Background: The molecular mechanism underlying the regulation of cellulase production by T. reesei is unclear.
Results: The absence of sugar transporter Stp1 enhanced cellulase gene induction whereas the absence of Crt1 abolished cellulase gene expression.
Conclusion: Crt1 is essential in cellulase gene induction independent of intracellular sugar delivery.
Significance: These data shed light on the mechanism by which T. reesei senses and transmits cellulose signal.
Keywords: Cellulase, Fungi, Gene Transcription, Sugar Transport, Transporters, Trichoderma reesei
Abstract
Proper perception of the extracellular insoluble cellulose is key to initiating the rapid synthesis of cellulases by cellulolytic Trichoderma reesei. Uptake of soluble oligosaccharides derived from cellulose hydrolysis represents a potential point of control in the induced cascade. In this study, we identified a major facilitator superfamily sugar transporter Stp1 capable of transporting cellobiose by reconstructing a cellobiose assimilation system in Saccharomyces cerevisiae. The absence of Stp1 in T. reesei resulted in differential cellulolytic response to Avicel versus cellobiose. Transcriptional profiling revealed a different expression profile in the Δstp1 strain from that of wild-type strain in response to Avicel and demonstrated that Stp1 somehow repressed induction of the bulk of major cellulase and hemicellulose genes. Two other putative major facilitator superfamily sugar transporters were, however, up-regulated in the profiling. Deletion of one of them identified Crt1 that was required for growth and enzymatic activity on cellulose or lactose, but was not required for growth or hemicellulase activity on xylan. The essential role of Crt1 in cellulase induction did not seem to rely on its transporting activity because the overall uptake of cellobiose or sophorose by T. reesei was not compromised in the absence of Crt1. Phylogenetic analysis revealed that orthologs of Crt1 exist in the genomes of many filamentous ascomycete fungi capable of degrading cellulose. These data thus shed new light on the mechanism by which T. reesei senses and transmits the cellulose signal and offers potential strategies for strain improvement.
Introduction
Since its initial isolation as a decomposer of cellulosic materials, Trichoderma reesei (telemorph Hypocrea jecorina) has been developed into one of the most prolific cellulase producers in industry. Its high capacity to secret large amounts of lignocellulosic enzymes that release fermentable sugars from plant cell walls holds the potential for production of environmentally clean and renewable source of energy. The cellulase mixture of T. reesei has been shown to consist of at least three types of enzymes that act upon the insoluble substrate to achieve the efficient conversion of crystalline cellulose to glucose (1).
Despite the tremendous progress that has been made in the extensive characterization of cellulosic enzymes in T. reesei, the molecular mechanism underlying the regulation of cellulase production by T. reesei is still insufficiently understood. For rapid launching of the cellulase machinery, T. reesei has to sense the presence of the insoluble polysaccharide cellulose outside the cell (2–5). Although the identity of the true “inducer” and the ensuing intracellular inductor cascade is still speculative, several lines of evidence have been presented suggesting that the soluble oligosaccharides released from cellulose or their derivatives may well function as the actual molecules that trigger cellulase induction (4). Specifically, both cellobiose, the major soluble end product of cellulose, and its transglycosylating derivative, sophorose, have been shown to induce cellulase gene expression and activity in T. reesei (3, 6–8). Moreover, an uptake system of cellobiose has been shown to be present in T. reesei as well as in another cellulolytic fungi Neurospora crassa (3, 6, 9). Although the identity of the cellobiose permease in T. reesei and its role in cellulase induction have been elusive, deletion for one of the major facilitator superfamily (MFS)3 sugar transporters in N. crassa seems to compromise its growth on cellobiose and cellulose (9), pointing to a role of the uptake of cellobiose in regulating cellulase gene expression.
We report here the identification of a MFS sugar transporter from T. reesei capable of transporting cellobiose and glucose. A strain carrying a deletion for this transporter behaves differentially in induced cellulase gene expression on cellulose versus cellobiose. We further report the isolation of another putative MFS transporter. Strains lacking the gene of this transporter were almost deprived of the capability to express cellulase genes on induction by cellulose or lactose. We discuss a possible role of these transporters in induced cellulase biosynthesis.
EXPERIMENTAL PROCEDURES
Strains, Medium, and Cultivations
The wild-type strain T. reesei TU-6 (ATCC MYA-256) (10), a uridine-auxotrophic derivative of T. reesei QM9414 (ATCC 26921) with a mutant pyr4 (orotidine 5′-phosphate decarboxylase-encoding) was used throughout this work. Strains were grown in 1-liter Erlenmeyer flasks on a rotary shaker (200 rpm) at 28 °C in the minimal medium as described by Mandels et al. (8) using glycerol (1%, w/v), Avicel (1%, w/v), lactose (1%, w/v), oat spelts xylan (1%, w/v), or cellobiose (0.5%, w/v) as the sole carbon source as indicated. Saccharomyces cerevisiae strains W303 and EBY.VW4000 (11) were grown in synthetic complete medium with glucose and maltose as the carbon source, respectively. Uracil or leucine was lacking for plasmid selection. Escherichia coli DH5α was used for plasmid construction and amplification.
For analysis of cellulase production, T. reesei strains were precultured on glycerol for 36 h and grown for another 12 h in the same fresh medium. Mycelia were then harvested by filtration. Equal amounts of mycelia were transferred to a fresh medium containing the respective carbon sources after being washed twice with medium without carbon source, and incubation was continued for the indicated time period. When sophorose (1 mm) was used as an inducer, resting cell cultivations were performed as described previously (12).
Construction of Plasmids for Expression in Yeast
For expression of Stp1 and Crt1 in yeast, the coding sequences of stp1 and crt1 were amplified from the cDNA of T. reesei with respective primers both with EcoRI and HindIII sites. The digested fragment of stp1 was ligated into pRS426ADH, which was derived from pRS426 containing the S. cerevisiae ADH-1 promoter and terminator to obtain pRS426ADHstp1. The digested fragment of crt1 was ligated into pRS426CYC, which was also derived from pRS426 containing the S. cerevisiae CYC-1 promoter and ADH-1 terminator to obtain pRS426CYCcrt1. For expression of CDT1 and β-glucosidase GH1–1 from N. crassa, the coding sequences of cdt1 and gh1-1 were amplified from the cDNA of N. crassa. After digestion with EcoRI and HindIII, cdt1 was ligated into the same sites of pRS426ADH to obtain pRS426cdt1. The fragment of gh1-1 digested with HindIII and SmaI was ligated into pRS425ADH containing the S. cerevisiae ADH-1 promoter and terminator to construct pRS425ADHgh1-1.
To determine the subcellular localization of Stp1, pRS426ADHstp1-gfp was constructed and expressed in S. cerevisiae. The coding sequence of stp1-gfp was inserted into the EcoRI and HindIII sites of pRS426ADH after fusion by overlap-extension PCR (13). For determination of the subcellular localization of Crt1, the coding sequence of crt1-gfp was inserted into the EcoRI and HindIII sites of pRS426CYC to construct pRS426crt1-gfp.
Yeast Growth Assay
The respective constructs were used to transform yeast strains by the high efficiency method of Gietz et al. (14). Transformants were grown in liquid medium under selective conditions, harvested during logarithmic growth, washed, adjusted to the same cell density, and transferred to liquid minimal medium with 1% cellobiose or glucose as the sole carbon source. The growth curve was determined by measuring the absorbance at 600 nm and reported as the mean of three independent experiments. For plate growth assay, yeast cells with serial dilutions were spotted onto the plate and incubated at 30 °C.
Disruption of the stp1, bgl1 and crt1 Genes of T. reesei
To delete the specific genes of T. reesei, the coding regions were replaced by either the T. reesei pyr4 gene or hph (hygromycin B phosphotransferase-encoding) gene. To delete stp1, the 2.7-kb pyr4 fragment with an HpaI site at both ends was amplified from pFGI (15) and then ligated into the same site of pDonorTM221 (Invitrogen) to obtain pDonorpyr4. Two DNA fragments corresponding to approximately 2 kb up- and downstream of the noncoding region of stp1 were amplified from the genomic DNA of T. reesei and ligated into pDonorpyr4 by BP cloning to yield the disruption vector pDonorstp1pyr4, which was used to transform T. reesei after linearization with I-SceI.
