Abstract
The aim of this study was to compare the effects of four different topical antimicrobial dressings on a multi-drug resistant Pseudomonas aeruginosa contaminated full-thickness burn wound rat model. A total of 40 adult male Wistar albino rats were used. The control group (group 1), silver sulfadiazine (1%) group 2, chlorhexidine acetate (0.5%) group 3, citric acid (3%) group 4, and silver-coated dressing group 5 were compared to assess the antibacterial effects of a daily application to a 30% full-skin thickness burn wound seeded 10 minutes earlier with 108 CFU (colony forming unit)/0.5 mL of a multi-drug resistant Pseudomonas aeruginosa strain. Five groups (1 control group and 4 treatment groups) were compared. The administration of third-degree burns to all rats was confirmed based on histopathologic data. The tissue cultures from groups 2 and 5 exhibited significant differences compared to those of the other 3 groups, whereas no significant differences were observed between groups 1, 3, and 4. The effectiveness of the treatments was as follows: 1% silver sulfadiazine > silver-coated dressing > 3% citric acid > 0.5% chlorhexidine acetate > control group. Our results supported the efficacy of topical therapy by silver sulfadiazine and silver-coated dressing on infections caused by multi-drug resistant Pseudomonas spp.
Keywords: Burn, Multi-drug resistant, Pseudomonas aeruginosa, Topical antibacterial agent, Citric acid, Silver sulfadiazine, Silver-coated dressing, Chlorhexidine acetate
Burn injury is a type of trauma that anyone may encounter at least once in their lifetime. Large and complicated burns may lead to shock, sepsis, multi-organ failure, and eventually death. Small and less severe burns may influence extremity functions and result in diminished quality of life. Infection is the greatest cause of mortality in long-term burn patients.1 Gram-negative bacteria are usually responsible for wound infections in burn patients. Of these bacteria, Pseudomonas spp. constitute the most resistant and predominant strains.1–3 Pseudomonas aeruginosa infections in hospitals mainly affect the patients in intensive care units and those having catheterization, burn, and/or chronic illnesses.4 These species spread among patients via cross contamination in a hospital environment. Treatment of Pseudomonas aeruginosa infections is challenging because the causative Pseudomonas aeruginosa strains are highly resistant to antimicrobial therapy, or they are able to gain resistance over time to all effective antibiotherapies.5 The most important mechanisms responsible for this multiple resistance are decreased permeability of outer wall to antibiotics and active transport of antibiotic molecules inside out.5–8 Due to database of National Nosocomial Infections Surveillance System, Pseudomonas aeruginosa seems to be the second most common agent leading to burn infections However most studies report Pseudomonas aeruginosa as the most common.9–10
The denaturated proteins within the burn eschar create a medium suitable for growth of microorganism. Diminished microcirculation caused by vascular injury blocks host defense mechanisms and administered antibiotics from reaching sufficient concentrations at burn site.11 Consequently, even if the correct are antibiotics administered concerning the sensitivity of isolated microorganism from cultures, clinic recovery may not happen and infection leads to mortality. Systemic antibiotic agents do not always effectively reach the burned area depending on the physiopathologic nature of a burn wound. Therefore, topical agents play an essential role in treating patients with deeper and wider burn wounds. Topical medications such as sulfamylon (mafenide acetate), silver nitrate, aquacel silver, Contreet antimicrobial foam, PolyMem Silver dressings, boric acid, acetic acid, 3% citric acid, 0.5% chlorhexidine acetate (Bactigrass), silver sulfadiazine 1% (Silverdin), and silver-coated dressing (Acticoat) are used clinically to treat Pseudomonas aeruginosa infections, which are frequently encountered in burn intensive care units and often lead to mortality.12–17 Several topical and systemic antibacterial agents are used to treat infections in burn patients, and different drug groups produce distinct clinical responses. Most studies investigating such responses have examined topical agents individually. Moreover, few studies have compared responses to different topical agents, and this is particularly true for citric acid. Here, we evaluated the efficacy of citric acid for treating severe burn wounds.15,18–19 No experimental studies have evaluated the effects of antibiotics used to treat infections caused by the multi-drug resistant Pseudomonas aeruginosa. Therefore, we evaluated the effectiveness of topical antimicrobial agents [3% citric acid, 0.5% chlorhexidine acetate (Bactigrass), 1% silver sulfadiazine (Silverdin) and silver-coated dressing (Acticoat)] on multidrug-resistant Pseudomonas aeruginosa infections established in experimental rats.
