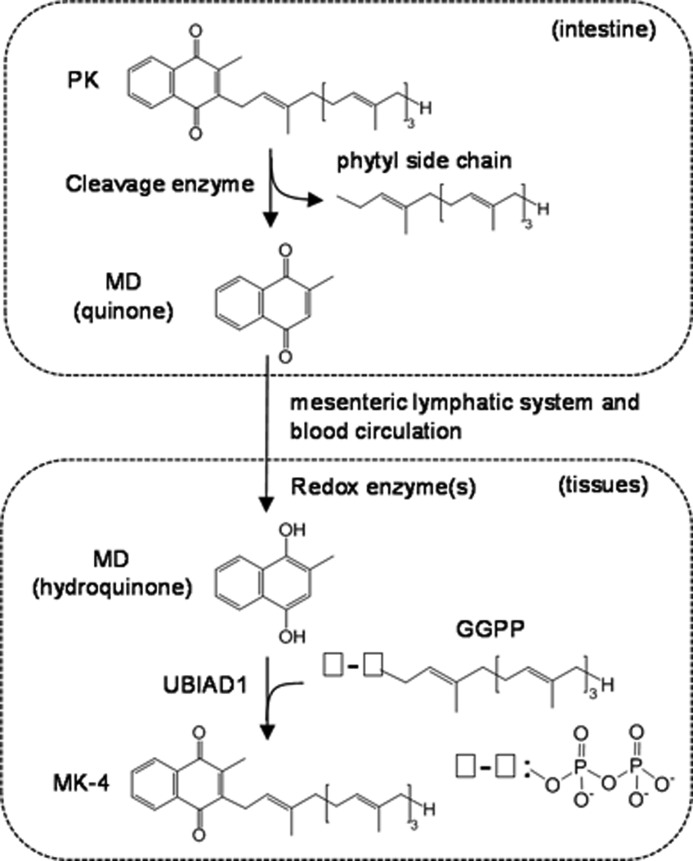
Background: Menadione is an intermediate in phylloquinone to menaquinone-4 conversion in mammals.
Results: Menadione is released from phylloquinone in the intestine and converted to menaquinone-4 in tissues after being reduced.
Conclusion: Menadione is a catabolic product of phylloquinone and circulating precursor of tissue menaquinone-4.
Significance: Determining how phylloquinone is metabolized in the body is crucial for understanding vitamin K biology.
Keywords: Enzymes, Intestine, Metabolism, Rat, Vitamin K, UBIAD1, Conversion, Menadione, Menaquinone-4, Phylloquinone
Abstract
Mice have the ability to convert dietary phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) and store the latter in tissues. A prenyltransferase enzyme, UbiA prenyltransferase domain-containing 1 (UBIAD1), is involved in this conversion. There is evidence that UBIAD1 has a weak side chain cleavage activity for phylloquinone but a strong prenylation activity for menadione (vitamin K3), which has long been postulated as an intermediate in this conversion. Further evidence indicates that when intravenously administered in mice phylloquinone can enter into tissues but is not converted further to menaquinone-4. These findings raise the question whether phylloquinone is absorbed and delivered to tissues in its original form and converted to menaquinone-4 or whether it is converted to menadione in the intestine followed by delivery of menadione to tissues and subsequent conversion to menaquinone-4. To answer this question, we conducted cannulation experiments using stable isotope tracer technology in rats. We confirmed that the second pathway is correct on the basis of structural assignments and measurements of phylloquinone-derived menadione using high resolution MS analysis and a bioassay using recombinant UBIAD1 protein. Furthermore, high resolution MS and 1H NMR analyses of the product generated from the incubation of menadione with recombinant UBIAD1 revealed that the hydroquinone, but not the quinone form of menadione, was an intermediate of the conversion. Taken together, these results provide unequivocal evidence that menadione is a catabolic product of oral phylloquinone and a major source of tissue menaquinone-4.