To delete bgl1 and crt1 genes in T. reesei, a hygromycin resistance cassette containing the gapdh (glyceraldehyde-3-phosphate dehydrogenase) promoter from T. reesei and hph gene from pRLMex30 (16) was ligated into pMD19-T (Takara, Japan) to obtain pMDhph. DNA fragments with approximately 2.0-kb 5′- and 3′-flanking sequences of the bgl1 gene or crt1 gene were successively inserted into pMDhph resulting in pMDbgl1hph and pMDcrt1hph, respectively. After linearization with SacI, the disruption vector pMDbgl1hph was used to transform WT TU-6 and the Δstp1 strain to obtain the Δbgl1 and Δstp1Δbgl1 mutants, respectively. Similarly, the deletion cassette for crt1 was released from pMDcrt1hph by SmaI digestion and used to transform TU-6 and Δstp1 to obtain Δcrt1 and Δstp1Δcrt1, respectively. To achieve the overexpression of crt1, the coding sequences of crt1 were placed under a constitutive pki promoter. DNA fragments corresponding to the pki promoter and cbh2 terminator from T. reesei were amplified from pRLMex30 and ligated into pUC19 to obtain pUCpki-cbh2. The coding sequence of crt1-gfp was further inserted to generate pUCpki-crt1-gfp-cbh2, which was used to co-transform the TU-6 strain with pFGI.
Transformation of T. reesei was carried out essentially as described previously (12). Transformants were selected on minimal medium either for uridine prototroph or for resistance to hygromycin (120 μg/ml). Anchored PCR and Southern blot analysis were used to verify the correct integration events.
High Performance Liquid Chromatography and High Performance Ion Chromatography Analyses
Analysis of cellobiose content in the medium by HPLC was performed as follows. Samples were desalted by incubating with bed resin TMD-8 (Sigma) overnight and filtered with a 0.22-μm membrane. Then the salt-free supernatants were applied onto a Bio-Rad Aminex HPX-42A carbohydrate column and analyzed by LC-10AD HPLC (Shimadzu, Japan) equipped with a RID-10A refractive index detector. The column was maintained at 78 °C and eluted with double-distilled water at a flow rate of 0.4 ml/min.
For cellobiose transport assays, the respective strains pre-cultured on glycerol were harvested by filtration and transferred to the same medium but with no carbon source for 1 h with shaking to maximally avoid the interference from membrane associated β-glucosidase-mediated hydrolysis. Mycelia used for sophorose transport assay was similarly pre-cultured but transferred to the medium with 1% lactose for 6 h with shaking. Mycelia were then harvested and washed with the culture medium without any carbon source. After extensive washing with 20 mm sodium citrate buffer (pH 5.0), mycelia were resuspended in the same buffer plus cycloheximide (75 μg/ml) and 150 μm cellobiose or 80 μm sophorose. The amount of sugar remaining in the supernatant at indicated time points was determined by Dionex ICS-5000 high performance ion chromatography) (Thermo Scientific) with a Thermo PA10 column eluted with 50 mm NaOH at a flow rate of 0.7 ml/min.
Fluorescence Microscopy
S. cerevisiae W303 strain carrying pRS426ADHstp1-gfp and pRS426CYCcrt1-gfp plasmids expressing Stp1 and Crt1 with C-terminal GFP fusions, respectively, were cultured on synthetic complete medium plate with 1% glucose as carbon source. A single colony was then transferred onto a piece of cover glass and resuspended with drops of TE buffer, and fluorescence images were taken using a Nikon Eclipse 80i fluorescence microscope.
Nucleic Acid Isolation and Hybridization
Fungal mycelia were harvested by filtration, washed with water, and frozen in liquid nitrogen. Fungal genomic DNA was extracted according to the instructions of E.Z.N.A.TM Fungal DNA Miniprep Kit (Omega Biotech). Total RNA was extracted with the TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Southern hybridization and Northern analysis were performed with the digoxigenin nonradioactive system from Roche Applied Science as described previously (12). Relative transcription levels were analyzed semiquantitatively by densitometry using ImageJ software. The values were normalized by densitometry of the cbh1 signal to that of the 18S rRNA control.
Quantitative RT-PCR
Total RNAs were further treated with TURBO DNA-free kit (Ambion) to remove DNA according to the manufacturer's instructions. Reverse transcription was carried out using the PrimeScript RT reagent kit (Takara) according to the manufacturer's instructions. Quantitative PCR were performed on a Bio-Rad myIQ2 thermocycler (Bio-Rad). Amplification reactions were performed in triplicate with a total reaction volume of 20 μl using the SYBR Green Supermix (Takara) with a 250 nm concentration each of forward and reverse primers and template cDNA. Data analysis was performed using the Relative Quantitation/Comparative CT (ΔΔCT) method and was normalized to the endogenous control actin with expression on glycerol as the reference sample.
Transcriptome Analysis
Mycelia of WT were precultured on glycerol and transferred to no carbon source for 2 h, or to glucose and Avicel for another 7.5 h. The Δstp1 strain was similarly transferred to Avicel for 7.5 h after preculture on glycerol. Total RNAs were extracted using TRIzol reagent according to the manufacturer's instructions, and then purified using the DNase I (Takara) to remove DNA. The RNA quality and quantity were determined using a Nanodrop spectrophotometer (Eppendorf). RNA sequencing (RNA-Seq; quantification) was performed by staff at the Beijing Genome Institute (BGI; Shenzhen, China). Poly(A) mRNA from total RNA was purified from total RNA using oligo(dT) magnetic beads and fragmented with buffered zinc solution. Hexamer primers were used to synthesize first-strand cDNA and second-strand cDNA from these short fragments using reverse transcriptase and DNA polymerase I. Short double-stranded cDNA fragments were purified with QiaQuick PCR extraction kit (Qiagen), resolved with EB buffer for end reparation and adding poly(A), and then ligated to sequencing adapters. After purification by agarose gel electrophoresis, suitable fragments were enriched by PCR amplification, and the library was sequenced using Illumina HiSeqTM 2000. Clean reads were deposited in the NCBI Sequence Read Archive under the accession number of SRP024316. Gene expression levels were calculated using the reads per kilobase per million reads (RPKM) method (17), thereby eliminating the effects of different gene lengths and sequencing levels so that the calculated gene expressions can be directly compared among samples. Log2Ratio indicates the degree of differential expression between two samples and was the ratio of RPKM values for the treatment and control samples. The false discovery rate was used to determine the p value threshold in multiple testing (18). False discovery rates ≤ 0.001 were used to judge the significance of values. Gene accession numbers were annotated according to version 2 of the T. reesei genome assembly, and ambiguous cases were annotated manually. Proteins involved in protein processing and secretion were referred to those described by Foreman et al. (19), and secretion signal peptide sequence was predicted by SignalP3.0.
Phylogenetic Analysis
Amino acid sequences of orthologs of Stp1 and Crt1 were obtained from online databases. Multiple sequence alignments were performed with ClustalW (20), and the neighbor-joining tree was generated with Mega 5 (21) with 1000 bootstraps.
Cellulase and Protein Analysis
The assays of cellulase activity were carried out in 200 μl of reaction mixture containing 50 μl of culture supernatant and 50 μl of the respective substrates (5 mm p-nitrophenol-d-cellobioside (pNPC) plus 0.1% σ-gluconolactone, 5 mm p-nitrophenyl-β-d-glucopyranoside (pNPG), 1% sodium carboxymethyl cellulose (CMC-Na)) in 50 mm sodium acetate (pH 5.0). The reactions were incubated for 30 min at 45 °C for analyzing cellobiohydrolase activity and β-glucosidase activity, and at 50 °C for CMCase activity analysis. The cellulase activity unit is defined as the amount of enzyme that liberates 1 μmol of pNP or glucose permin. Determination of total Avicel hydrolysis activity was performed at 50 °C in 50 mm sodium acetate (pH 5.0) with 1% Avicel cellulose as substrate. One unit of Avicelase activity is defined as the amount of enzyme that degrades 1 g of Avicel in 48 h. Measurement of xylanase activity was performed as described by Guo et al. (22) with incubation temperature at 50 °C.
SDS-PAGE and Western blotting were performed according to standard protocols (23). Detection of cellobiohydrolase CBH1 was performed by immunoblotting using a polyclonal antibody raised against amino acids (426–446) of CBH1 as described previously (12).