Material and Methods
Protocol
This study was conducted in the Experimental Animal Raising and Research Laboratory of Baskent University Faculty of Medicine, after approval of the local ethics committee. All experimental manipulations were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animals
A total of 40 male Wistar albino rats (12–13 weeks of age, weighing 250–300 g) were acclimated for 1 week before the experiments. The animals were kept in individual cages, housed at room temperature and given standard rat chow. Only water was provided in the 12 hours preceding the experiments.
Establishment of anesthesia and postoperative analgesia
All rats were anesthetized using a combination of 7 mg/kg intramuscular xylazine hydrochloride (Rompun; Bayer, Istanbul, Turkey) and 50 mg/kg ketamine hydrochloride (Ketalar; Eczacıbasi Warner-Lambert Pharmaceutical Industry, Levent, Istanbul, Turkey). Rats in the established burn model group were injected subcutaneously with 0.02 μg/kg fentanyl citrate (fentanyl citrate ampoule Abbott, Beckon, Istanbul, Turkey) twice daily after the procedure, thus, achieving anesthesia. All rats were killed through cervical dislocation under anesthesia.
Establishment of the burn model
Once the appropriate anesthesia was achieved, the back of each rat was shaved, followed by cleansing of the shaved area with Povidone-iodine twice. A standard dimensioned brass plate was prepared to inflict 30% identical burns. The brass plate was placed on a flame for 2 minutes and then applied to the back skin of each animal for 10 seconds to inflict a full thickness third-degree burn injury (Fig. 1). Presence of third-degree burns in the injuries created was histopathologically proved. The animals were resuscitated by administering 2 mL of lactated Ringer's solution intraperitoneally.
Fig. 1.
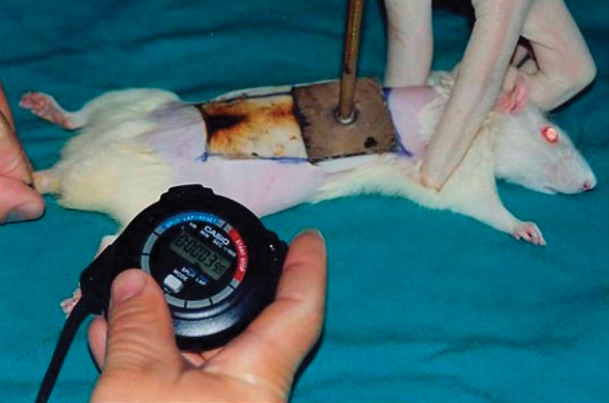
Creating a third-degree burn with a brass plate.
Establishment of the infected burn model
Multi-drug resistant Pseudomonas aeruginosa strains (1 × 108) isolated from 10 burn patients who were followed at the Baskent University Faculty of Medicine Burn Unit between 2008 and 2009 were used. Only one clinically significant Pseudomonas aeruginosa isolate from each patient was included in the study. The frozen bacterial solutions were subcultured on blood and EMB (eosin methylene blue) agar plates. After then the colonies were subcultured onto appropriate agar mediums for identification. The plates were incubated at 37°C in ambient air for 16–18 hours. The bacteria were identified to the genus level by conventional methods using an automated system. The fluid obtained through this method, which contained a colony of 1 × 108 multi-drug resistant Pseudomonas aeruginosa in every 0.5 mL, was administered to the burn site on the back of each rat (Fig. 2). Treatment started at 24 hours after burn injury. The antimicrobial susceptibilities were studied according to the Clinical and Laboratory Standards Institute criteria.20 For this study the pseudomonas strains resistant to three of the following antibiotic classes were considered as “multidrug resistant” in accordance with the published articles: carbapenems, piperacillin-tazobactam, third- or fourth-generation cephalosporins, aminoglycosides, and quinolones.
Fig. 2.
