Abstract
As there have been many multidrug regimens introduced in colorectal cancer treatment, hypersensitivity is more often encountered than in the past. Though most allergic adverse events of oxaliplatin are mainly classified as type I reaction, a limited number of case reports of type IV reaction (delayed-type hypersensitivity) have been reported. A 73-year-old man was hospitalized for receiving the third cycle of FOLFOX4 plus bevacizumab. Forty-two hours after administration, he had dyspnea and hemoptysis. Acute respiratory distress syndrome was suspected, and the patient underwent mechanical ventilation and steroid pulse therapy. Delayed-type hypersensitivity is induced by induction of inflammation via IL-1, TNF-α and IL-6. The serum level of IL-6 in patients with advanced colorectal cancers is usually greater than the normal range. Therefore, delayed-type hypersensitivity may be easily induced in those patients. We should pay special attention to delayed-type hypersensitivity in advanced colorectal cancer patients undergoing FOLFOX treatment.
Keywords: Colorectal cancer, Chemotherapy, oxaliplatin, Delayed-type hypersensitivity
There have been many multidrug regimens in colorectal cancer treatment, such as FOLFOX, XELOX, FOLFIRI and 5FU/LV. Moreover, some molecular targeted agents are advocated to use concomitantly to improve patients' outcomes.
Oxaliplatin is a third-generation platinum compound; one of its adverse events is allergic reaction. Most allergic reactions of oxaliplatin are classified as type I, but type IV reaction (delayed-type hypersensitivity reaction) may also occur.1,2
Bevacizumab, a recombinant humanized monoclonal antibody, has been approved for metastatic or recurrent colorectal cancer in combination with multidrug regimens. Its adverse events, which include intestinal perforation, hypertension, bleeding from the nose and the tumor, thrombosis of artery and vein, and poor wound healing, are different from those of other anticancer drugs.
Although adverse events of late have been more complicated than before, there have been limited reports focusing on interactions of chemotherapeutic agents.
We experienced severe adverse events in FOLFOX + bevacizumab treatment.
Case Report
A 73-year-old man was admitted to our hospital because of bowel obstruction after colonoscopy in March 2007. An abdominal computed tomography (CT) revealed that the whole intestine was considerably dilated because of the sigmoid colon tumor. Multiple metastatic nodules were noted in the bilateral lobes of the liver. He underwent transverse colostomy for intestinal obstruction. Then he electively underwent sigmoidectomy in the following month. Pathology showed tubular adenocarcinoma of the sigmoid colon, and TNM staging was T3N2M1, stage IV.
He received chemotherapy using tegafur/uracil and folinic acid followed by CPT-11 for liver metastases. Although serum carbohydrate antigen 19-9 (CA19-9) levels decreased initially, CT revealed progression of liver metastases in June 2008. Chemotherapy of FOLFOX4 (bolus 5-FU, 400 mg/m2 and oxaliplatin: 85 mg/m2 and 22 h infusion of 5-FU, 600 mg/m2), plus bevacizumab (5 mg/kg, Chugai Pharma, Tokyo, Japan) was initiated as the third line treatment in August 2008 (Fig.1). He had grade 4 neutropenia and leukopenia until 2 cycles. Then, the patient received the third cycle of FOLFOX4 (bolus 5-FU, 400 mg/m2 and oxaliplatin: 68 mg/m2 and 22 h infusion of 5-FU, 600 mg/m2), plus bevacizumab in September 2008.