Introduction
Mice have the ability to convert dietary vitamin K1 (phylloquinone (PK)2) into vitamin K2 (menaquinone-4 (MK-4)) and store the latter in many tissues (1). An enzyme, UbiA prenyltransferase domain-containing 1 (UBIAD1), catalyzes this conversion (2). It has been shown that UBIAD1 has a weak side chain cleavage activity for PK, but it has a strong prenylation activity for vitamin K3 (menadione (MD)). MD has long been postulated as an intermediate or a catabolic product of PK in this conversion process (2), although it is believed to be biologically active by virtue of its conversion into MK-4 in the body (3–6). UBIAD1 is accordingly believed to generate MK-4 by the prenylation of MD in tissues, although it is not known whether UBIAD1 generates MD from PK in tissues. Thijssen et al. (7) reported that orally administered, but not subcutaneously injected, PK in humans was catabolized to MD and excreted in the form of conjugates in urine. Recently, Al Rajabi et al. (8) reported that deuterium-labeled PK-derived MD existed in the serum and urine of the rats fed a diet containing deuterium-labeled PK. These results suggest that PK generates MD in the course of intestinal absorption. To identify the locations where release of MD from PK occurs and where MD is converted to MK-4, it is necessary to identify PK-derived MD in the mesenteric lymph and/or portal vein of rats who are orally administered PK and to demonstrate a relationship between the concentrations of MD in the lymph and/or portal vein and concentrations of MK-4 in tissues.
In the present study, we conducted cannulation experiments in rats at four sites, including catheterization into the inferior vena cava (IVC), portal vein (PV), thoracic lymph duct (TLD), and bile duct (BD), to identify and measure deuterium-labeled PK (PK-d7)-derived MD in the lymph and blood of rats orally administered PK-d7. Unlike PK and MK-4, accurate measurement of MD in biological fluids by physicochemical means is difficult because of its high volatility. Thus, we developed a novel method for the measurement of MD (putative MD-d7) using rhUBIAD1 as a source of converting enzyme and deuterium-labeled geranylgeranyl pyrophosphate (GGPP-d5) as a source of the side chain in the final product (putative MK-4-d12). In this method, MD-d7 in the lymph and serum was converted to MK-4-d12, which was quantitatively detected by atmospheric pressure chemical ionization-liquid chromatography-tandem mass spectrometry (APCI-LC-MS/MS). We also examined the time course changes in serum PK and MD concentrations after oral administration of PK to healthy adult volunteers. Furthermore, to elucidate the conversion mechanism of PK to MK-4, we sought to identify the chemical structure of the product by high resolution mass spectrometry (HR-MS) and 1H NMR after incubation of MD-d3 with rhUBIAD1 in the presence of GGPP.
Here, we demonstrate unequivocally that tissue MK-4 mainly originated from MD released from oral PK during intestinal absorption and was delivered to tissues through the mesenteric lymphatic system. Furthermore, we were able to observe time course changes in the serum concentrations of PK, MK-4, and MD in healthy adult volunteers orally administered PK that were similar to those observed in rat cannulation experiments. During the geranylgeranylation of MD to MK-4, the hydroquinone, but not the quinone form of MD, was the substrate for UBIAD1.
EXPERIMENTAL PROCEDURES
Materials
PK and MK-4 were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Deuterium-labeled MD (MD-d3 and MD-d8) were purchased from C/D/N Isotopes Inc. (Quebec, Canada). PK capsules (Kaywan; 20 mg of PK/capsule) were purchased from Eisai Co., Ltd. (Japan). PK epoxide, MK-4 epoxide, 18O-labeled PK ([18O]PK), and 18O-labeled MK-4 ([18O]MK-4) were synthesized in our laboratory as reported previously (9). PK-d7, MK-4-d7, MK-4-d12, their respective epoxides, and GGPP-d5 were also synthesized in our laboratory as reported previously (10, 11). The synthesis and unambiguous physicochemical assignments of these deuterium-labeled vitamin K compounds have been described elsewhere (10, 11). Culture media and antibiotics were purchased from Nacalai Tesque (Kyoto, Japan). Deuterium-labeled chloroform (CDCl3; 99.8%; NMR analytical grade) was obtained from EURISO-TOP (Gif-Sur-Yvette, France). Organic solvents of HPLC grade were purchased from Nacalai Tesque.
