Background: State transition balances the excitation pressure between the two photosystems of plants.
Results: The organization of photosystem II supercomplexes and megacomplexes is the same in state 1 and state 2.
Conclusion: Phosphorylation is not sufficient to induce the disassembly of the supercomplexes.
Significance: This work helps to understand how plants optimize light harvesting under ever changing light conditions.
Keywords: Photosynthesis, Photosystem II, Plant, Plant Biochemistry, Protein Phosphorylation, State Transitions, Supramolecular Organization
Abstract
Plants are exposed to continuous changes in light quality and quantity that challenge the performance of the photosynthetic apparatus and have evolved a series of mechanisms to face this challenge. In this work, we have studied state transitions, the process that redistributes the excitation pressure between photosystems I and II (PSI/PSII) by the reversible association of LHCII, the major antenna complex of higher plants, with either one of them upon phosphorylation/dephosphorylation. By combining biochemical analysis and electron microscopy, we have studied the effect of state transitions on the composition and organization of photosystem II in Arabidopsis thaliana. Two LHCII trimers (called trimers M and S) are part of the PSII supercomplex, whereas up to two more are loosely associated with PSII in state 1 in higher plants (called “extra” trimers). Here, we show that the LHCII from the extra pool migrates to PSI in state 2, thus leaving the PSII supercomplex and the semicrystalline PSII arrays intact. In state 2, not only is the mobile LHCII phosphorylated, but also the LHCII in the PSII supercomplexes. This demonstrates that PSII phosphorylation is not sufficient for disconnecting LHCII trimers S and M from PSII and for their migration to PSI.
Introduction
Oxygenic photosynthetic organisms harvest light via two large pigment-protein assemblies called photosystems I (PSI)2 and II (PSII). The two photosystems, connected through the plastoquinol pool and the cytochrome b6f complex, work in series to transform light energy into chemical energy (1). Both photosystems have a specific absorption spectrum. In plants and green algae, PSII is rich in chlorophyll b (absorption maxima ∼475 nm and 650 nm) which is coordinated by light-harvesting complexes, whereas PSI is rich in chlorophyll a (absorption maxima ∼440 nm and 680 nm). In addition, PSI has chlorophylls that can harvest light at wavelengths longer than 700 nm (2). For optimal performance of linear electron transport, the excitation pressure on the two photosystems needs to be balanced. In natural fluctuating light environments, a mechanism known as state transitions serves to redistribute the excitation energy between PSI and PSII (3, 4). In plants and algae, this is achieved by the migration of part of the major light harvesting complex (LHCII) between PSI and PSII. State 1 (St1) is traditionally defined as the condition in which PSI is preferentially excited and all LHCII is associated with PSII. When light conditions change, favoring PSII excitation (St2), a mobile pool of LHCII moves from PSII and associates with PSI (5). This process is triggered by phosphorylation of LHCII by the Stn7 kinase, which is activated upon oxidative reduction of the plastoquinol pool (6, 7). Conversely, in state 2 (St2), preferential excitation of PSI oxidizes the plastoquinol pool inactivating the Stn7 kinase. The constitutively active LHCII phosphatase, (TAP38/PPH1 (8, 9)), dephosphorylates the mobile LHCII, which moves to PSII. Recently, we showed that LHCII is associated with both photosystems in most natural light conditions, and it disconnects from PSI only upon far red illumination or high light stress, this is contrary to the common assumption that LHCII is only associated to PSI as short term response (10). If exposed to different light qualities for a long time, plants can adjust their PSI to PSII stoichiometry (11–15). Instead, long term changes in light intensity lead to changes in the antenna size of both PSI and PSII by regulating the level of the major light harvesting complex (LHCII), which functions as an antenna for both photosystems (10).
PSII is composed of a core complex, where the primary photochemistry takes place, and a peripheral antenna system that is encoded by the Lhcb1–6 genes (16). LHCII is present as a trimer composed of a combination of the Lhcb1–3 gene products. A threonine in the N terminus of Lhcb1 and Lhcb2 is the target for Stn7 (17). The minor Lhcbs consist of three monomers, Lhcb4–6, also named CP29, CP26, and CP24. Lhcbs associate with dimeric PSII cores to form PSII supercomplexes. The largest PSII supercomplex observed in Arabidopsis thaliana contains one copy of each minor complex and two LHCII trimers per core (C), which are indicated as S (strongly bound) and M (moderately bound), forming the C2S2M2 supercomplex. Several PSII supercomplexes can associate to form megacomplexes (18). Depending on light growth conditions, up to two more LHCII trimers per PSII core can be present in the thylakoids (18–20), but their physical interaction with the PSII supercomplex is rather weak or possibly absent in the sense that part of these complexes are probably not in direct contact with the core, although they are still able to transfer energy to it via other antennas (21). We call these trimers extra LHCII (10). The grana membrane in St1 contains the PSII supercomplexes (mainly in the C2S2M2 configuration) and extra LHCII that are located in between the supercomplexes but probably also at the grana margins (19).