RESULTS
Isolation of a MFS Sugar Transporter Capable of Transporting Cellobiose
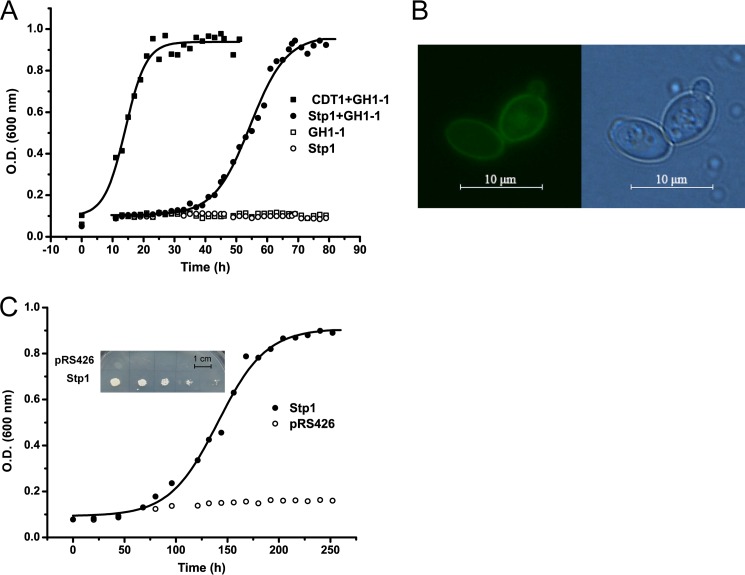
To identify the uptake system of cellobiose in T. reesei, we took advantage of the inability of S. cerevisiae to assimilate cellobiose. Expression of a functional cellobiose transporter together with an intracellular β-glucosidase, however, enables S. cerevisiae to grow with cellobiose as the sole carbon source (9). Several candidate genes encoding putative MFS sugar transporters in T. reesei with increased transcription by sophorose were thus co-expressed with gh1-1 encoding an intracellular β-glucosidase from N. crassa in S. cerevisiae. Homologs of the well characterized cellobiose transporters CDT-1 and CDT-2 from N. crassa were also included. Growth assays of the engineered yeast strains showed that, in contrast to the negligible growth of yeast strains expressing β-glucosidase GH1-1 only, co-expression of a MFS sugar transporter (Tr_47710), hereafter called Stp1, and GH1-1 enabled the transformed yeast cells to grow on cellobiose-containing minimal medium, indicating that Stp1 is capable of transporting cellobiose (Fig. 1A). The growth is, however, significantly delayed compared with the control strain expressing the reported high affinity cellobiose transporter CDT1 from N. crassa although a similar yield of biomass could be finally obtained.
FIGURE 1.
Identification of Stp1 (Tr_47710) capable of transporting cellobiose and glucose. A, cellobiose-mediated growth of S. cerevisiae W303 expressing Stp1 (Tr_47710), CDT1 (NCU00801), or no transporter. Strains also express the intracellular β-glucosidase GH1-1 (NCU00130) as indicated. B, intracellular localization of Stp1. GFP fused at the C terminus of Stp1 was used as a reporter to visualize their subcellular localization. Left, fluorescent image of GFP-fused Stp1; right, phase-contrast image of yeast cells. C, glucose-mediated growth of S. cerevisiae EBY.VW4000 expressing Stp1. Serial dilutions of yeast cells expressing Stp1 or containing the control empty vector were spotted on the synthetic complete agar plate for 6 days (insets) or inoculated in liquid synthetic complete medium with glucose as the sole carbon source.
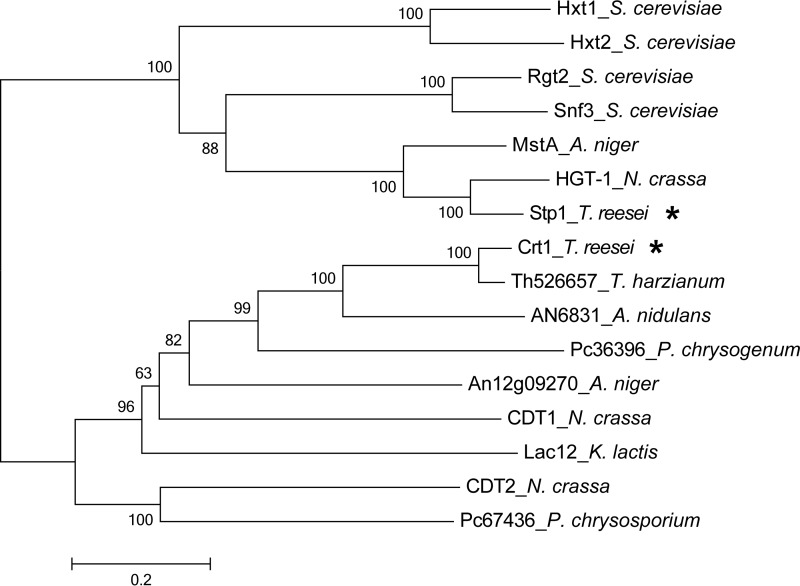
The 1665-bp cDNA sequence of Stp1 had a deduced product of 554 amino acids and was predicted to be a 12-transmembrane domain sugar transporter belonging to the MFS with N and C termini both located on the intracellular side. The expressed Stp1 with its C terminus fused with a green fluorescent protein (GFP) was found to be largely dispersed at the yeast cell periphery (Fig. 1B), indicating the membrane localization of Stp1. A high degree of similarity between Stp1 and fungal glucose transporters was observed by means of the BLASTX algorithm. Phylogenetic analysis further revealed that Stp1 did not group with the well characterized cellobiose transporters from N. crassa (CTD1 and CTD2), but rather was closely related to some characterized glucose transporter proteins including HGT-1 from N. crassa (24), MstA from Aspergillus niger (25), as well as glucose sensors Rgt2 and Snf3 from S. cerevisiae (26) (Fig. 2).
FIGURE 2.
Phylogenetic analysis of Stp1 and Crt1 and their homologs. Sequence alignments were performed with ClustalW, and the neighbor-joining tree was generated with Mega 5. Numbers on the tree branches represent the bootstrap support calculated per 1000 bootstrap replicates. Stp1 and Crt1 are indicated by stars.
To test whether Stp1 is also capable of transporting glucose, it was expressed in a yeast strain (EBY.VW4000) with all hexose transporter genes deleted, and therefore glucose consumption and transport activity are completely abolished (11). As expected, no growth of the EBY.VW4000 carrying an empty vector was observed with glucose as the sole carbon source. In contrast, expression of Stp1 enabled the growth of strain EBY.VW4000, indicating that Stp1 is also functional to transport glucose (Fig. 1C).