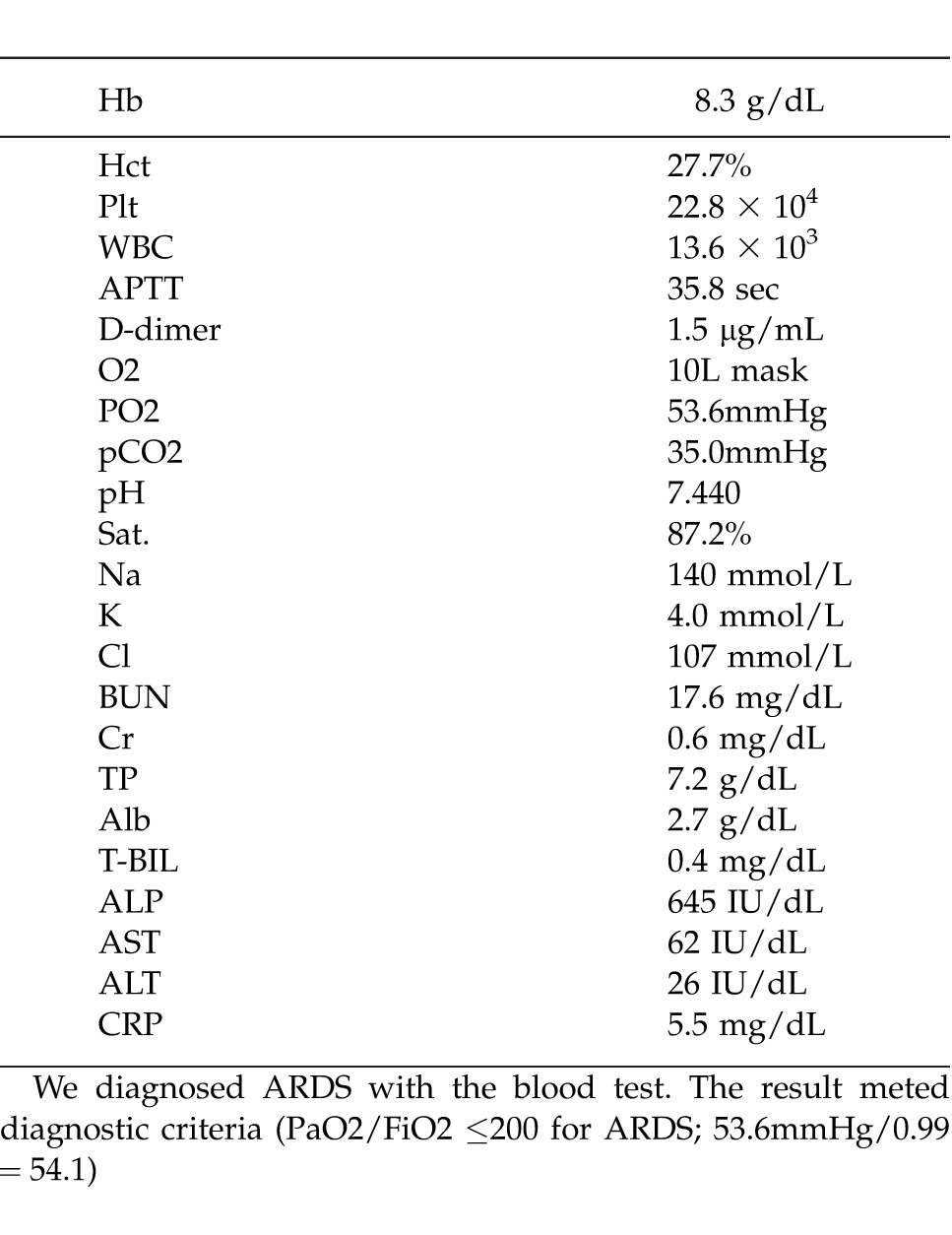
He had grade 1 nausea on day 2, and developed dyspnea with wheezing at 4 am on day 3, after 42 hours of administration. He had hemoptysis at 9 am on the same day. Chest X-ray and CT revealed severe infiltrated shadows at the bilateral hilum of the lung, but neither pulmonary arterial thrombosis nor metastatic lesions were observed. The presence of acute hypoxia, bilateral infiltrative shadow, central venous pressure of 10 mmHg, and PaO2/FiO2 (53.6mmHg/0.99 = 54.1) ≤200 indicated acute respiratory distress syndrome (ARDS).