Regarding the mobile LHCII involved in state transitions, it is known that the association of LHCII with PSI occurs on the PsaH and PsaL side (22) and involves one LHCII trimer (23). However, less is known about the association of this mobile LHCII with PSII in St1. Although several models have been proposed of how state transitions influence PSII organization, the identity of the trimer that moves from PSII to PSI is still under debate. The involvement of trimer M (and even S) has been suggested in various models (24, 25), but it is controversially discussed in literature. On the one hand, Lhcb3, which is a component of trimer M (26, 27), was not found in the stroma lamellae in St2 (28) or in the PSI-LHCII supercomplex (10, 29). On the other hand, upon St1 → St2 transition an increased fraction of C2S2M1 and a decreased fraction of C2S2M2 complexes was observed, suggesting that trimer M goes from PSII to PSI (23). This was further supported by the finding that the rate of state transitions was enhanced in CP24 and Lhcb3 KO mutants, where trimer M is not (KO CP24) or less (KO Lhcb3) stably associated with the PSII supercomplex (30, 31). More recently, it was proposed that remodeling of PSII supercomplexes is required for state transitions (24), as has been suggested before for the green alga Chlamydomonas reinhardtii (32), whereas Tikkanen et al. (33) propose that both PSI and PSII move to the grana margins in St2 where they share LHCII. In this study, we combine biochemical and electron microscopy analysis to investigate the effect of state transitions on the PSII supercomplex organization and to determine which LHCII trimer(s) is/are involved in this process.
EXPERIMENTAL PROCEDURES
Plant Material
A. thaliana (Columbia) WT plants were grown at 100 μEinstein/m2/s, 70% humidity, 22 °C, and 8 h/16 h day/night regime (Plant Climatics Percival Growth Chamber, Model AR-36L, Germany). Only leaves fully exposed to the light were used for thylakoid isolation.
Plant Treatment and Thylakoid Isolation
Leaves were harvested either directly after overnight dark adaptation (St1) or after 50 min of 20 μE/m2/s white light treatment (St2). Harvested leaves were directly transferred to ice water. Thylakoid membranes were prepared according to Ref. 34 with the addition of 10 mm of NaF to all buffers, to inhibit phosphatase activity. The membrane was resuspended in 20 mm Hepes, pH 7.5, 0.4 m sorbitol, 15 mm NaCl, 5 mm MgCl2, and 10 mm NaF, quickly frozen in liquid N2, and stored at 193 K until use. The chlorophyll a/b ratio (measured as described (35)) was the same for St1 and St2 thylakoids, indicating that no changes in PSI/PSII ratio had occurred during the 50 min of light treatment.
77 K Fluorescence Measurements
The 77 K emission spectra were measured in 66% (w/v) glycerol, 10 mm Hepes, pH 7.5, 5 mm MgCl2, 15 mm NaCl, and 10 mm NaF with a Fluorolog (Jobin-Yvon) with 3.5-nm bandwidth in excitation and 2-nm bandwidth in emission. The OD680 of the sample was <0.1, and the diameter of the circular cuvette was 4 mm.
PAGE
First or second dimension denaturing PAGE was performed with the Tris-Tricine system (36) at a 14.5% acrylamide concentration. Blue native PAGE was performed as described in Ref. 37, with the 25BTH20G buffer and an acrylamide/bisacrylamide ratio of 32:1 in both stacking (3.5%) and resolving (4 to 14%) gel. The final chlorophyll concentration was 0.5 mg/ml, and the final detergent concentration was 1% α- or β-n-dodecyl-d-maltoside (α/β-DM) or 1% digitonin/0.1% α-DM as used in Ref. 29. It should be noted that the very small amount of α-DM added to digitonin does not have any effect in the solubilization of the membrane but only helps the solubilization of digitonin (29). The cathode buffer was supplemented with 0.02% Coomassie Blue G (Serva). Second dimension PAGE was performed as described in Ref. 37. Pro-Q Diamond Phosphoprotein Gel Stain (Molecular Probes) was used as described in the user manual with fluorescence detection, except that the samples were not desalted and delipidated. We confirmed that the signal was linear with the protein concentration. Gels were stained with 0.025% Coomassie Blue G in 10% acetic acid as described in Ref. 36. The Coomassie and Pro-Q Diamond stained gels were imaged with ImageQuant LAS-4000 (GE Healthcare).
EM and Image Analysis
Thylakoid membranes were solubilized in 20 mm Bis-Tris buffer, pH 6.5, 5 mm MgCl2 using digitonin (0.5 mg of chlorophylls/ml, 0.5% digitonin) for 20 min at 4 °C with slow stirring, followed by centrifugation in an Eppendorf table centrifuge for 10 min. The pellet, which contained the non-solubilized grana thylakoid membranes, was washed one more time using the same buffer, centrifuged for 5 min, and then used for EM analysis. Specimens were prepared by negative staining with 2% uranyl acetate on glow-discharged carbon-coated copper grids. Electron microscopy was performed (34) with 80,000× magnification and semi-automated data acquisition (38). Sub-areas (256 × 256 pixels) of PSII arrays selected from individual electron micrographs were analyzed separately. To determine a density of PSII complexes in a membrane area, mid-mass positions of the PSII core complexes were marked manually using Groningen Image Processing software (GRIP).