The Absence of Stp1 Results in Differential Cellulolytic Response in T. reesei on Cellulose versus on Cellobiose
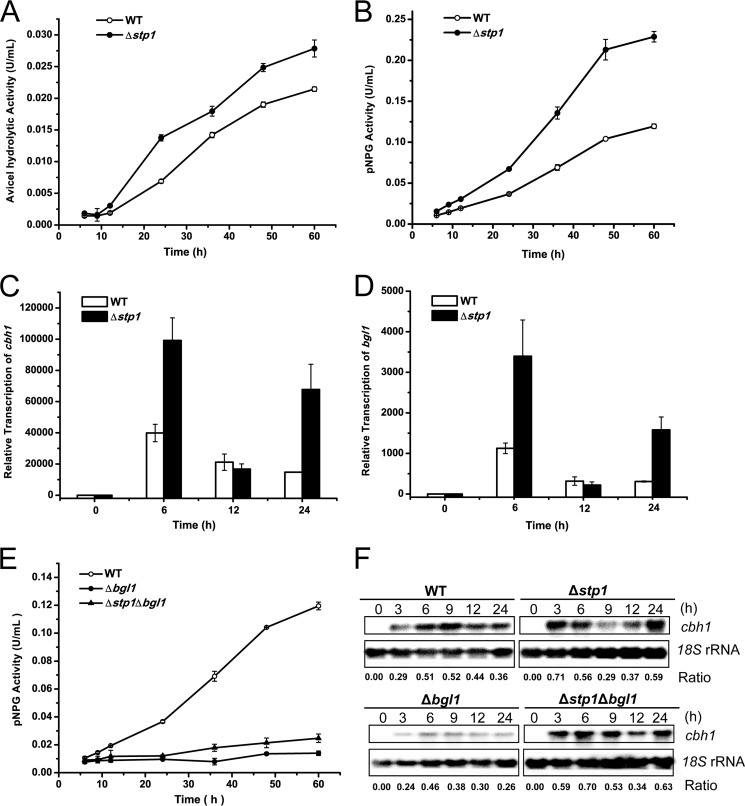
To investigate the functional involvement of the sugar transporting activity associated with Stp1 in cellulase gene expression in T. reesei, stp1-null mutant (Δstp1) was generated by replacing the coding region of stp1 with the orotidine-5-decarboxylase gene pyr4 in T. reesei TU-6. Although the Δstp1 strain grew at a rate lower than that of WT on glucose, it displayed a growth rate similar to that of WT on cellobiose although the final cell yield was lower than WT (data not shown). Analysis of Avicel and pNPG hydrolytic activity of the supernatant from the Δstp1 culture on cellulose demonstrated that the absence of Stp1 resulted in a significant increase in extracellular cellulase and β-glucosidase activity (Fig. 3, A and B). Examination of the endogenous mRNA by quantitative RT-PCR revealed that, compared with WT, deletion of stp1 enhanced the productive expression of cbh1 and bgl1 on cellulose (Fig. 3, C and D). To determine the relative contribution by the increased extracellular β-glucosidase activity to the enhanced cellulolytic response to cellulose in the Δstp1 strain, simultaneous deletion of the major extracellular β-glucosidase encoding gene, bgl1, was carried out, and the transcriptional response of the Δstp1Δbgl1, Δbgl1 strains to cellulose was compared. Whereas the absence of Bgl1 dramatically decreased the extracellular β-glucosidase activity (Fig. 3E), a strain carrying deletions for stp1 and bgl1 showed relative expression levels of cbh1 similar to that of the Δstp1 strain (Fig. 3F), indicating that the observed transcriptional response to cellulose was specific for the absence of Stp1, although the exact mechanism awaits further elucidation.
FIGURE 3.
Disruption of stp1 results in enhanced cellulase induction on cellulose. A and B, extracellular Avicel hydrolytic activity and β-glucosidase activity of the supernatant from the WT and Δstp1 cultures on 1% (w/v) Avicel for the indicated time period. C and D, gene expression of cbh1 and bgl1 analyzed by quantitative RT-PCR after induction on Avicel. Relative gene expression levels were normalized to 1 when incubated with glycerol. Expression levels of actin were used as an endogenous control in all samples. Values are the mean of three biological replicates. Error bars are the S.D. from these replicates. E, extracellular β-glucosidase activity from the supernatant of WT T. reesei and strains lacking Bgl1 on 1% Avicel. F, Northern blot analysis of cbh1 mRNA of WT, Δstp1, Δbgl1, and Δstp1Δbgl1 strains after induction for the indicated time period on Avicel. The values below the panels indicate the ratio of the intensity of the cbh1 signal as measured by densitometry to that of the reference 18S rRNA.
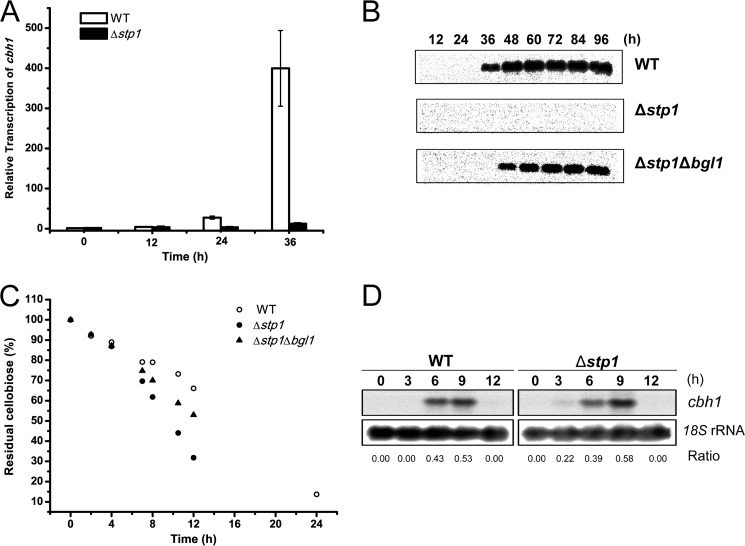
Because low concentrations of cellobiose or inhibition of extracellular hydrolysis of cellobiose by β-glucosidase inhibitor have been shown to trigger cellulase gene expression in T. reesei (3, 6, 7, 12), we also determined the effect of the absence of Stp1 on cellulase induction by cellobiose. In contrast to WT, the productive expression of cbh1 was compromised with cellobiose as the sole carbon source (Fig. 4, A and B). To find out whether the defective induction as displayed by the Δstp1 strain on cellobiose was due to the inefficient intracellular transport of cellobiose, the amount of residual cellobiose in the culture supernatant was analyzed. In comparison with WT, cellobiose in the culture supernatant of Δstp1 mutant was consumed at a much faster rate (Fig. 4C). Further examination of the extracellular pNPG hydrolytic activity revealed a 2-fold higher activity in the absence of Stp1 during the initial growth (data not shown), suggesting that the more rapid consumption of cellobiose may well result from the increased hydrolysis by β-glucosidases. In accordance with this assumption, simultaneous deletion of bgl1 slowed down the consumption rate of cellobiose and partially restored the productive expression of cbh1 in the Δstp1Δbgl1 strain (Fig. 4, B and C). Moreover, the relative expression level of cbh1 was not compromised by the absence of Stp1 in response to sophorose (Fig. 4D), indicating that Stp1 may not play a crucial role in transmitting the inducing signal via its transporting activity.
FIGURE 4.
Disruption of stp1 comprised cellulase induction on cellobiose but not on sophorose. A, quantitative RT-PCR analysis of gene expression of cbh1 after induction on 0.5% (w/v) cellobiose for different time periods as indicated. Values are the mean of three biological replicates. Error bars are the S.D. from these replicates. B, Western blot analysis of CBH1 secreted into the culture supernatant on 0.5% cellobiose. An equal amount of culture supernatant relative to biomass was loaded for all strains. C, HPLC analysis of residual cellobiose in the culture supernatant of WT, Δstp1, and Δstp1Δbgl1 strains cultured on 0.5% cellobiose for the indicated time period. Values are average from three independent experiments. D, Northern blot analysis of cbh1 mRNA of WT and Δstp1 strains induced with 1 mm sophorose in a resting cell system. The values below the panels indicate the ratio of the intensity of the cbh1 signal as measured by densitometry to that of the reference 18S rRNA.
