):125–129. [PubMed] [Google Scholar]
- 2.Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg. 1985;72(1):70–71. doi: 10.1002/bjs.1800720127. [DOI] [PubMed] [Google Scholar]
- 3.Voeller GR. Innovations in ventral hernia repair. Surg Technol Int. 2007;16:117–122. [PubMed] [Google Scholar]
- 4.Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Anthony T, Bergen PC, Kim LT, Henderson M, Fahey T, Rege RV, et al. Factors affecting recurrence following incisional herniorrhaphy. World J Surg. 2000;24(1):95–100. doi: 10.1007/s002689910018. [DOI] [PubMed] [Google Scholar]
- 6.Hesselink VJ, Luijendijk RW, de Wilt JH, Heide R, Jeekel J. An evaluation of risk factors in incisional hernia recurrence. Surg Gynecol Obstet. 1993;176(3):228–234. [PubMed] [Google Scholar]
- 7.Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343(6):392–398. doi: 10.1056/NEJM200008103430603. [DOI] [PubMed] [Google Scholar]
- 8.Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240(4):578–583. doi: 10.1097/01.sla.0000141193.08524.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86(3):519–526. doi: 10.1097/00006534-199009000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner JS. Guideline for prevention of surgical wound infections, 1985. Centers for Disease Control and Prevention. Available at: http://wonder.cdc.gov/wonder/prevguid/p0000420/p0000420.asp. Accessed March 18, 2013. [Google Scholar]
- 13.Sullivan D, Chung KC, Eaves FF, III, Rohrich RJ. The level of evidence pyramid: indicating levels of evidence in Plastic and Reconstructive Surgery articles. Plast Reconstr Surg. 2011;128(1):311–314. doi: 10.1097/PRS.0b013e3182195826. [DOI] [PubMed] [Google Scholar]
- 14.Bellows CF, Albo D, Berger DH, Awad SS. Abdominal wall repair using human acellular dermis. Am J Surg. 2007;194(2):192–198. doi: 10.1016/j.amjsurg.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Chavarriaga LF, Lin E, Losken A, Cook MW, Jeansonne LO, White BC, et al. Management of complex abdominal wall defects using acellular porcine dermal collagen. Am Surg. 2010;76(1):96–100. [PubMed] [Google Scholar]
- 16.Diaz JJ, Jr, Guy J, Berkes MB, Guillamondegui O, Miller RS. Acellular dermal allograft for ventral hernia repair in the compromised surgical field. Am Surg. 2006;72(12):1181–1187. [PubMed] [Google Scholar]
- 17.Diaz JJ, Jr, Conquest AM, Ferzoco SJ, Vargo D, Miller P, Wu YC, et al. Multi-institutional experience using human acellular dermal matrix for ventral hernia repair in a compromised surgical field. Arch Surg. 2009;144(3):209–215. doi: 10.1001/archsurg.2009.12. [DOI] [PubMed] [Google Scholar]
- 18.Helton WS, Fisichella PM, Berger R, Horgan S, Espat NJ, Abcarian H. Short-term outcomes with small intestinal submucosa for ventral abdominal hernia. Arch Surg. 2005;140(6):549–560. doi: 10.1001/archsurg.140.6.549. [DOI] [PubMed] [Google Scholar]
- 19.Kolker AR, Brown DJ, Redstone JS, Scarpinato VM, Wallack MK. Multilayer reconstruction of abdominal wall defects with acellular dermal allograft (AlloDerm) and component separation. Ann Plast Surg. 2005;55(1):36–41. doi: 10.1097/01.sap.0000168248.83197.d4. [DOI] [PubMed] [Google Scholar]
- 20.Limpert JN, Desai AR, Kumpf AL, Fallucco MA, Aridge DL. Repair of abdominal wall defects with bovine pericardium. Am J Surg. 2009;198(5):e60–e65. doi: 10.1016/j.amjsurg.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Lin HJ, Spoerke N, Deveney C, Martindale R. Reconstruction of complex abdominal wall hernias using acellular human dermal matrix: a single institution experience. Am J Surg. 2009;197(5):599–603. doi: 10.1016/j.amjsurg.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Parker DM, Armstrong PJ, Frizzi JD, North JH., Jr Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg. 2006;63(4):255–258. doi: 10.1016/j.cursur.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Schuster R, Singh J, Safadi BY, Wren SM. The use of acellular dermal matrix for contaminated abdominal wall defects: wound status predicts success. Am J Surg. 2006;192(5):594–597. doi: 10.1016/j.amjsurg.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Shah BC, Tiwari MM, Goede MR, Eichler MJ, Hollins RR, McBride CL, et al. Not all biologics are equal! Hernia. 2011;15(2):165–171. doi: 10.1007/s10029-010-0768-7. [DOI] [PubMed] [Google Scholar]
- 25.Campbell KT, Burns NK, Rios CN, Mathur AB, Butler CE. Human versus non-cross-linked porcine acellular dermal matrix used for ventral hernia repair: comparison of in vivo fibrovascular remodeling and mechanical repair strength. Plast Reconstr Surg. 2011;127(6):2321–2332. doi: 10.1097/PRS.0b013e318213a053. [DOI] [PubMed] [Google Scholar]
- 26.Janfaza M, Martin M, Skinner R. A preliminary comparison study of two noncrosslinked biologic meshes used in complex ventral hernia repairs. World J Surg. 2012;36(8):1760–1764. doi: 10.1007/s00268-012-1576-2. [DOI] [PubMed] [Google Scholar]
- 27.Choi JJ, Palaniappa NC, Dallas KB, Rudich TB, Colon MJ, Divino CM. Use of mesh during ventral hernia repair in clean-contaminated and contaminated cases: outcomes of 33,832 cases. Ann Surg. 2012;255(1):176–180. doi: 10.1097/SLA.0b013e31822518e6. [DOI] [PubMed] [Google Scholar]
- 28.Itani KMF, Rosen M, Vargo D, Awad SS, DeNoto G, Butler CE, et al. Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH study. Surgery. 2012;152(3):498–505. doi: 10.1016/j.surg.2012.04.008. [DOI] [PubMed] [Google Scholar]