The overlay of the C2S2M2 projection maps on the micrographs of the grana membranes with randomly distributed PSII complexes was based on a cross-correlation alignment using GRIP software. To enhance a contrast of PSII complexes and to facilitate their localization in the grana membrane, high frequency noise was filtered out.
RESULTS
State Transitions
In this study, the plants were brought to St1 by dark adaptation, whereas St2 was induced by 50 min of low intensity white light treatment, conditions that are physiological for the plant. To check whether this treatment was successful, the level of LHCII phosphorylation in the thylakoids was evaluated by Pro-Q Diamond Phosphorylation gel stain (Fig. 1A). As expected, LHCII was far less phosphorylated after dark adaptation (St1) than after light adaptation (St2). Furthermore, the 77K emission spectrum shows enhanced 735 nm (PSI 77K fluorescence) emission in St2 thylakoids compared with those in St1, confirming that St1 and St2 were successfully induced.
FIGURE 1.
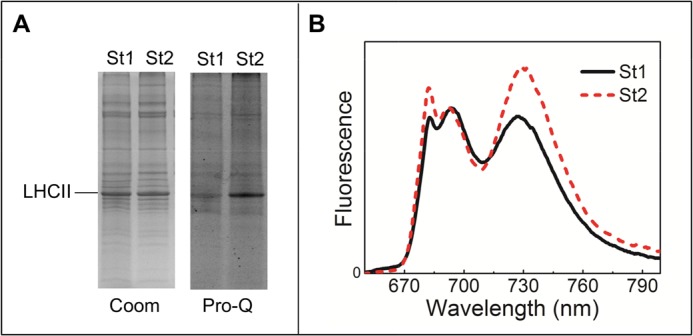
Analysis of St1 and St2 thylakoid analysis. A, Coomassie Blue (Coom) and Pro-Q Diamond Phosphorylation (Pro-Q) stained SDS-PAGE gel. Gels were loaded with St1 or St2 thylakoids (1 μg on chlorophyll basis). B, 77 K fluorescence of St1 and St2 thylakoids. Excitation was at 484 nm and normalized to the maximum in the 690–700 nm PSII core emission region.
Influence of State Transition on the Organization of PSII Supercomplexes
To compare the organization of PSII supercomplexes in St1 and St2, the thylakoid membranes in the two states were solubilized with β-DM or with the milder α-DM and loaded on a blue native gel. Freshly prepared thylakoids where used for this experiment to avoid freeze/thaw cycles that might affect membrane and supercomplex solubilization. PSII supercomplexes were present in equal amount in St1 and St2 for both solubilization conditions, indicating that the amount of the supercomplexes in intact St1 and St2 membranes is the same. This idea is further supported by using a very mild solubilization condition (1% digitonin, 0.1% α-DM, Fig. 2) that solubilizes only the grana margins, which is the region where the largest difference in the supercomplexes organization between St1 and St2 can be expected. Also, in this case, the amount of supercomplexes were identical in St1 and St2, indicating that not even the supercomplexes in the grana margin disassemble during state transitions. It should be added that when not using fresh thylakoids, the amount of supercomplexes decreases in St2 as compared with St1, especially upon treatment with the β-DM (data not shown), suggesting that the supercomplexes in St2 are less stable than those in St1.
FIGURE 2.
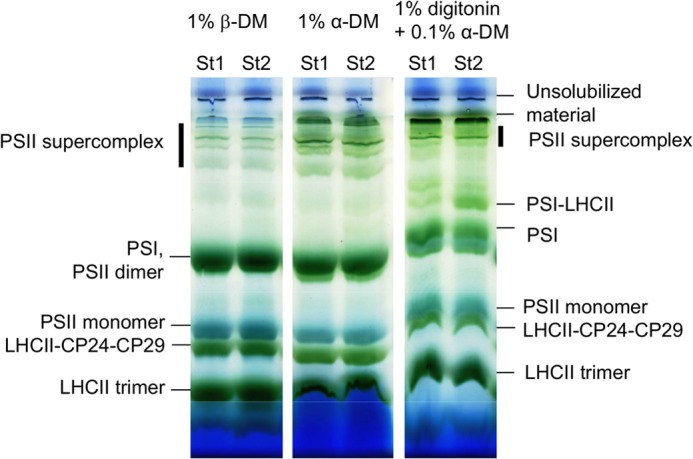
Blue native PAGE of thylakoids isolated from plants in St1 or St2. Solubilization conditions are indicated.
Organization of PSII Supercomplexes in the Membrane
The organization of PSII supercomplexes in the grana membrane was further investigated by EM. The analysis reveals pairs of grana membranes where the typical features of PSII core complex are observed (Fig. 3). The major part (>90%) of the inspected micrographs (555 for St1 and 480 for St2) showed a random organization of PSII supercomplexes in the membrane (Fig. 3, A and D), whereas the other fraction (∼7% in St1 and 9% in St2) showed a semi-crystalline organization (Fig. 3, B and E). The very similar amount of crystalline arrays in the two states is not in agreement with the recent model of Dietzel et al. (24), which suggests that disassembly of semi-crystalline PSII arrays is a prerequisite for the St1 → St2 transition.
FIGURE 3.