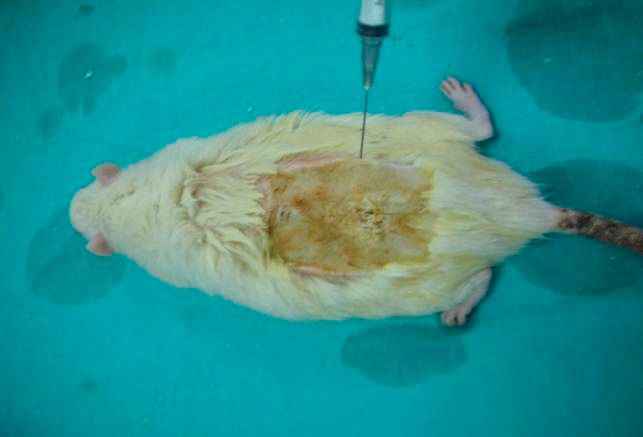
Showing Pseudomonas colonies on the burned surface.
Experimental groups
Once the infection model was designated, all rats were randomized into 5 groups by a surgeon not included in the study. Each group was composed of 8 rats. The groups were titled on the basis of the antibacterial agent to be applied to the burn site. Group 1 (n = 8), the control group, did not undergo application of any systemic or topical agent, rats in group 2 (n = 8), group 3 (n = 8), group 4 (n = 8), and group 5 (n = 8) were administered 1% silver sulfadiazine (Silverdin, Deva, Turkey), 0.5% chlorhexidine acetate (Bactigrass, Smith & Nephew, Warwickshire, UK), 3% citric acid, and silver-coated dressing (Acticoat, Smith & Nephew), respectively. Systemic antibacterial agent was not given to the any treatment groups. Ten minutes after the burn, each animal was seeded with 0.5 mL of broth containing 1 × 108 colony forming units of Pseudomonas aeruginosa (ATCC 27853; American Type Culture Collection, Rockville, Maryland) by swabbing. Growth of bacteria >104 cfu (colony forming unit) were considered significant. The burn sites of the rats in group I were secured with gauze, and 1% silver sulfadiazine was applied to the burn sites of the rats in group 2 via a sterile knife, after which the burn site was closed with sterile gauze. The burn sites of the rats in group 3 were secured with sterile gauze, preceded by the administration of Bactigrass. Citric acid was poured through a sterile injector over the burn sites of the rats in group IV, followed by closure of the burn site with sterile gauze. Acticoat was placed over the burn sites of the rats in group 5, followed by closure of the burn site with sterile gauze. Acticoat was soaked with distilled water three times per day. All dressings were fixed using a skin stapler. Dressings in all groups were changed once at the same hour every morning under anesthesia in all groups. The agents used in treatment were administered as appropriate for burn surface area. Appropriate anesthesia was achieved using the aforementioned anesthetic agents. On day 7 of the post-procedure follow-up, blood cultures were taken using samples from the left ventricle, and tissue cultures were taken from the burn site (quantitative), paravertebral muscles (quantitative), and lungs (quantitative) to evaluate the efficacy of the administered topical antimicrobial agents and bacterial translocation. Cultures were assessed in the microbiology laboratory and were calculated as 10× colonies per gram of tissue. Growth of bacteria >104 cfu (colony forming unit) were considered significant. Biopsies were obtained in an attempt to evaluate the histopathologic degree of the burns. All rats were sacrificed at the end of the procedure.
Statistical analysis
The statistical analysis was performed using the SPSS 11.0 software (SPSS, Inc., Chicago, Illinois, USA). Intergroup data were assessed with a 1-way analysis of variance (ANOVA), and post-hoc multiple comparisons tests were used to compare the five groups. A P-value < 0.05 was considered significant.
Results
No mortality occurred in any of the groups. Of the 40 rats, 31 lost weight. Five of the rats that maintained their weights were from group 2, three were from group 5, and one was from group 4.
1—Histopathologic evaluations
Hematoxylin and eosin staining was used to confirm the administration of third-degree burns on tissue specimens taken from the burned areas (Fig. 3).
Fig. 3.