Animals
Forty-two female C57BL/6 mice aged 9 weeks and three male Sprague-Dawley rats aged 8 weeks were obtained from Japan SLC Inc. (Hamamatsu, Japan). They were fed a commercially available normal laboratory chow (F-2 diet, Oriental Yeast Co. Ltd., Tokyo, Japan) for 1 week prior to the experiments. The mice were housed in groups of four, whereas the rats were individually housed in stainless steel wire cages with a 12-h light-dark cycle under controlled environmental conditions (temperature, 20 ± 2 °C; humidity, 50 ± 5%). The animals were allowed ad libitum access to diet and water for 1 week prior to the experiments. The protocols for the experiments were approved by the Guidelines for the Care and Use of Laboratory Animals of Kobe Pharmaceutical University. Sixteen male Sprague-Dawley rats aged 8 weeks (body weight, 350–400 g) were obtained from Japan SLC Inc. They were fed a commercially available normal laboratory chow (Labo MR Stock, NOSAN Corp., Yokohama, Japan) for 1 week prior to the experiments. They were individually housed in polypropylene cages with a 12-h light-dark cycle under controlled environmental conditions (temperature, 24 ± 2 °C; humidity, 50 ± 10%) and allowed ad libitum access to diet and water for 1 week prior to experiments. The protocols for the cannulation experiments were approved by the Guidelines for the Care and Use of Laboratory Animals of Japan SLC Inc.
Measurements of PK-d7, MK-4-d7, and Their Respective Epoxides in the Intestine, Liver, and Cerebrum of Mice Orally Administered PK-d7
At 0, 3, 6, 12, 24, 48, and 72 h after oral administration of PK-d7 as a single dose of 10 μmol/kg of body weight, blood was withdrawn by heart puncture under light diethyl ether anesthesia, and plasma was obtained by centrifugation at 3,000 rpm for 10 min. Immediately after death, the intestine, liver, and cerebrum were excised, immersed and rinsed in ice-cold saline, and stored at −70 °C until analysis. The tissue concentrations of PK-d7, MK-4-d7, and their respective epoxides were measured as reported previously (1). In brief, the tissues were thoroughly pulverized in anhydrous sodium sulfate (1:10, w/v) and transferred into brown glass tubes fitted with a Teflon-lined screw cap. Following the addition of 0.1 ml of ethanol containing [18O]PK and [18O]MK-4 as internal standards, 0.9 ml of ethanol, and 9 ml of acetone, the homogenates were thoroughly mixed using a Vortex mixer for 3 min and allowed to stand for 5 min. This procedure was repeated three times. The resulting mixture was centrifuged at 3,000 rpm for 5 min at 4 °C, and the upper layer was transferred into small brown glass tubes and evaporated to dryness under reduced pressure. The residue was dissolved in 2 ml of water and 6 ml of hexane, thoroughly mixed, and centrifuged at 3,000 rpm for 5 min at 4 °C. The upper layer was loaded onto a Sep-Pak Vac Silica cartridge column (Waters) and eluted with 5 ml of hexane to remove concomitants. The vitamin K-containing fraction was then eluted with 5 ml of hexane/diethyl ether (97:3). The eluate was evaporated under reduced pressure, and the residue was dissolved in 60 μl of methanol. An aliquot of this solution was subjected to APCI-LC-MS/MS. HPLC analyses were performed using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) consisting of a binary pump (LC-10AD liquid chromatograph), automatic solvent degasser (DGU-14A degasser), and autosampler (SIL-10AD autoinjector). Separations were performed on a reversed-phase C18 analytical column (Capcell PAK C18 UG120, 5 μm, 4.6-mm inner diameter × 250 mm; Shiseido, Tokyo, Japan) with a solvent system consisting of isocratic solvent A (25 min) and then a linear gradient from 0 to 50% ethanol (50 min). Solvent A contained methanol, 0.1% acetic acid aqueous (95:5, v/v) and was delivered at a flow rate of 1.0 ml/min. The column was maintained at 35 °C using a column oven (CTO-10AC column oven). The HPLC system was controlled by a SCL-10A System Controller (Shimadzu). Acetic acid functioned as an ion pair reagent during reversed-phase HPLC and facilitated the formation of protonated vitamin K [M + H] in the positive ion mode with APCI. The autosampler was maintained at 25 °C. MS was performed using an API3000-LC-MS/MS system (Applied Biosystems, Foster City, CA) equipped with an APCI electrospray interface. All MS data were collected in the positive ion mode. The following settings were used: corona discharge needle voltage, 5.5 kV; vaporizer temperature, 400 °C; sheath gas (high purity