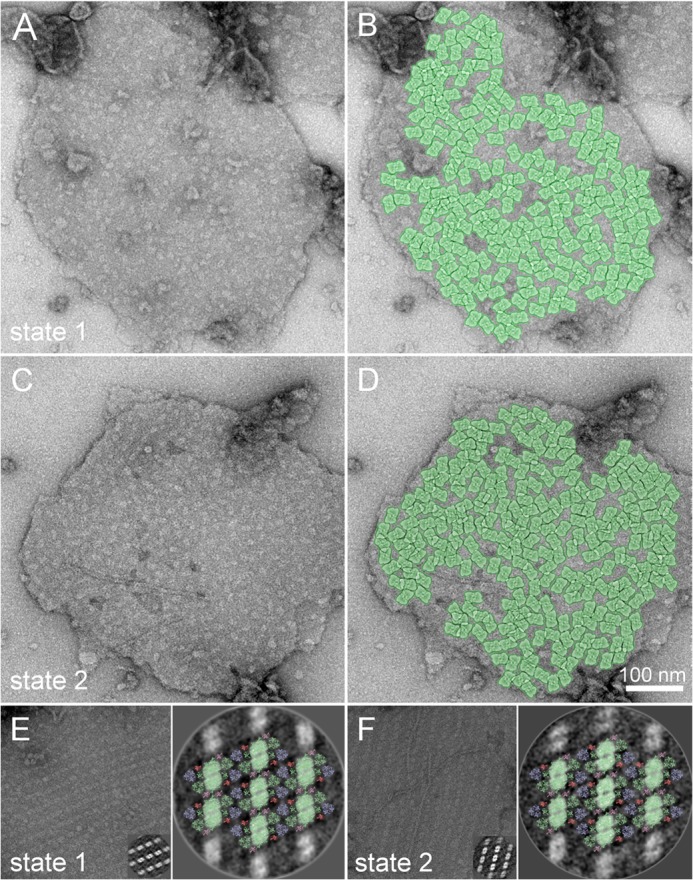
Organization of PSII complexes in the thylakoid membrane. Examples of electron micrographs of negatively stained pairs of thylakoid membranes with either a random (A, St1; C, St2) or ordered (E, St1; F, St2) organization of PSII complexes. To give an idea of the possible organization of the supercomplexes in the membrane, in B and D, a subset of PSII complexes in the membrane are highlighted by the overlay of the projection maps of C2S2M2 particles. Insets in E and F, single particle analysis of sub-areas of ordered arrays of PSII supercomplexes selected from the particular electron micrograph. Averaged projections of two-dimensional arrays of PSII complexes were assigned by fitting the structural pseudo-atomic model of the PSII C2S2M2 supercomplex according to Ref. 34 (PSII core complex, the minor antenna CP29 and S-type of light-harvesting trimer are depicted in light green; M-type of light harvesting trimer and minor antennae CP24 and CP26 are depicted in light blue, dark salmon, and light pink, respectively) (E, St1; F, St2).
To identify the type of PSII supercomplexes present in the semi-crystalline arrays in the two states, image analysis of sub-areas of 2D arrays followed by fitting of the projection map to an x-ray pseudo-atomic model of the PSII supercomplex (34) was performed. In both St1 and St2, all supercomplexes in the 2D arrays were of the C2S2M2 type (Fig. 3C,F).
To investigate the effect of state transitions on the organization of PSII in the random regions, the PSII density in the random area was determined. The white spot in the micrographs represent PSII core complexes which protrude out of the membrane. About 1200 PSII particles were manually selected from 8 micrographs for both St1 and St2. The average PSII density was 1549 ± 128 particles/μm2 (data are presented as mean ± S.D., n = 8 micrographs) in St1 and 1601 ± 146 particles/μm2 in St2, meaning that the PSII density does not significantly change during state transitions (there is no statistically significant difference at p = 0.05 level determined using the Student's t test), whereas differences were observed with the same method when the amount of LHCII changes (19). To give an idea of the possible organization of the supercomplexes in the membrane, the position of the PSII complexes in St1 and St2 membranes were highlighted by overlaying the projection map with the C2S2M2 supercomplex (see Methods, Fig. 3, B and D, respectively) (34). The pattern is very similar in both St1 and St2 indicating (i) a very similar supercomplex distribution and (ii) that the large majority of PSII complexes can be present in the form of the C2S2M2 supercomplex, in agreement with previous results obtained with cryo-electron microscopy (39). We stress here that it was not possible to investigate the PSII density in the grana margins. A change in the PSII density could occur in this region. In conclusion, the molecular and supramolecular organization of PSII supercomplexes does not change during state transitions; neither the occurrence/composition of the crystalline arrays (from which the megacomplexes originate) nor the grana region with randomly oriented supercomplexes were altered.