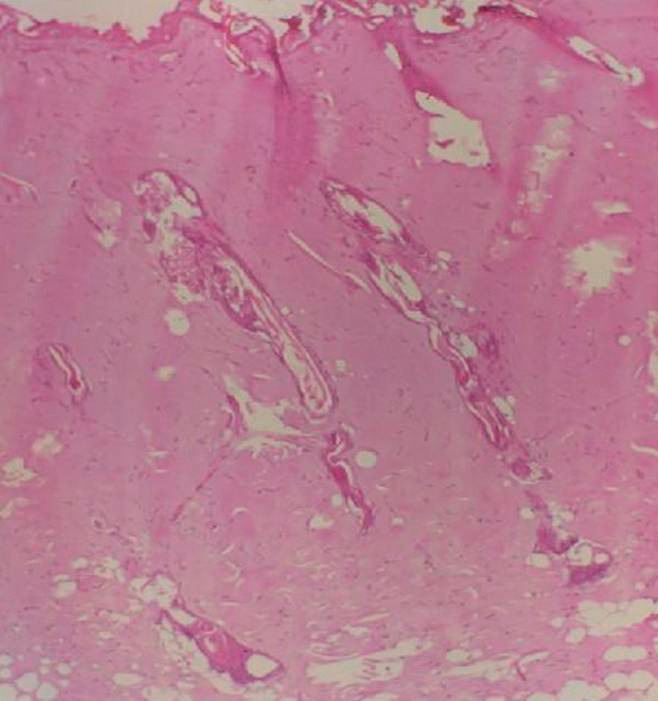
Hematoxylin and eosin (H&E) staining was used for histopathologic confirmation of presence of third-degree burns (H&E ×100).
2—Quantitative tissue cultures
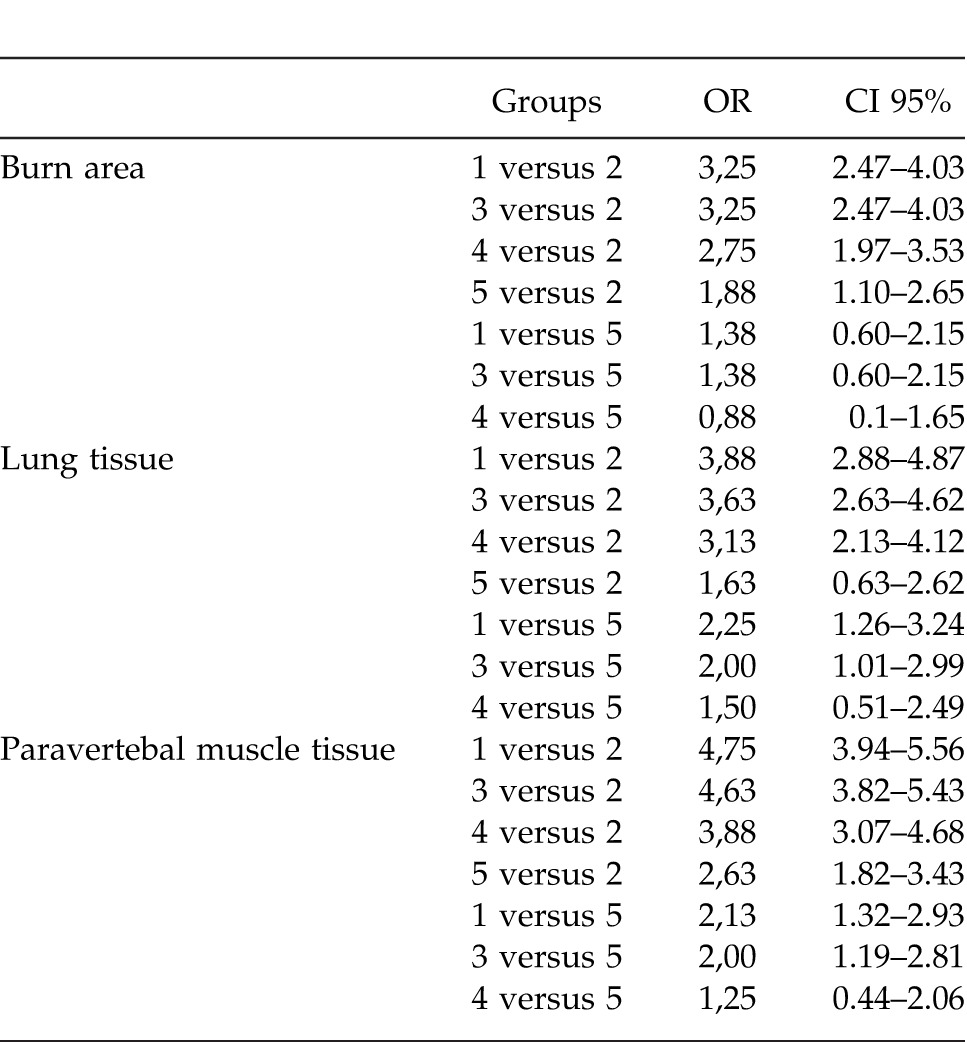
Table 1 summarizes the results of the quantitative tissue cultures from the paravertebral muscles, the lungs, and the burn injury sites. No significant difference was detected in cultures from the burn sites, the lung tissues, or the paravertebral muscles among groups 1, 3, and 4, whereas significant results were obtained from groups 2 and 5 (P < 0.05). One ANOVA test demonstrated a difference among the groups, a post-hoc test was utilized to identify from which group the difference stemmed. A post-hoc test revealed that the most efficacious agents were as follows: 1% silver sulfadiazine > silver-coated dressing > 3% citric acid > 0.5% chlorhexidine acetate > the control group. Table 2 summarizes some of the results obtained from the post-hoc analysis.