The patient underwent mechanical ventilation with tracheal intubation. Steroid pulse therapy was initiated with a dose of 500 mg/d of methylprednisolone sodium succinate (Pfizer, Tokyo, Japan) for the initial 3 days, and then a dose of 100 mg/d of prednisolone sodium succinate (Shionogi Pharma., Osaka, Japan) for 1 day. Although his respiratory condition rapidly improved within a few days, infiltrative shadow had remained during the 9 days from onset. Another steroid pulse therapy with a dose of 500 mg/d of methylprednisolone for 3 days followed by a dose of 50 mg/d of prednisolone for 3 days was performed. Sivelestat sodium hydrate (Ono Pharma, Osaka, Japan) with a dose of 300 mg/d was used for 14 days, and antibiotics, cefepime dihydrochloride hydrate (Bristol-Myers Squibb, Tokyo, Japan), 2 g/d for the initial 3 days, and biapenem (Meiji Seika, Tokyo, Japan), 0.9 g/d from the 10th day to the 13th day, were also given. His respiratory condition recovered on the 15th day (Fig. 2).
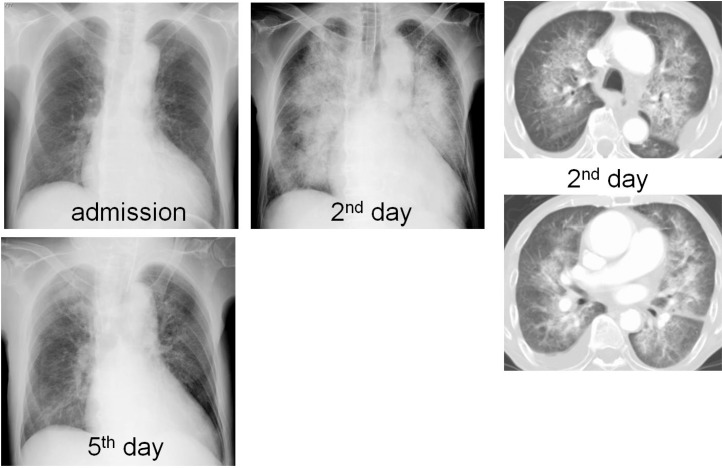
Fig. 2.
Chest X-ray and CT. Each X-ray showed at admission and after administration of bevacizumab. Chest X-ray and CT showed severe infiltrated shadows at bilateral pulmonary gates at second day and its change.
Chemotherapy was discontinued thereafter, and the patient died of cancer progression in November 2008. Autopsy was not performed.
Discussion
Introduction of FOLFOX and FOLFIRI regimens has dramatically prolonged survival in advanced colorectal cancer patients. Recently, various molecular targeted agents have been introduced into advanced cancer treatment to further prolong survival. Bevacizumab, a recombinant humanized monoclonal antibody against vascular endothelial growth factor, has been shown to have a synergistic efficacy when combined with other chemotherapy in various malignancies including colorectal cancer.3–5
The most common adverse events in FOLFOX and FOLFIRI are bone marrow suppression, gastrointestinal toxicities such as nausea, vomiting and diarrhea, hepatic and renal dysfunction, and neuropathy.6 As for respiratory adverse events, there were some case reports of interstitial pneumonia after FOLFOX treatment.7–9 However, the incidence of interstitial pneumonia and hemoptysis in FOLFOX was 0.18% and 0.04% (the pharmaceutical report of Yakult Honsha; http://www.yakult.co.jp/english/index.html), and 0.37% and 0.19% when combined with bevacizumab (the pharmaceutical report of Chugai Pharma; http://www.chugai-pharm.co.jp/hc/ss/english/index.html). Moreover, though ARDS induced by FOLFOX and FOLFIRI has rarely been documented, the incidence of ARDS when these regimens were combined with bevacizumab was only 0.19% (7/3610; http://www.chugai-pharm.co.jp/hc/ss/english/index.html).
Oxaliplatin is a third-generation platinum compound, and its antitumor effect originates from inhibition of DNA synthesis and induction of apoptosis by DNA-Pt compound.10 The primary adverse events are renal, auditory, and peripheral nerve disorder, and allergic reaction. The allergic reaction occurs suddenly and is sometimes lethal.
Most allergic reactions of oxaliplatin occur within a few minutes or hours after administration, and are classified as type I reaction.1,2 Moreover, there were some case reports classified as type II reaction (hemolysis and thrombocytopenia). However, type IV reaction (delayed-type reaction)1 2 is rare, and its mechanism is unclear.