Phosphorylation State of PSII Supercomplexes
To clarify which LHCII complexes are phosphorylated in the different states, the thylakoid membranes of plants in St1 and St2 were separated by blue native PAGE, and their protein content was analyzed in the second dimension by SDS-PAGE. The phosphorylated proteins were stained with Pro-Q Diamond stain, after which a Coomassie stain was used to visualize the total protein content (Fig. 4). The PSI-LHCII complex is not present in this gel, as it disassembles into PSI and LHCII trimers upon α-DM solubilization. The analysis shows that Lhcb1,2 present in the St2 LHCII trimers are phosphorylated. Indeed, it has been shown previously that the LHCII polypeptides associated with PSI are phosphorylated (10, 29) in agreement with the standard model that LHCII moves to PSI when phosphorylated and to PSII upon dephosphorylation (8, 9, 40, 41, 42). However, in contradiction with the general model, the gel shows that intact PSII supercomplexes are also phosphorylated in St2, confirming recent results of Grieco et al. (43). Thus, phosphorylated LHCII in the PSII supercomplex does not move to PSI, which means that the standard state transitions model needs to be revised.
FIGURE 4.
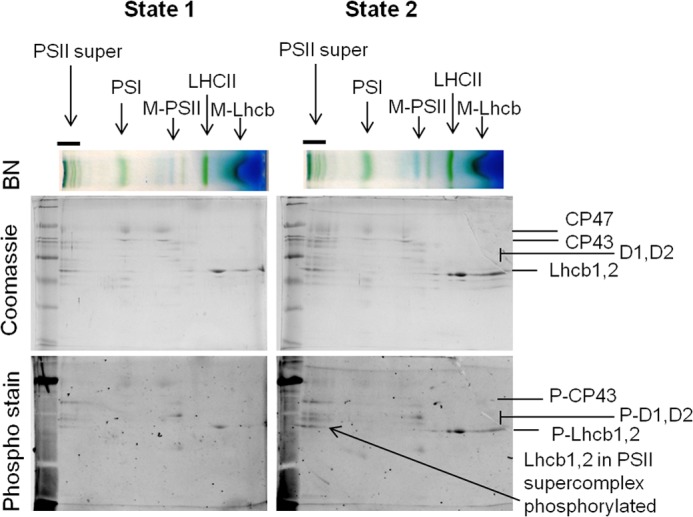
Phosphorylation state of PSII supercomplexes. St1 and St2 thylakoids were solubilized with 1% α-DM, and the supercomplexes were separated on blue native PAGE (BN, top) and the protein on SDS-PAGE. Total protein content was stained with Coomassie Blue (middle), and phosphorylated proteins were visualized with Diamond Pro-Q Phosophostain (bottom). Identities of PS supercomplexes (top; PSII super, PSII supercomplex, M-PSII, monomeric PSII core), and important PSII proteins (middle) and phosphorylated PSII proteins (bottom) are indicated.
DISCUSSION
The Mobile Pool of LHCII Involved in State Transitions Consists of the Extra (Non-PSII Supercomplex) LHCII
In A. thaliana two LHCII trimers (S and M) per monomeric PSII core are located in the PSII supercomplex (18, 34), whereas up to two extra LHCIIs per PSII monomer are present in the thylakoid membrane (19, 20). Our investigation of their role in state transitions shows that the involvement of trimer M and S can be excluded: the absence of trimer S would lead to the full disassembly of PSII supercomplexes, which does not occur as we demonstrate here. Mobility of trimer M can also be excluded, as Lhcb3, a marker for this trimer, is not a component of the mobile LHCII fraction (10, 29). These findings lead to the conclusion that the extra LHCIIs are the mobile antenna, which can migrate between PSI and PSII, in agreement with the proposal of Kyle et al. (44). It is likely that these extra LHCIIs are located near the grana-margins in St1, such that transfer into the stroma lamellae (St2) only needs a short migration distance. This might explain why the PSII density inside the PSII grana does not change upon state transitions (Fig. 3) (44).
The migration of the extra LHCIIs has clear advantages for the plant. In the PSII supercomplex, the short distance between the trimers and the PSII core allows for fast excitation-energy transfer and thus high PSII operating efficiency (45). The extra LHCIIs are located more distantly from the PSII core, which leads to slower, and thus less efficient, excitation-energy migration to the core (10). Thus, evolution has “chosen” to send the least efficient LHCII antenna to PSI, whereas the efficient PSII supercomplex remains fully functional.
Phosphorylated LHCII Does Not Have to Move to PSI
According to the accepted model of state transitions, the phosphorylation of LHCII induces its dissociation from PSII and its movement/association to PSI. Only in mutants lacking PSI (46) or the LHCII anchor point in PSI (22) was it observed that phosphorylated LHCII could not dock to PSI and stayed in the grana, supporting the “molecular recognition model” (22, 47). However, this observation has several possible explanations: (i) phosphorylation involves only the extra LHCII, which in the absence of PSI cannot move to PSI; (ii) phosophorylation involves also trimer M and S (the trimers that are part of the supercomplex) and induces their dissociation from the core, although in the absence of PSI, they remain in the grana; (iii) trimer M and S get phosphorylated but do not dissociate from the core. Here we show that both the LHCII of the PSII supercomplex as well as the extra trimers get phosphorylated upon St1 to St2 transition but that phosphorylated trimer M and S remain associated with the PSII core. And this in WT plants, when the docking side on PSI is available. This is in agreement with previous results (48), which showed that there are at least two pools of LHCII that can be phosphorylated, of which only one moves to the stroma lamellae. Our data clearly indicate that the association of trimers S and M with the PSII core is too strong to be broken by phosphorylation in the membrane. However, phosphorylation can be instrumental in breaking the weak interactions between the PSII supercomplex and the extra LHCII trimers, thus allowing the latter to move to PSI.