Table 1.
Comparison of the quantitative tissue cultures of the burned area, the lung tissue, and the paravertebral muscle tissue. Measures are expressed as 10x colonies/gram
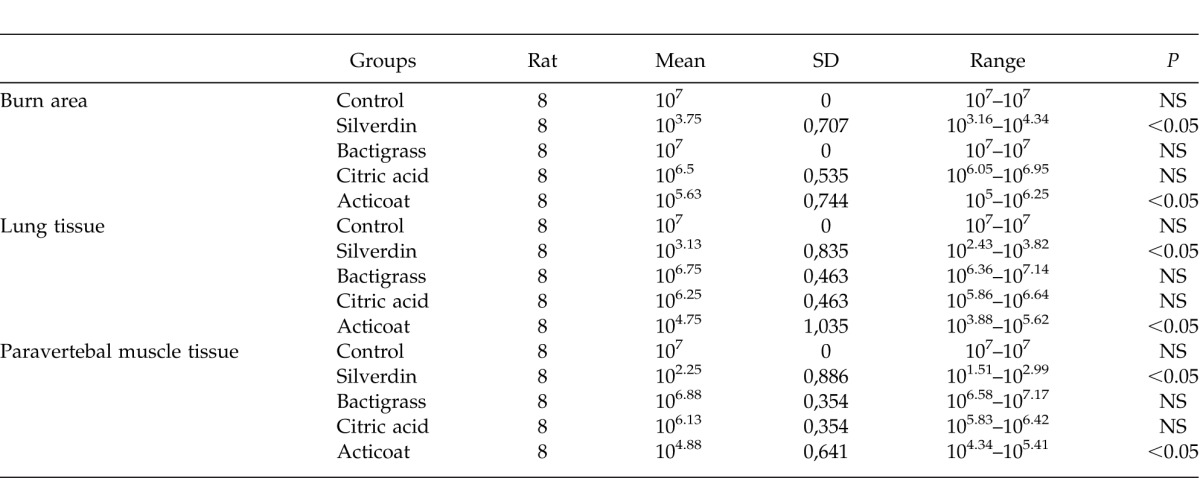
Table 2.
Odds ratios and 95% confidence intervals obtained from the post-hoc analysis. Only the results from the comparison of groups 2 and 5 between themselves and with the other groups are presented
3—Blood cultures
Bacterial growth was evident on 38 of 40 blood cultures obtained from the rats. No significant differences in bacterial growth were detected among the groups.