Transcriptional Profile of the Δstp1 Mutant on Avicel
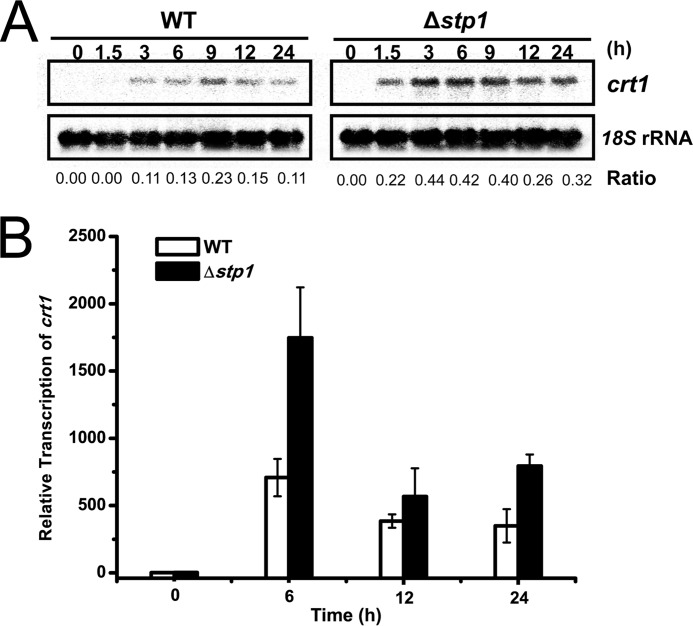
To better understand the differences in the process by which WT and the Δstp1 mutant sense and respond to cellulose, the full genomic response in both strains were assessed using next-generation RNA-Seq to profile genome-wide mRNA abundance when exposed to Avicel. 7.5 h after transfer to Avicel, 755 genes were identified that were significantly induced in WT cultures compared with those shifted to either no carbon or glucose conditions (-fold change ≥2). The 755-gene set was thus referred as “Avicel regulon,” which included 17 predicted cellulases and 28 predicted hemicellulase genes (supplemental Database S1 and supplemental Table S1). Additional genes in the regulon included 98 genes encoding secreted proteins, 21 genes encoding putative transcription factors, 31 genes encoding putative transporters with 17 putative MFS sugar transporters, and 30 genes encoding proteins involved in protein processing and secretion. Also included in the Avicel regulon were 282 genes annotated as conserved hypothetical proteins or with no predicted function. Of note, stp1 was not included in the Avicel regulon because it was down-regulated by approximately 6-fold on Avicel compared with that on no carbon. The stp1 deletion strain showed an expression profile somewhat different from that of WT on Avicel. A total of 671 genes were differentially expressed (-fold change ≥2) compared with WT on Avicel, including 139 up-regulated genes and 532 down-regulated genes. In addition, a total of 1099 genes showed a moderate difference in the expression levels (1< -fold change <2) including 166 moderately up-regulated genes and 933 moderately down-regulated genes in the absence of Stp1 (Fig. 5A). Hierarchy clustering of expression patterns for the genes within the Avicel regulon resulted in the identification of five major groups of genes with similar expression patterns: Stp1-up-regulated, Stp1-down-regulated, Stp1-moderately up-regulated, Stp1-moderately down-regulated, and Stp1-independent (Fig. 5, A and B, and supplemental Database S1). Whereas 474 genes in the regulon remained a WT induction (Stp1-independent) and 122 other genes had a significant down-regulation in the Δstp1 mutant (Stp1-up-regulated), 39 genes in the Stp1-down-regulated group including 5 cellulase genes, 2 hemicellulase genes, 2 proteins encoding transporters, 16 genes encoding hypothetical proteins, and 15 genes encoding other proteins were found to have expression levels greater than in WT. Another 59 genes dominated by 11 cellulase genes, 6 hemicellulase genes, and 6 genes encoding putative transporters (Stp1-moderately down-regulated group) were moderately up-regulated by the absence of Stp1. The up-regulation of the above genes including xyr1, which has been shown to play a major role in the regulation of cellulases in T. reesei (27), may contribute to the observed enhanced cellulolytic response in the Δstp1 mutant on Avicel. Of specific interest, the two transporters (Tr_80058 and Tr_3405) up-regulated in the absence of Stp1 were annotated as MFS sugar transporters, and the up-regulation of Tr_3405 within the WT Avicel regulon was confirmed by Northern blot and quantitative RT-PCR (Fig. 6).
FIGURE 5.
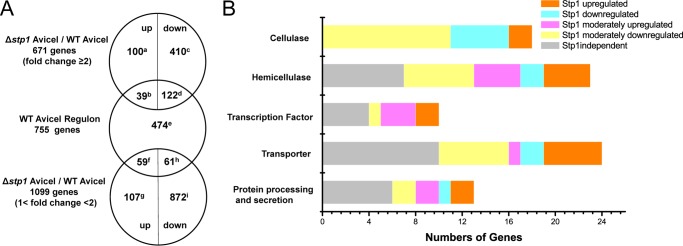
Differential transcription pattern of WT and Δstp1 strain in response to Avicel as measured by RNA-Seq. A, summary of genes differentially expressed in the absence of Stp1 versus WT on Avicel. The profiling data were filtered to obtain the set of 2974 genes with a -fold change of 2 or more on Avicel compared with under no carbon conditions. Among them, 2301 genes displaying the highest expression on glucose compared with no carbon were then subtracted from the above set except those 82 genes still showing a lower transcription level (<2) compared with on Avicel. This identified a 755-gene set as the Avicel regulon. Genes displaying a -fold change ≥2 and between 1 and 2 in the absence of Stp1 on Avicel were further compared with the regulon to identify the genes that were common and distinct among the sets. Numbers refer to the number of genes in the set. The full corresponding genes are listed in supplemental Database S1. B, distribution of genes within the Avicel regulon in functional categories among groups identified based on the relative dependence on Stp1. Stp1-up-regulated, Stp1-down-regulated, Stp1-moderately up-regulated, Stp1-moderately down-regulated and Stp1-independent groups are represented by 122d, 39b, 61h, 59f, and 474e in A, respectively.
FIGURE 6.
Deletion of stp1 leads to increased transcription of Tr_3405 on Avicel. Northern blotting (A) and quantitative RT-PCR (B) were both performed to analyze gene expression of Tr_3405 of WT and Δstp1. The values below the panels in A indicate the ratio of the intensity of the crt1 signal as measured by densitometry to that of the reference 18S rRNA. Values in B are the mean of three biological replicates. Error bars are the S.D. from these replicates.
Tr_3405 Is Essential for Cellulase Gene Expression in T. reesei
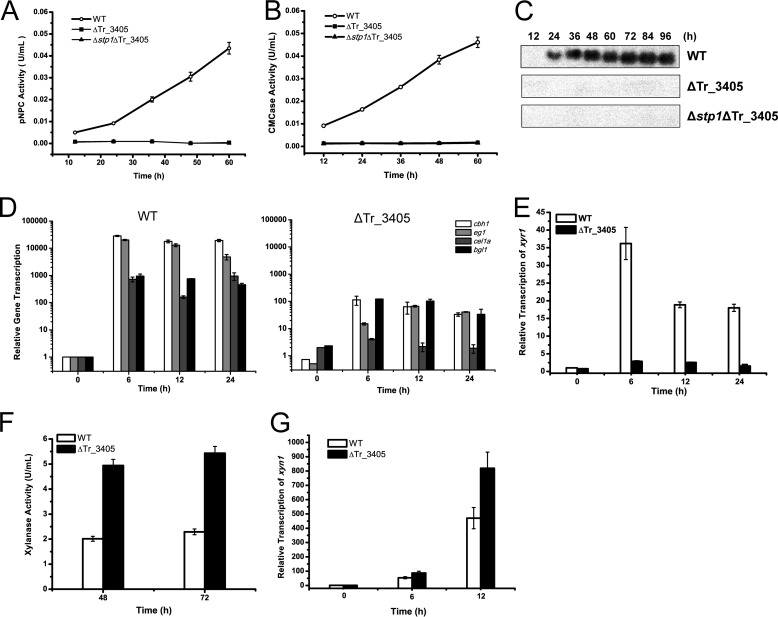
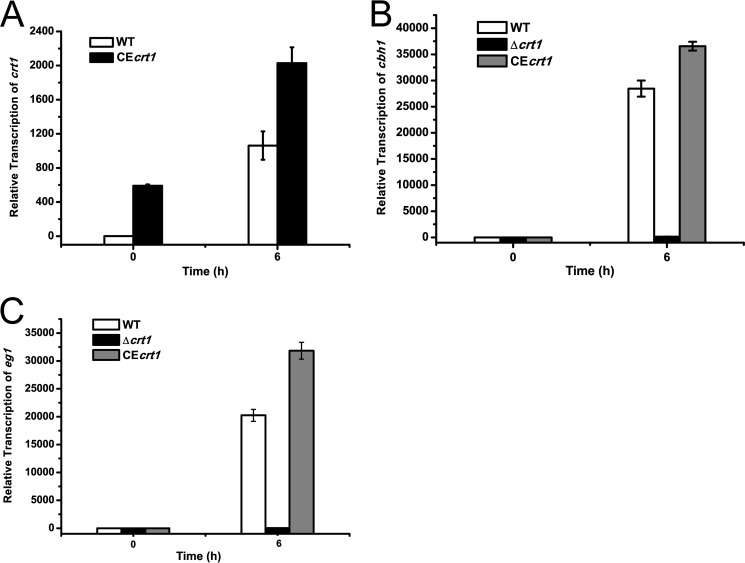
To assess the involvement of the above putative sugar transporters in the cellulolytic response of T. reesei, mutants lacking the coding sequences of Tr_3405 and Tr_80058 were obtained by targeted gene replacement in WT and the Δstp1 strains. In contrast to Tr_80058, the Tr_3405 deletion strains exhibited severe growth defect on Avicel, but not on glucose or glycerol (data not shown). The deletion strains pregrown on glycerol and then transferred to Avicel showed no detectable activity when assayed for cleavage of pNPC and CMC-Na in the culture supernatant (Fig. 7, A and B). In accordance with a recent report (28), the cellulolytic response of the mutant strain was also compromised on lactose (data not shown). Correspondingly, secretion of CBH1 into the culture supernatant from the deletion strains in response to Avicel was abolished as assayed by Western blotting (Fig. 7C). Transcriptional analysis revealed that the defective cellulase gene induction occurred on the transcription level because the deletion strain did not show any significant induction of cbh1, eg1, bgl1, or cel1a expression (Fig. 7D). Compared with WT, the induced transcription of xyr1 was also significantly reduced on cellulose in the mutant strain (Fig. 7E). However, when the deletion mutant was shifted to xylan, it displayed enhanced hemicellulase activity and expression levels of xylanase gene xyn1 (Fig. 7, F and G). Tr_3405 was provisionally named crt1, for cellulose response transporter. To test whether the enhanced expression of cellulase genes in the Δstp1 strain was in part due to the increased expression level of crt1, a WT strain constitutively expressing crt1 driven by the pki promoter was constructed (CEcrt1). In comparison with WT, the expression of crt1 in the CEcrt1 strain occurred early on glycerol and reached a level 2-fold higher than that of WT 6 h after shift to Avicel (Fig. 8A). Simultaneous examination of the endogenous cbh1 and eg1 mRNA revealed a 1.5-fold higher expression in the CE strain (Fig. 8, B and C).