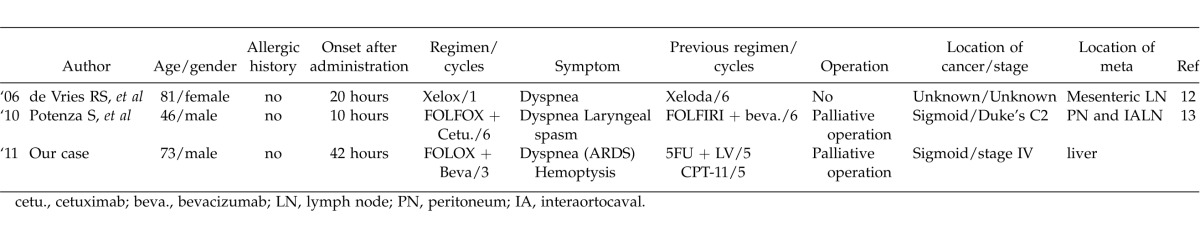
Already, there have been two case reports about respiratory delayed-type hypersensitivity due to oxaliplatin with the detailed information about the clinical course. As with our case, de Vries et al reported that severe dyspnea occurred after 20 hours of oxaliplatin administration as a delayed allergic reaction,11 and Potenza et al reported that severe dyspnea and laryngeal spasm occurred after 10 hours of administration.12 To our knowledge, our patient was the third case with respiratory complications caused by delayed-type hypersensitivity (Table 2).
Table 2.
The list of delayed hypersensitivity reactions in use of oxaliplatin
Delayed-type hypersensitivity is induced by induction of inflammation via IL-1, TNF-α and IL-6.13 In patients with advanced colorectal cancers, the serum level of IL-6 is usually greater than the normal range.14,15 Therefore, it can be speculated that delayed-type hypersensitivity may be easily induced in patients with advanced cancer.
The US Food and Drug Administration (FDA) has constructed the Adverse Event Report System (AERS). The FDA's Multi-Item Gamma Poisson Shrinker (MGPS) is an algorithm to detect possible synergistic interactions between drugs and adverse events from the AERS database, and Excess2 is a statistical index of its algorithm showing the grade of interactions among three or more items. High value of Excess2 (>1) suggests a strong interaction.16 Sakaeda et al reported that Excess2 in severe hypersensitivity reactions occurring after co-administration of bevacizumab and oxaliplatin-based chemotherapy was 5.38, suggesting that bevacizumab possibly affected severe oxaliplatin-induced hypersensitivity reactions.17 These findings were not confirmed in the present case because the serum level of IL-6 was not examined.
In conclusion, in chemotherapy using FOLFOX plus bevacizumab for far-advanced colorectal cancer, it is important to pay special attention to respiratory adverse events, even in patients without lung disease or lung metastases.
Acknowledgments
The authors received no financial support for this article.
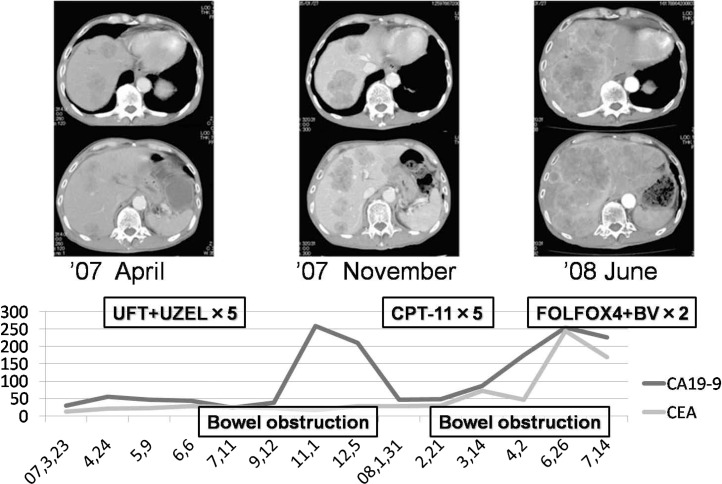
Fig. 1.
Clinical course after operation. Though we changed chemotherapy, UFT+UZEL and CPT-11, each CT showed metastatic liver tumor gradually progressed. After operation, we started UFT/USEL 5 times, CPT-11 twice and FOLFOX4 + bevacizumab twice. During each chemotherapy, bowel obstruction occurred and after that tumor markers increased.