Discussion
Today burn induced mortality and morbidity are still an important health issue. Consequently, significant improvements were made regarding the continuum starting with first aid and projecting toward the rehabilitation of burn patients. Tangential debridement and grafting is a universally accepted approach.21 Most significant medical improvements target control of local and systemic infection in burn patients. Protein denaturation, breaking of skin integrity, generalized immunosuppression, and diminished local microcirculation are factors leading to local and systemic infections in burn patients and causes of high mortality and morbidity rates observed in this group of patients.9
Pseudomonas aeruginosa, Staphylococus aureus, and Escherichia coli currently occupy the top ranks among factors responsible for the etiology of burn wound infections.2,22,23 Pseudomonas aeruginosa is noted as the most common bacterial agent in burn patients in developing countries includes Turkey.24,25 Pseudomonas strains are gram-negative bacilli, and have been recognized as the most detrimental bacterial agent in burn wounds since the 1960s. However, their eradication is challenging due to their resistance to many antibiotics.5,26 The development of resistance to broad-spectrum antibiotics is inevitable when treating infections accompanying trauma such as burns. Therefore, the development of topical agents for treating Pseudomonas aeruginosa infections, which is frequently problematic in burn intensive care units, is of increasing interest. Topical medications such as boric acid, acetic acid, 3% citric acid, 0.5% chlorhexidine acetate (Bactigrass), silver-coated dressing (Acticoat), and 1% silver sulfadiazine (Silverdin) have been used to treat burn wounds infected with Pseudomonas aeruginosa.12–16,18,19,25,27 However, no study has compared the effectiveness of topical antimicrobial agents against multidrug-resistant Pseudomonas aeruginosa infections. Similarly, no experimental study has investigated the effectiveness of citric acid against Pseudomonas aeruginosa infections in burn patients. Accordingly, we compared the effectiveness of topical antimicrobial agents used to treat multidrug-resistant Pseudomonas aeruginosa infections. We found that the topical antimicrobial agents administered to groups 2 (silver sulfadiazine) and 5 (silver-coated dressing) were significantly more efficacious compared to the other groups.
Silver sulfadiazine (1%, Silverdin) is one of the topical antibacterial agents used most commonly for the prevention of burn wound infection. The silver ion binds with the DNA of an organism and, consequently, releases the sulfonamide, which interferes with the intermediary metabolic pathway of the microbe. It is most effective against Pseudomonas aeruginosa, the enterics, and equally as effective as any antifungal drug against Candida albicans. Recently, Pseudomonas aeruginosa resistance to silver sulfadiazine has been reported. The results of this experimental study showed that silver sulfadiazine was a more potent antipseudomonal agent than other topical agents. It prevented the colonization of the Pseudomonas aeruginosa in all of the tissues, including eschar, which the other agents could not achieve.14
Silver-coated dressing (Acticoat) is used widely for wound management, particularly in burns. It consists of 2 sheets of high-density polyethylene mesh coated with nanocrystalline silver. When moistened with water, the nanocrystalline silver continues to release silver ions onto the wound surface.13,14 These dressings have potent antibacterial activity against most of gram-negative and gram-positive bacteria, yeast, filamentous fungi, and viruses.13,14
Citric acid has antiseptic property as indicated by microbiologic studies and by rapid clearing up of infected surfaces. The use of citric acid as a topical agent for effective elimination of Pseudomonas aeruginosa from the burn wound has been reported. Citric acid was also found effective against other bacteria isolated from various types of wound infections.18 Conversely, we found that citric acid was not effective at treating infections caused by resistant Pseudomonas aeruginosa strains. Although our results differ from those previously reported, that the colonies in our study were composed of multidrug-resistant bacteria should be considered.
Despite a reduction in bacterial colonization, the results failed to document complete eradication of bacteria from specimens taken from burn sites, lungs, paravertebral muscles, and the left ventricle. Pruitt and Foley28 performed a tissue biopsy culture of patients with burn wound infections and reported a 75% mortality rate in those with cultures exhibiting 1 × 105 colonies per gram of tissue. Another review reported that burn wound infection is unlikely in patients exhibiting a positive tissue culture if <1 × 105 microorganisms per gram of tissue, whereas those showing 1 × 105–108 microorganisms were not always interpreted as burn wound infections, and tissue biopsy cultures growing >1 × 108 microorganisms per gram of tissue showed a high proportion of burn wound sepsis.9 Therefore, we used 1 × 108 colonies of Pseudomonas aeruginosa to initiate burn wound sepsis. This number of colonies has been used in previous studies.14 Our results clearly demonstrated that fewer than 1 × 108 colonies of Pseudomonas aeruginosa were present following treatment in Groups 2 and 5. Despite incomplete eradication of the bacteria in these groups, the bacterial colonization remaining in these tissues was likely insufficient to trigger sepsis. Therefore, inhibiting a likely clinical sepsis scenario and decreasing the mortality and morbidity rates are achievable in patients with large burned areas.