FIGURE 7.
Disruption of crt1 (Tr_3405) abolished cellulase gene expression on Avicel but not on xylan. A and B, extracellular hydrolytic activity on pNPC and CMC-Na from the culture supernatant of WT, ΔTr_3405, and Δstp1ΔTr_3405 strains for the indicated time period on 1% (w/v) Avicel. C, Western blot analysis of CBHI secreted into the culture supernatant of the above three strains. An equal amount of culture supernatant relative to biomass was loaded for all strains. D and E, gene expression of cbh1, eg1, cel1a, bgl1, and xyr1 analyzed by quantitative RT-PCR. Relative gene expression levels were normalized to 1 when incubated with glycerol. Expression levels of actin were used as an endogenous control in all samples. Values are the mean of three biological replicates. Error bars are the S.D. from these replicates. F and G, extracellular xylanase activity from the culture supernatant from WT and the ΔTr_3405 strain (F) and gene expression level of xyn1 as determined by quantitative RT-PCR (G) when induced on 1% (w/v) xylan.
FIGURE 8.
Increased gene expression of crt1, cbh1, and eg1 of CEcrt1 strain. Quantitative RT-PCR was performed to analyze gene transcription of crt1 (A), cbh1 (B), and eg1 (C) of T. reesei WT, Δcrt1, and CEcrt1 strains after induction on 1% Avicel for the indicated time period.
Crt1 Mediates the Cellulolytic Signaling Independent of Sugar Transporting Activity
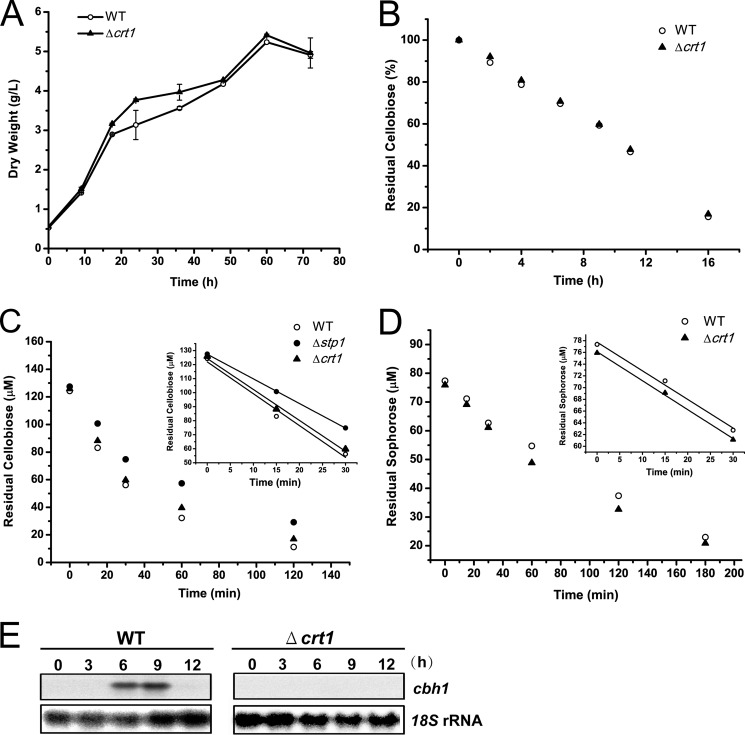
The 1530-bp cDNA sequence of Crt1 had a deduced product of 509 amino acids. Similar to Stp1, Ctr1 was predicted to be a sugar transporter with 12 deduced transmembrane helices belonging to the MFS family. But unlike stp1, the relative expression level of crt1 was significantly induced after shift to Avicel compared with no carbon. Orthologs of Crt1 are widely distributed across filamentous ascomycete fungi. Phylogenetic analysis showed that Crt1 roughly clustered with cellobiose transporters CDT1 and CDT2 from N. crassa and appeared to be a sister clade to lactose permease LAC12 from Kluyveromyces lactis (Fig. 2). In contrast to CDT1 and CTD2, whose absence has been shown to have a significant effect on cellobiose consumption (9), a T. reesei strain carrying a deletion for crt1 grew and consumed cellobiose at a rate similar to that of WT with cellobiose as the sole carbon source (Fig. 9, A and B), indicating that the absence of crt1 does not interfere with the assimilation of cellobiose. Compared with Δstp1, hardly any difference in extracellular pNPG hydrolytic activity was observed between WT and the Δcrt1 strain (data not shown). Reconstitution of Crt1 into S. cerevisiae did not support the growth on cellobiose as Stp1 did, although Crt1 fused with GFP was properly localized on the membrane (data not shown). Further determination of cellobiose consumption by WT and the mutant strains in a resting system allowing for no growth and protein synthesis revealed that, compared with Stp1 whose absence slightly decreased the uptake of cellobiose, the absence of Crt1 displayed hardly any difference in the uptake of cellobiose from that of WT (Fig. 9C). Moreover, a similar uptake of sophorose was obtained for WT and the Δcrt1 strain (Fig. 9D). In contrast to Stp1, the absence of crt1 abolished the transcriptional response to sophorose (Fig. 9E). Altogether, these data suggest that Crt1 plays an essential role in transmitting the cellulase gene-inducing signal independent of intracellular sugar delivery.
FIGURE 9.
The absence of Crt1 did not interfere with the overall sugar uptake but compromises cellulase induction by sophorose. A, growth curves of T. reesei WT and the Δcrt1 strain with 0.5% (w/v) cellobiose as the sole carbon source. Error bars indicate S.D. B, HPLC analysis of residual cellobiose in the culture supernatant of WT and Δcrt1 strain cultured on 0.5% cellobiose. Values are the mean of three independent replicates. C and D, cellobiose and sophorose consumption by T. reesei. The indicated T. reesei strains were incubated with the respective sugars in a buffered system with cycloheximide (75 μg/ml) for the indicated time intervals. Values are the mean of two independent experimental replicates. Insets show the sugar consumption during the initial 30 min. E, Northern blot analysis of cbh1 mRNA of WT and Δcrt1 strains induced with 1 mm sophorose in resting cell system with 18S rRNA as the reference.
DISCUSSION
Successful perception of the existence of insoluble cellulose outside the cell is critical for T. reesei to initiate the rapid production of the enzymatic machinery needed to sustain its growth on the breakdown products from cellulose. In this study, we identified a MFS transporter Stp1 capable of delivering cellobiose as shown in a reconstructed cellobiose transport system in S. cerevisiae. Deletion of stp1, however, enhanced the induced expression of cellulases on crystalline cellulose. We further identified another potential MFS sugar transporter that is critical for expression of cellulase genes in response to cellulose or lactose. To the best of our knowledge, this is the first report of a potential sugar transporter critical for cellulolytic response to cellulose in T. reesei.