Table 1.
The blood test at the onset of ARDS
References
- 1.Syrigou E, Syrigos K, Saif MW. Hypersensitivity reactions to oxaliplatin and other antineoplastic agents. Curr Allergy Asthma Rep. 2008;8(1):56–62. doi: 10.1007/s11882-008-0011-0. [DOI] [PubMed] [Google Scholar]
- 2.Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol. 2009;102(3):179–87. doi: 10.1016/S1081-1206(10)60078-6. [DOI] [PubMed] [Google Scholar]
- 3.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 6.Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol. 2005;23:4553–4560. doi: 10.1200/JCO.2005.17.749. [DOI] [PubMed] [Google Scholar]
- 7.Muneoka K, Shirai Y, Sasaki M, Wakai T, Sakata J, Hatakeyama K. Interstitial pneumonia arising in a patient treated with oxaliplatin, 5-fluorouracil, and, leucovorin (FOLFOX) Int J Clin Oncol. 2009;14:457–459. doi: 10.1007/s10147-008-0863-2. [DOI] [PubMed] [Google Scholar]
- 8.Trisolini R. Lazzari Agli L, Tassinari D, Rondelli D, Cancellieri A, Patelli M, et al. Acute lung injury associated with 5-fluorouracil and oxaliplatinum combined chemotherapy. Eur Respir J. 2001;18:243–245. [PubMed] [Google Scholar]
- 9.Jung KH, Kil SY, Choi IK, Seo JH, Shin C, Kim YS, et al. Interstitial lung diseases in patients treated with oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX) Int J Tuberc Lung Dis. 2006;10:1181–1183. [PubMed] [Google Scholar]
- 10.Koopman M, Venderbosch S, Nagtegaal ID, van Krieken JH, Punt CJ. A review on the use of molecular markers of cytotoxic therapy for colorectal cancer, what have we learned? Eur J Cancer. 2009;45:1935–1949. doi: 10.1016/j.ejca.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 11.de Vries RS, Mattijssen EJ, van Sorge AA. Serious delayed hypersensitivity reaction to oxaliplatin. Ann Oncol. 2006;17(11):1723–1724. doi: 10.1093/annonc/mdl116. [DOI] [PubMed] [Google Scholar]
- 12.Potenza S, Nasti G, Ottaiano A, Filippelli A, Rossi F, Capuano A. Severe respiratory symptoms to oxaliplatin infusion: a case report of delayed hypersensitivity reaction. Invest New Drugs. 2010;28(2):185–186. doi: 10.1007/s10637-009-9250-8. [DOI] [PubMed] [Google Scholar]
- 13.Bonefeld CM, Larsen JM, Dabelsteen S, Geisler C, White IR, Menné T, et al. Consumer available permanent hair dye products cause major allergic immune activation in an animal model. Br J Dermatol. 2010;162(1):102–107. doi: 10.1111/j.1365-2133.2009.09417.x. [DOI] [PubMed] [Google Scholar]
- 14.Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients–a summary of published results. Int J Colorectal Dis. 2010;25(2):135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 15.Nikiteas NI, Tzanakis N, Gazouli M, Rallis G, Daniilidis K, Theodoropoulos G, et al. Serum IL-6, TNFalpha and CRP levels in Greek colorectal cancer patients: prognostic implications. World J Gastroenterol. 2005;11(11):1639–1643. doi: 10.3748/wjg.v11.i11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szarfman A, Machado SG, O'Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA's spontaneous reports database. Drug Safety. 2002;25(6):381–392. doi: 10.2165/00002018-200225060-00001. [DOI] [PubMed] [Google Scholar]
- 17.Sakaeda T, Kadoyama K, Yabuuchi H, Niijima S, Seki K, Shiraishi Y, et al. Platinum agent-induced hypersensitivity reactions: data mining of the public version of the FDA Adverse Event Reporting System, AERS. Int J Med Sci. 2011;8(4):332–338. doi: 10.7150/ijms.8.332. [DOI] [PMC free article] [PubMed] [Google Scholar]