Remodeling of PSII Supercomplexes and Megacomplexes Is Not Required for State Transitions
In this work, we have investigated several important aspects of state transition in higher plants at the level of supercomplexes, looking at their composition and organization. However, note that other mechanisms, such as thylakoid membrane reorganization, might also occur during state transitions. The results of this study are summarized in Fig. 5: the composition and organization of the PSII supercomplexes does not show significant differences between St1 and St2. The LHCII that migrates to the stroma lamellae in St2 is a subpopulation of extra LHCII, which is probably located close to the grana margin in St1, as suggested by the absence of changes in the PSII density in the grana in the two conditions. In St2, not only the mobile LHCII complexes are phosphorylated but also the LHCII trimers, which are associated with the core to form the PSII supercomplexes. This indicates that phosphorylation is not sufficient to induce the movement of LHCII from grana to stroma as suggested previously (22). Moreover, the phosphorylation is not sufficient to detach the LHCII trimers from the PSII supercomplex. The population of extra LHCII is weakly connected with PSII supercomplexes as indicated by the fact that they transfer energy relatively slowly to the reaction center of PSII in St1 (21) and that it has thus far not been possible to purify a PSII supercomplex from plants with these trimers attached (34). It is therefore likely that this connection is sufficiently weak to be broken by phosphorylation. At the same time, it can be expected that phosphorylated LHCII has a higher affinity for PSI, such that the effect is synergetic. The presence of the migrating LHCII in the grana margins also means that the LHCII do not have to migrate very far during state transitions, thus limiting the possible danger caused by the presence of “free” pigment-protein complexes in the membrane. In addition, we have recently shown that in basically all natural light conditions for plants, a part of the mobile LHCII population is associated with PSI (10). It can thus be concluded that the association of LHCII with PSI has evolved from a short term response as observed in green algae, where it involves up to 80% of the LHCII population (46) and has a large effect on the PSII organization (32), to a long term response in plants, where LHCII is an antenna of PSI in most light conditions.
FIGURE 5.
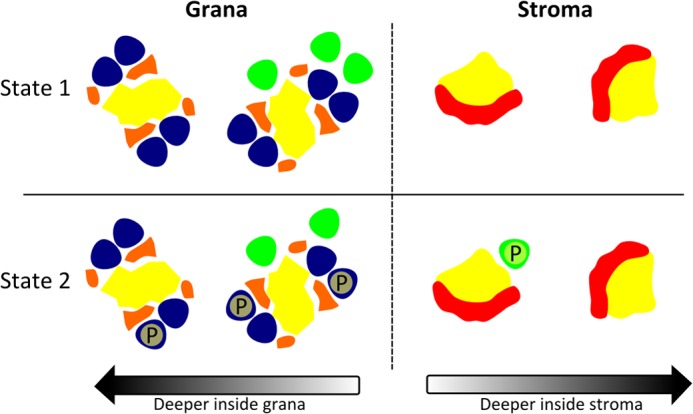
Schematic model of LHCII phosphorylation during state transitions in higher plants. The PSI and PSII core are depicted in yellow; in PSII, trimers M and S are colored blue; the extra LHCII trimers are colored green, the minor PSII antenna is colored orange, and the Lhca PSI antenna is colored red. Phosphorylation of LHCII is indicated with a P. The extra LHCIIs are mainly associated with the PSII C2S2M2 supercomplexes located nearby the stroma. Phosphorylation of PSII supercomplex LHCII trimers and extra LHCII trimers (St2) results in the migration of the latter to PSI in the stroma, whereas the C2S2M2 supercomplex remains intact. The mobile LHCII trimer will most likely associate with PSI complexes located nearby the grana.
Acknowledgment
We thank Laura M. Roy for help with the membrane preparations and for helpful comments on the manuscript.
This work was supported by the Netherlands Organization for Scientific Research (NWO), Earth and Life Sciences (ALW), through a Vici grant and the European Research Council (ERC) starting/consolidator Grant 281341 (to R. C.), and Grant ED0007/01/01 Centre of the Region Haná for Biotechnological and Agricultural Research (to R. K.).
- PSI
- photosystem I
- α/β-DM
- α- or β-n-dodecyl-d-maltoside
- St1
- state 1
- St2
- state 2
- LHCII
- major light-harvesting complex of Photosystem II.