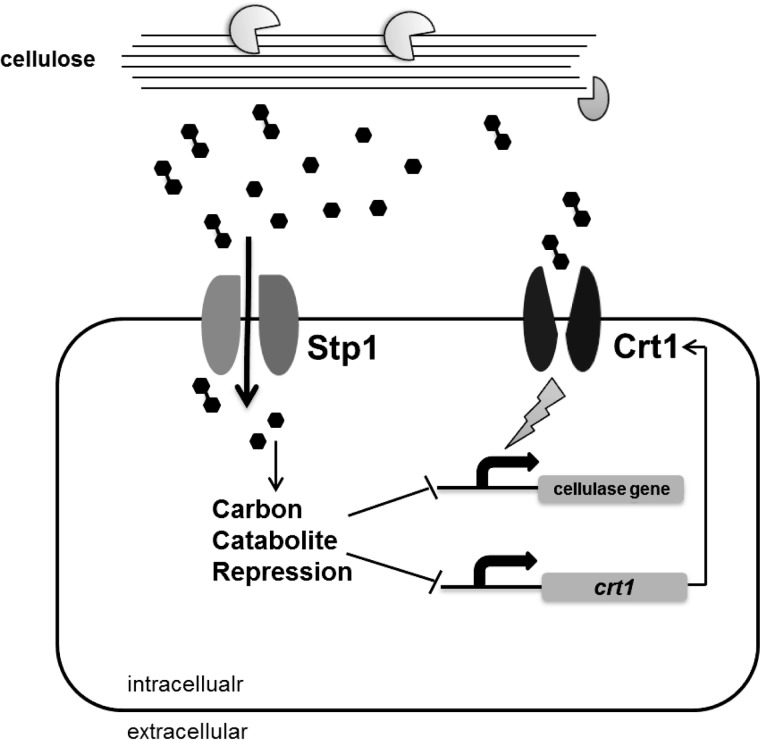
A low efficiency uptake system for cellobiose whose permease activity can be inhibited by glucose has been shown to be present in T. reesei (3, 6) though its identity and its role in cellulase induction have been elusive. In this study, Stp1 (Tr_47710) was found to be capable of transporting both cellobiose and glucose. It was previously found to be significantly up-regulated by sophorose and was annotated as a high affinity glucose transporter (19). Indeed, although the structural basis for Stp1-mediated transport of both glucose and cellobiose warrants further elucidation, the observation that the Δstp1 strain displayed a more severe growth defect on glucose indicated that Stp1 more likely participated in the assimilation of glucose in T. reesei. The observed delay in the uptake of cellobiose in the absence of Stp1 may also partly result from the competitive inhibition of accumulated glucose during the transport assay. Our results further revealed that deletion of stp1 did not compromise the cellulolytic response to sophorose, but resulted in differential responses of the Δstp1 mutant to cellulose and cellobiose induction. Compared with a 755-gene set that specifically responded to crystalline cellulose (Avicel regulon) in WT T. reesei, the absence of Stp1 resulted in a different expression pattern in response to Avicel with a bulk of genes outside the regulon displaying differential expression. While the effect of these differentially expressed genes on the enhanced induction in the Δstp1 strain awaits further dissection, the increased expression of almost all cellulase and hemicellulase genes as well as the key cellulolytic regulator xyr1 and crt1 identified in this study may well account for the observed enhanced cellulolytic response in the absence of Stp1. Another hypothesis regarding the enhanced cellulase gene expression on Avicel is that the relief of carbon catabolite repression is related to Stp1-mediated glucose uptake during incubation with cellulose. In the presence of cellobiose, however, the inducing effect of cellobiose was counteracted by the rapid consumption of cellobiose, probably resulting from the increased extracellular β-glucosidase-mediated hydrolysis in the absence of Stp1.
Other than direct import of the degradation products from cellulose into cytoplasm, sensing these products may also involve a specific membrane anchored receptor (6, 29, 30). In the present study, a putative sugar transporter Crt1 belonging to the major facilitator superfamily was also identified. Despite the complete loss of induced cellulase gene expression and cellulase secretion in the Δcrt1 strain in response to cellulose or lactose, growth and hemicellulase expression were normal on xylan. Moreover, the absence of Crt1 abolished the potent induction of cellulase gene expression by sophorose. These findings correlate well with the observation that transcription of xyr1, which has been shown to be necessary for regulation of almost all cellulase and hemicellulase genes, was compromised in the Δcrt1 strain in response to cellulose, but not to xylan. Orthologs of crt1 are found to exist in genomes of many filamentous ascomycete fungi, particularly those in association with plants, suggesting conserved regulatory mechanisms involved in cellulose destruction. Deletion for one of the orthologs cdt2 in N. crassa also compromises its growth on cellulose (9); but unlike CDT2, the absence of Crt1 does not seem to interfere with the assimilation of cellobiose in T. reesei. Our data are in accordance with results published while this work was in preparation that Crt1 participates in the lactose-induced cellulase gene expression; but our work contrasts with their point that Ctr1 is dispensable for sophorose-mediated induction (28). The reason for this discrepancy is at present unclear but may result from different strains used for construction of the deleted mutants where a Δtku70 strain was used in the reported study. We also noticed that unpublished data from another report suggested that Tr_3405 plays a pivotal role in the cellulase induction without being directly involved in the uptake of cellulase inducers or lactose (31). These notions are consistent with our findings that the absence of Ctr1 resulted in no apparent difference in the uptake of cellobiose when assayed within a short period in a resting system allowing for no growth and protein synthesis. Although extracellular hydrolysis of cellobiose by β-glucosidases associated with plasma membrane other than Bgl1 may still contribute to the observed decrease of cellobiose in the transport assay, the fact that Crt1 does not support the growth of reconstructed S. cerevisiae on cellobiose together with the observation that similar consumption of sophorose occurred for WT and the Δcrt1 strain suggested that the overall intracellular sugar delivery is not compromised in the absence of Crt1. The reported interference with the consumption of lactose by the absence of Tr_3405 was most likely due to the compromised extracellular β-galactosidase activities because the assay was carried out in a culturing system lasting for up to 40 h (31). At this stage of analysis, however, we cannot draw the conclusion that Crt1 does not participate in transporting cellobiose or sophorose given that Ctr1 may probably represent only a low efficiency transporter and its absence does not affect the overall transporting activity. Nevertheless, the fact that the Δcrt1 strain displayed nearly the same assimilation of cellobiose and sophorose as WT did suggests that Crt1 may largely transmit the inducing signal independent of intracellular sugar delivery (Fig. 10). Although it has been difficult to convincingly distinguish the signaling function from the transport function, the discovery that some transporters also act as receptors, or transceptors, to achieve a signaling function rather than nutrient transport has been reported in other organisms (32–35). Similar to Stp1, the mechanism and structural basis for how Crt1 mediates the signaling from two structurally different sugars are worth detailed investigation. Further identification of residues around the substrate binding site used for transport and signaling or screening constitutively active mutants in the absence of agonists would help elucidate the molecular mechanism of signal sensing and transmitting by Crt1 in regulation of genes involved in plant cell wall deconstruction.
FIGURE 10.
Model of cellulase induction mediated by membrane sugar transporters Stp1 and Crt1. Upon induction with cellulose, cellobiose is released from cellulose by the synergistic action of exoglucanases and endoglucanases. A cellobiose and glucose permease, Stp1, somehow represses the induced expression of major cellulase genes. Crt1, however, does not seem to interfere with cellobiose assimilation by T. reesei, but plays an essential role in the cellulolytic signaling.
Acknowledgment
We thank Prof. Dr. Eckhard Boles of Goethe University Frankfurt for providing the EBY.VW4000 strain.
This work was supported by National Basic Research Program of China Grant 2011CB707402, National Natural Science Foundation Grants 31270116 and 31300059, New Century Excellent Talents in University Grant NCET-10-0546, Shandong Provincial Funds for Distinguished Young Scientists Grant JQ201108, and Independent Innovation Foundation of Shandong University Grant 2012GN021.

This article contains supplemental Database S1 and Table S1.
- MFS
- major facilitator superfamily
- CMC-Na
- sodium carboxymethyl cellulose
- pNPC
- p-nitrophenol-d-cellobioside
- pNPG
- p-nitrophenyl-β-d-glucopyranoside
- RNA-Seq
- RNA sequencing.