Systemic antibiotherapy used for prophylactic or empiric reasons in burn patients both challenges the more traditional treatments of Pseudomonas aeruginosa infections that commonly manifest in the following weeks and are characterized by multidrug resistance, as well as highlight the significance of topical agent administration during this period. We suggest that administering topical agents other than 1% silver sulfadiazine, which is the first-choice agent for many burn intensive care units, could increase the number of resistant Pseudomonas aeruginosa colonies, resulting in increased morbidity and mortality. We also feel that early administration of silver-containing antimicrobial agents has proven efficacy in the treatment of burns and is one of the most integral components of therapy together with early debridement and resuscitation treatments.
Results of our study demonstrated that citric acid therapy, which was not evaluated with an experimental study, has no significant effect on antibiotic resistant Pseudomonas aeruginosa infections. In our opinion, regarding the favorable outcomes reported as case reports in literature, factors other than citric acid might have played a role. We suggest this because citric acid was experimentally shown to be ineffective on Pseudomonas aeruginosa infections having effective colony counts.
An additional consideration is that the 1% silver sulfadiazine treatment is substantially less expensive than the cost of developing sepsis. Preventing infections of burn wounds is of utmost importance; however, antibiotics used in a frivolous manner lead to the emergence of infections, increased drug resistance, and the failure of most topical and systemic medications to eradicate these bacteria, thus increasing morbidity and mortality.
Acknowledgments
This study was supported by the Research Fund of Baskent University (DA08/27). The authors declare that there is no conflict of interest.
References
- 1.Ranjbar R, Owlia P, Saderi H, Mansouri S, Jonaidi-Jafari N, Izadi M, et al. Characterization of Pseudomonas aeruginosa strains isolated from burned patients hospitalized in a major burn center in Tehran, Iran. Acta Med Iran. 2011;49(10):675–679. [PubMed] [Google Scholar]
- 2.Azzopardi EA, Azzopardi SM, Boyce DE, Dickson WA. Emerging gram-negative infections in burn wounds. J Burn Care Res. 2011;32(5):570–576. doi: 10.1097/BCR.0b013e31822ac7e6. [DOI] [PubMed] [Google Scholar]
- 3.Bayram Y, Parlak M, Aypak C, Bayram I. Three-year review of bacteriological profile and antibiogram of burn wound isolates in Van, Turkey. Int J Med Sci. 2013;10(1):19–23. doi: 10.7150/ijms.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yetkin G, Otlu B, Cicek A, Kuzucu C, Durmaz R. Clinical, microbiologic, and epidemiologic characteristics of Pseudomonas aeruginosa infections in a University hospital, Malatya, Turkey. Am J Infect Control. 2006;34(4):188–192. doi: 10.1016/j.ajic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Livermore DM. Multiple mechanisms of antimicrobial resistance in pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34(5):634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 6.Fluit AC, Verhoef J, Schmitz FJ. Antimicrobial resistance in European isolates of Pseudomonas aeruginosa. European SENTRY Participants. Eur J Clin Microbiol Infect Dis. 2000;19(5):370–374. doi: 10.1007/s100960050497. [DOI] [PubMed] [Google Scholar]
- 7.Pollack M. Pseudomonas aeruginosa. In: Mandell GL, JE Bennett, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone; 2000. pp. 2310–2335. In. eds. [Google Scholar]
- 8.Bertrand X, Thouverez M, Talon D, Boillot A, Capellier G, Floriot C, et al. Endemicity, molecular diversity and colonisation routes of pseudomonas aeruginosa in intensive care units. Intensive Care Med. 2001;27(8):1263–1268. doi: 10.1007/s001340100979. [DOI] [PubMed] [Google Scholar]
- 9.Mayhall CG. Nosocomial burn wounds. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 275–286. In. ed. [Google Scholar]