REFERENCES
- 1. Nelson N., Yocum C. F. (2006) Structure and Function of Photosystems I and II. Annu. Rev. Plant Biol. 57, 521–565 [DOI] [PubMed] [Google Scholar]
- 2. Croce R., Zucchelli G., Garlaschi F. M., Bassi R., Jennings R. C. (1996) Excited state equilibration in the Photosystem I light-harvesting I complex: P700 is almost isoenergetic with its antenna. Biochemistry 35, 8572–8579 [DOI] [PubMed] [Google Scholar]
- 3. Bonaventura C., Myers J. (1969) Fluorescence and Oxygen Evolution from Chlorella pyrenoidosa. BBA 189, 366–383 [DOI] [PubMed] [Google Scholar]
- 4. Murata N. (1969) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. BBA 172, 242–251 [DOI] [PubMed] [Google Scholar]
- 5. Allen J. F. (1992) Protein phosphorylation in regulation of photosynthesis. BBA 1098, 275–335 [DOI] [PubMed] [Google Scholar]
- 6. Allen J. F., Bennett J., Steinback K. E., Arntzen C. J. (1981) Chloroplast protein-phosphorylation couples plastoquinone redox state to distribution of excitation-energy between photosystems. Nature 291, 25–29, 10.1038/291025a0 [DOI] [Google Scholar]
- 7. Larsson U. K., Jergil B., Andersson B. (1983) Changes in the lateral distribution of the light-harvesting chlorophyll-a/b–protein complex induced by its phosphorylation. Eur. J. Biochem. 136, 25–29 [DOI] [PubMed] [Google Scholar]
- 8. Pribil M., Pesaresi P., Hertle A., Barbato R., Leister D. (2010) Role of Plastid Protein Phosphatase TAP38 in LHCII Dephosphorylation and Thylakoid Electron Flow. PLoS Biol. 8, e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shapiguzov A., Ingelsson B., Samol I., Andres C., Kessler F., Rochaix J. D., Vener A. V., Goldschmidt-Clermont M. (2010) The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 107, 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wientjes E., van Amerongen H., Croce R. (2013) LHCII is an antenna of both photosystems after long-term acclimation. Bba-Bioenergetics 1827, 420–426 [DOI] [PubMed] [Google Scholar]
- 11. Chow W. S., Melis A., Anderson J. M. (1990) Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc. Natl. Acad. Sci. U.S.A. 87, 7502–7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melis A. (1991) Dynamics of Photosynthetic Membrane-Composition and Function. Biochim. Biophys. Acta 1058, 87–106 [Google Scholar]
- 13. Hogewoning S. W., Wientjes E., Douwstra P., Trouwborst G., van Ieperen W., Croce R., Harbinson J. (2012) Photosynthetic quantum yield dynamics: from photosystems to leaves. Plant Cell 24, 1921–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allen J. F., Pfannschmidt T. (2000) Balancing the two photosystems: photosynthetic electron transfer governs transcription of reaction centre genes in chloroplasts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allen J. F., Santabarbara S., Allen C. A., Puthiyaveetil S. (2011) Discrete Redox Signaling Pathways Regulate Photosynthetic Light-Harvesting and Chloroplast Gene Transcription. PLoS One 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jansson S. (1999) A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4, 236–240 [DOI] [PubMed] [Google Scholar]
- 17. Bennett J. (1991) Protein-Phosphorylation in Green Plant Chloroplasts. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 281–311 [Google Scholar]
- 18. Dekker J. P., Boekema E. J. (2005) Supramolecular Organization of Thylakoid Membrane Proteins in Green Plants. Biochim. Biophys. Acta 1706, 12–39 [DOI] [PubMed] [Google Scholar]
- 19. Kouřil R., Wientjes E., Bultema J. B., Croce R., Boekema E. J. (2013) High-light vs. low-light: Effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim. Biophys. Acta 1827, 411–419 [DOI] [PubMed] [Google Scholar]
- 20. Peter G. F., Thornber J. P. (1991) Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J. Biol. Chem. 266, 16745–16754 [PubMed] [Google Scholar]
- 21. Wientjes E., van Amerongen H., Croce R. (2013) Quantum Yield of Charge Separation in Photosystem II: Functional Effect of Changes in the Antenna Size upon Light Acclimation. J. Phys. Chem. B 117, 11200–11208 [DOI] [PubMed] [Google Scholar]
- 22. Lunde C., Jensen P. E., Haldrup A., Knoetzel J., Scheller H. V. (2000) The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408, 613–615 [DOI] [PubMed] [Google Scholar]
- 23. Kouril R., Zygadlo A., Arteni A. A., de Wit C. D., Dekker J. P., Jensen P. E., Scheller H. V., Boekema E. J. (2005) Structural characterization of a complex of photosystem I and light-harvesting complex II of Arabidopsis thaliana. Biochemistry 44, 10935–10940 [DOI] [PubMed] [Google Scholar]
- 24. Dietzel L., Bräutigam K., Steiner S., Schüffler K., Lepetit B., Grimm B., Schöttler M. A., Pfannschmidt T. (2011) Photosystem II supercomplex remodeling serves as an entry mechanism for state transitions in Arabidopsis. Plant Cell 23, 2964–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minagawa J. (2011) State transitions-The molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim. Biophys. Acta 1807, 897–905 [DOI] [PubMed] [Google Scholar]
- 26. Bassi R., Dainese P. (1992) A supramolecular light-harvesting complex from chloroplast photosystem-II membranes. Eur. J. Biochem. 204, 317–326 [DOI] [PubMed] [Google Scholar]