REFERENCES
- 1. Lynd L. R., Weimer P. J., van Zyl W. H., Pretorius I. S. (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kubicek C. P., Mühlbauer G., Grotz M., John E., Kubicek-Pranz E. M. (1988) Properties of a conidial-bound cellulase enzyme system from Trichoderma reesei. J. Gen. Microbiol. 134, 1215–1222 [Google Scholar]
- 3. Kubicek C. P., Messner R., Gruber F., Mandels M., Kubicek-Pranz E. M. (1993) Triggering of cellulase biosynthesis by cellulose in Trichoderma reesei: involvement of a constitutive, sophorose-inducible, glucose-inhibited β-diglucoside permease. J. Biol. Chem. 268, 19364–19368 [PubMed] [Google Scholar]
- 4. Kubicek C. P., Messner R., Gruber F., Mach R. L., Kubicek-Pranz E. M. (1993) The Trichoderma cellulase regulatory puzzle: from the interior life of a secretory fungus. Enzyme Microb. Technol. 15, 90–99 [DOI] [PubMed] [Google Scholar]
- 5. Mach R. L., Seiboth B., Myasnikov A., Gonzalez R., Strauss J., Harkki A. M., Kubicek C. P. (1995) The bgl1 gene of Trichoderma reesei QM 9414 encodes an extracellular, cellulose-inducible β-glucosidase involved in cellulase induction by sophorose. Mol. Microbiol. 16, 687–697 [DOI] [PubMed] [Google Scholar]
- 6. Fritscher C., Messner R., Kubicek C. P. (1990) Cellobiose metabolism and cellobiohydrolase I biosynthesis by Trichoderma reesei. Exp. Mycol. 14, 451–461 [Google Scholar]
- 7. Vaheri M. P., Vaheri M. E. O., Kauppinen V. S. (1979) Formation and release of cellulolytic enzymes during growth of Trichoderma reesei on cellobiose and glycerol. Appl. Microbiol. Biotechnol. 8, 73–80 [Google Scholar]
- 8. Mandels M., Parrish F. W., Reese E. T. (1962) Sophorose as an inducer of cellulase in Trichoderma reesei. J. Bacteriol. 83, 400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galazka J. M., Tian C., Beeson W. T., Martinez B., Glass N. L., Cate J. H. (2010) Cellodextrin transport in yeast for improved biofuel production. Science 330, 84–86 [DOI] [PubMed] [Google Scholar]
- 10. Gruber F., Visser J., Kubicek C. P., de Graaff L. H. (1990) The development of a heterologous transformation system for the cellulolytic fungus Trichoderma reesei based on a pyrG-negative mutant strain. Curr. Genet. 18, 71–76 [DOI] [PubMed] [Google Scholar]
- 11. Wieczorke R., Krampe S., Weierstall T., Freidel K., Hollenberg C. P., Boles E. (1999) Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 464, 123–128 [DOI] [PubMed] [Google Scholar]
- 12. Zhou Q., Xu J., Kou Y., Lv X., Zhang X., Zhao G., Zhang W., Chen G., Liu W. (2012) Differential involvement of β-glucosidases from Hypocrea jecorina in rapid induction of cellulase genes by cellulose and cellobiose. Eukaryotic Cell 11, 1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heckman K. L., Pease L. R. (2007) Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2, 924–932 [DOI] [PubMed] [Google Scholar]
- 14. Gietz D., St Jean A., Woods R. A., Schiestl R. H. (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20, 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gruber F., Visser J., Kubicek C. P., de Graaff L. H. (1990) Cloning of the Trichoderma reesei pyrG gene and its use as a homologous marker for a high-frequency transformation system. Curr. Genet. 18, 447–451 [DOI] [PubMed] [Google Scholar]
- 16. Mach R. L., Schindler M., Kubicek C. P. (1994) Transformation of Trichoderma reesei based on hygromycin B resistance using homologous expression signals. Curr. Genet. 25, 567–570 [DOI] [PubMed] [Google Scholar]
- 17. Mortazavi A., Williams B. A., McCue K., Schaeffer L., Wold B. (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 [DOI] [PubMed] [Google Scholar]
- 18. Benjamini Y., Drai D., Elmer G., Kafkafi N., Golani I. (2001) Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284 [DOI] [PubMed] [Google Scholar]
- 19. Foreman P. K., Brown D., Dankmeyer L., Dean R., Diener S., Dunn-Coleman N. S., Goedegebuur F., Houfek T. D., England G. J., Kelley A. S., Meerman H. J., Mitchell T., Mitchinson C., Olivares H. A., Teunissen P. J., Yao J., Ward M. (2003) Transcriptional regulation of biomass-degrading enzymes in the filamentous fungus Trichoderma reesei. J. Biol. Chem. 278, 31988–31997 [DOI] [PubMed] [Google Scholar]
- 20. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) ClustalW and ClustalX version 2.0. Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 21. Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo B., Chen X. L., Sun C. Y., Zhou B. C., Zhang Y. Z. (2009) Gene cloning, expression and characterization of a new cold-active and salt-tolerant endo-β-1,4-xylanase from marine Glaciecola mesophila KMM 241. Appl. Microbiol. Biotechnol. 84, 1107–1115 [DOI] [PubMed] [Google Scholar]
- 23. Sambrook J., Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, 3rd Ed., p. 18.54, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 24. Xie X., Wilkinson H. H., Correa A., Lewis Z. A., Bell-Pedersen D., Ebbole D. J. (2004) Transcriptional response to glucose starvation and functional analysis of a glucose transporter of Neurospora crassa. Fungal Genet. Biol. 41, 1104–1119 [DOI] [PubMed] [Google Scholar]
- 25. Vankuyk P. A., Diderich J. A., MacCabe A. P., Hererro O., Ruijter G. J., Visser J. (2004) Aspergillus niger mstA encodes a high-affinity sugar/H+ symporter which is regulated in response to extracellular pH. Biochem. J. 379, 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moriya H., Johnston M. (2004) Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc. Natl. Acad. Sci. U.S.A. 101, 1572–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stricker A. R., Grosstessner-Hain K., Würleitner E., Mach R. L. (2006) Xyr1 (xylanase regulator 1) regulates both the hydrolytic enzyme system and d-xylose metabolism in Hypocrea jecorina. Eukaryotic Cell 5, 2128–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ivanova C., Bååth J. A., Seiboth B., Kubicek C. P. (2013) Systems analysis of lactose metabolism in Trichoderma reesei identifies a lactose permease that is essential for cellulase induction. PLoS One 8, e62631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szakmary K., Wottawa A., Kubicek C. P. (1991) Origin of oxidized cellulose degradation products and mechanism of their promotion of cellobiohydrolase I biosynthesis in Trichoderma reesei. J. Gen. Microbiol. 137, 2873–2878 [Google Scholar]
- 30. Znameroski E. A., Coradetti S. T., Roche C. M., Tsai J. C., Iavarone A. T., Cate J. H., Glass N. L. (2012) Induction of lignocellulose-degrading enzymes in Neurospora crassa by cellodextrins. Proc. Natl. Acad. Sci. U.S.A. 109, 6012–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Porciuncula Jde O., Furukawa T., Shida Y., Mori K., Kuhara S., Morikawa Y., Ogasawara W. (2013) Identification of major facilitator transporters involved in cellulase production during lactose culture of Trichoderma reesei PC-3-7. Biosci. Biotechnol. Biochem. 77, 1014–1022 [DOI] [PubMed] [Google Scholar]
- 32. Kriel J., Haesendonckx S., Rubio-Texeira M., Van Zeebroeck G., Thevelein J. M. (2011) From transporter to transceptor: signaling from transporters provokes re-evaluation of complex trafficking and regulatory controls: endocytic internalization and intracellular trafficking of nutrient transceptors may, at least in part, be governed by their signaling function. BioEssays 33, 870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Donaton M. C., Holsbeeks I., Lagatie O., Van Zeebroeck G., Crauwels M., Winderickx J., Thevelein J. M. (2003) The Gap1 general amino acid permease acts as an amino acid sensor for activation of protein kinase A targets in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 50, 911–929 [DOI] [PubMed] [Google Scholar]
- 34. Popova Y., Thayumanavan P., Lonati E., Agrochão M., Thevelein J. M. (2010) Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc. Natl. Acad. Sci. U.S.A. 107, 2890–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rutherford J. C., Chua G., Hughes T., Cardenas M. E., Heitman J. (2008) A Mep2-dependent transcriptional profile links permease function to gene expression during pseudohyphal growth in Saccharomyces cerevisiae. Mol. Biol. Cell 19, 3028–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]