- 10.Song W, Lee KM, Kang HJ, Shin DH, Kim DK. Microbiologic aspects of predominant bacteria isolated from the burn patients in Korea. Burns. 2001;27(2):136–139. doi: 10.1016/s0305-4179(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 11.Order Se, Mason AD, Switzer WF, Moncrief JA. Arterial vascular occlusion and devitalization of burn wounds. Ann Surg. 1965;161(4):502–508. doi: 10.1097/00000658-196504000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryssel H, Germann G, Riedel K, Reichenberger M, Hellmich S, Kloeters O. Suprathel-acetic acid matrix versus acticoat and aquacel as an antiseptic dressing: an in vitro study. Ann Plast Surg. 2010;65(4):391–395. doi: 10.1097/SAP.0b013e3181d6e2f2. [DOI] [PubMed] [Google Scholar]
- 13.Acar A, Uygur F, Diktaş H, Evinç R, Ulkür E, Oncül O, et al. Comparison of silver-coated dressing (Acticoat®), chlorhexidine acetate 0.5% (Bactigrass®) and nystatin for topical antifungal effect in Candida albicans-contaminated, full-skin-thickness rat burn wounds. Burns. 2011;37(5):882–885. doi: 10.1016/j.burns.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Ulkür E, Oncül O, Karagöz H, Celiköz B, Cavuşlu S. Comparison of silver-coated dressing (ActionatTM), chlorhexidine acetate 0.5% (Bactigrass®), silver sulfodiazine %1 (Silverdin®) for topical antibacterial effect in pseudomonas-aeruginosa-contaminated, full-skin thickness burn wounds in rats. J Burn Care Rehabil. 2005;26(5):430–433. doi: 10.1097/01.bcr.0000176879.27535.09. [DOI] [PubMed] [Google Scholar]
- 15.Nagoba BS, Gandhi RC, Wadher BJ, Deshmukh SR, Gandhi SP. Citric acid treatment of severe electric burns complicated by multiple antibiotic resistant Pseudomonas aeruginosa. Burns. 1998;24(5):481–483. doi: 10.1016/s0305-4179(98)00052-7. [DOI] [PubMed] [Google Scholar]
- 16.Ryssel H, Kloeters O, Germann G, Schäfer T, Wiedemann G, Oehlbauer M. The antimicrobial effect of acetic acid–an alternative to common local antiseptics? Burns. 2009;35(5):695–700. doi: 10.1016/j.burns.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Kujath P, Hugelcshaffer C. Pseudomonas aeruginosa: pathogenicity, prevention and therapeutic approaches. Zentralbl Chir. 1987;112(9):558–563. [PubMed] [Google Scholar]
- 18.Nagoba BS, Gandhi RC, Hartalkar AR, Wadher BJ, Selkar SP. Simple, effective and affordable approach for the treatment of burns infections. Burns. 2010;36(8):1242–1247. doi: 10.1016/j.burns.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Nagoba BS, Deshmukh SR, Wadher BJ, Mahabaleshwar L, Gandhi RC, Kulkarni PB, et al. Treatment of superficial pseudomonal infections with citric acid: an effective and economical approach. J Hosp Infect. 1998;40(2):155–157. doi: 10.1016/s0195-6701(98)90095-0. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing; 15th Informational Supplement. Wayne, PA: 2008. CLSI/NCCLS document M100-S17. [Google Scholar]
- 21.Burke JF. Burn treatment's evolution in the 20th century. J Am Coll Surg. 2005;200(2):151–153. doi: 10.1016/j.jamcollsurg.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Agnihotri N, Gupta V, Joshi RM. Aerobic bacterial isolates from burn wound infections and their antibiograms: a five-year study. Burns. 2004;30(3):241–243. doi: 10.1016/j.burns.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song W, Lee KM, Kang HJ, Shin DH, Kim DK. Microbiologic aspects of predominant bacteria isolated from the burn patients in Korea. Burns. 2001;27(2):136–139. doi: 10.1016/s0305-4179(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 25.Estahbanati HK, Kashani PP, Ghanaatpisheh F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns. 2002;28(4):340–348. doi: 10.1016/s0305-4179(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 26.Artz CP, Moncrief JA, Pruitt BA., Jr. Burns . Topics in Acute Care and Trauma. Philadelphia, PA: WB Saunders; 1979. A Team Approach; pp. 45–94. In: Campbell MK, Covey MH, eds. [Google Scholar]
- 27.Fraser JF, Bodman J, Sturgess R, Faoagali J, Kimble RM. An in vitro study of the anti-bacterial efficacy of a 1% silver sulphadiazine and 0.2 chlorhexidine Digluconate cream, 1% silver sulphadiazine cream and a silver coated dressing. Burns. 2004;30(1):35–41. doi: 10.1016/j.burns.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Pruitt BA, Jr, Foley FD. The use of biopsies in burn patient care. Surgery. 1973;73(6):887–897. [PubMed] [Google Scholar]