- 27. Hankamer B., Nield J., Zheleva D., Boekema E., Jansson S., Barber J. (1997) Isolation and biochemical characterisation of monomeric and dimeric photosystem II complexes from spinach and their relevance to the organisation of photosystem II in vivo. Eur. J. Biochem. 243, 422–429 [DOI] [PubMed] [Google Scholar]
- 28. Bassi R., Giacometti G., Simpson D. (1988) Changes in the organization of stroma membranes induced by in vivo state 1-state 2 transition. Biochim. Biophys. Acta 935, 152–165 [Google Scholar]
- 29. Galka P., Santabarbara S., Khuong T. T., Degand H., Morsomme P., Jennings R. C., Boekema E. J., Caffarri S. (2012) Functional analyses of the plant photosystem I-light-harvesting complex II supercomplex reveal that light-harvesting complex II loosely bound to photosystem II is a very efficient antenna for photosystem I in state II. Plant Cell 24, 2963–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kovács L., Damkjaer J., Kereïche S., Ilioaia C., Ruban A. V., Boekema E. J., Jansson S., Horton P. (2006) Lack of the light-harvesting complex CP24 affects the structure and function of the grana membranes of higher plant chloroplasts. Plant Cell 18, 3106–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Damkjaer J. T., Kereïche S., Johnson M. P., Kovacs L., Kiss A. Z., Boekema E. J., Ruban A. V., Horton P., Jansson S. (2009) The photosystem II light-harvesting protein Lhcb3 affects the macrostructure of photosystem II and the rate of state transitions in Arabidopsis. Plant Cell 21, 3245–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iwai M., Takahashi Y., Minagawa J. (2008) Molecular remodeling of photosystem II during state transitions in Chlamydomonas reinhardtii. Plant Cell 20, 2177–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tikkanen M., Nurmi M., Suorsa M., Danielsson R., Mamedov F., Styring S., Aro E. M. (2008) Phosphorylation-dependent regulation of excitation energy distribution between the two photosystems in higher plants. Biochim. Biophys. Acta 1777, 425–432 [DOI] [PubMed] [Google Scholar]
- 34. Caffarri S., Kouril R., Kereïche S., Boekema E. J., Croce R. (2009) Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 28, 3052–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Croce R., Canino G., Ros F., Bassi R. (2002) Chromophore organization in the higher-plant photosystem II antenna protein CP26. Biochemistry 41, 7334–7343 [DOI] [PubMed] [Google Scholar]
- 36. Schägger H. (2006) Tricine-SDS-PAGE. Nat. Protoc. 1, 16–22 [DOI] [PubMed] [Google Scholar]
- 37. Järvi S., Suorsa M., Paakkarinen V., Aro E. M. (2011) Optimized Native Gel Systems for Separation of Thylakoid Protein Complexes: Novel Super- and Mega-Complexes. Biochem. J. 439, 207–214 [DOI] [PubMed] [Google Scholar]
- 38. Oostergetel G. T., Keegstra W., Brisson A. (1998) Automation of specimen selection and data acquisition for protein electron crystallography. Ultramicroscopy 74, 47–59 [Google Scholar]
- 39. Kouřil R., Oostergetel G. T., Boekema E. J. (2011) Fine structure of granal thylakoid membrane organization using cryo electron tomography. Biochim. Biophys. Acta 1807, 368–374 [DOI] [PubMed] [Google Scholar]
- 40. Telfer A., Allen J. F., Barber J., Bennett J. (1983) Thylakoid protein-phosphorylation during state-1-state-2 transitions in osmotically shocked pea-chloroplasts. Biochim. Biophys. Acta 722, 176–181 [Google Scholar]
- 41. Allen J. F. (1992) How Does Protein-Phosphorylation Regulate Photosynthesis. Trends Biochem. Sci. 17, 12–17 [DOI] [PubMed] [Google Scholar]
- 42. Bellafiore S., Barneche F., Peltier G., Rochaix J. D. (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895 [DOI] [PubMed] [Google Scholar]
- 43. Grieco M., Tikkanen M., Paakkarinen V., Kangasjärvi S., Aro E. M. (2012) Steady-State Phosphorylation of Light-Harvesting Complex II Proteins Preserves Photosystem I under Fluctuating White Light. Plant Physiol. 160, 1896–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kyle D. J., Staehelin L. A., Arntzen C. J. (1983) Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch. Biochem. Biophys. 222, 527–541 [DOI] [PubMed] [Google Scholar]
- 45. Broess K., Trinkunas G., van Hoek A., Croce R., van Amerongen H. (2008) Determination of the Excitation Migration Time in Photosystem II - Consequences for the Membrane Organization and Charge Separation Parameters. Biochim. Biophys. Acta 1777, 404–409 [DOI] [PubMed] [Google Scholar]
- 46. Delosme R., Olive J., Wollman F. A. (1996) Changes in light energy distribution upon state transitions: An in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1273, 150–158 [Google Scholar]
- 47. Allen J. F., Forsberg J. (2001) Molecular recognition in thylakoid structure and function. Trends Plant Sci. 6, 317–326 [DOI] [PubMed] [Google Scholar]
- 48. Larsson U. K., Sundby C., Andersson B. (1987) Characterization of 2 Different Subpopulations of Spinach Light-Harvesting Chlorophyll a/B-Protein Complex (Lhc-Ii) - Polypeptide Composition, Phosphorylation Pattern and Association with Photosystem-Ii. Biochim. Biophys. Acta 894, 59–68 [Google Scholar]