nitrogen) pressure, 50 p.s.i.; auxiliary gas, none; and transfer capillary temperature, 220 °C. The electron multiplier voltage was set at 850 eV. Identification and quantification were based on MS/MS using a multiple reaction monitoring mode. The range for the parent scan was 400–500 atomic mass units. Multiple reaction monitoring transitions, collision energy, retention time, and the corresponding segment used for each analyte were as follows: MK-4: precursor ion, m/z 445.4; collision energy, 31 V; product ion(s), m/z 187.2; retention time, 20.8 min; MK-4-epoxide: precursor ion, m/z 461.3; collision energy, 31 V; product ion(s), m/z 161.0; retention time, 14.6 min; MK-4-d7: precursor ion, m/z 452.3; collision energy, 31 V; product ion(s), m/z 194.2; retention time, 19.6 min; MK-4-d7 epoxide: precursor ion, m/z 468.4; collision energy, 31 V; product ion(s), m/z 168.4; retention time, 14.3 min; [18O]MK-4: precursor ion, m/z 449.3; collision energy, 29 V; product ion(s), m/z 191.2; retention time, 20.1 min; PK-d7: precursor ion, m/z 458.5; collision energy, 35 V; product ion(s), m/z 194.3; retention time, 38.3 min; PK-d7 epoxide: precursor ion, m/z 474.5; collision energy, 25 V; product ion(s), m/z 168.3; retention time, 26.7 min; and [18O]PK: precursor ion, m/z 455.4; collision energy, 35 V; product ion(s), m/z 191.3; retention time, 39.0 min. Calibration using internal standardization was performed using linear regression at five different concentrations: 12.5, 50, 200, 800, and 1,600 ng/ml.
Measurements of PK-d7, MK-4-d7, and Their Respective Epoxides in the Incubation Mixture of Rat Intestinal Luminal Contents and PK-d7
Immediately after death by exsanguination under light diethyl ether anesthesia, small and large intestinal luminal contents from three male Sprague-Dawley rats were collected and separately combined in amounts of ∼3 and 6 g, respectively, avoiding contamination with intestinal villous cells, and 0.3 g of each was transferred into a tube and suspended in 0.6 ml of Plusgrow II culture medium (pH 7.0). Approximately 0.9 ml of Plusgrow II culture medium alone was used as a control. After addition of 100 μl of a solution containing 218.8 nmol of PK-d7 dissolved in 10% ethanol, 0.1% SDS, and 0.1% Nonidet P-40 in water, the resulting solution was incubated at 37 °C for 1 h, and PK-d7, MK-4-d7, and their respective epoxides were measured using the APCI-LC-MS/MS method described above.
Cannulation Experiments in Rats
Cannulation experiments were conducted at The Bio Technical Center, Japan SLC Inc. (Shizuoka, Japan). Sixteen Sprague-Dawley rats were divided into four groups of four rats, and each group was used for IVC, PV, TLD, or BD cannulation. All cannulation techniques were based on previous reports (12–15) with slight modifications. In brief, all rats were fasted overnight and anesthetized with isoflurane prior to surgery. For IVC, the rats were cannulated through the external jugular vein. The right jugular vein was separated from the surrounding neck musculature, and the cephalad end of the external jugular vein was tied. A polyurethane catheter (outside diameter, 0.64 mm; inside diameter, 0.30 mm; Bio Research Center Corp., Nagoya, Japan) was caudally advanced to 3.5 cm into the superior vena cava close to the right atrium. The catheter was subcutaneously tunneled and exteriorized at the back between the shoulder blades. Except when collecting blood samples, the distal end of the tubing was sealed with a stainless steel plug. For PV, a 0.5–1-cm segment of portal vein was teased free from the mesentery, and the same type of polyurethane catheter (as used in IVC) was inserted into the portal vein by sliding along the underside of the guide needle. The cannula was subcutaneously tunneled and exteriorized at the back between the shoulder blades. For TLD, the thoracic duct was located and separated from the psoas muscle and dorsal aorta. The same type of polyurethane catheter (as used in IVC) was inserted into the thoracic lymph duct. The cannula was exteriorized at the abdomen and inserted into the collection vessel. For BD, the common bile duct was cannulated at both the hepatic and ileal ends using a continuous 20-cm length of tube. The intact bile cannula was exteriorized at the abdomen and inserted into the collection vessel. After these surgical procedures, the rats were monitored until they regained full consciousness. There was a 1-h period of recovery and stabilization with continuous blood, lymph, and bile collection before initiation of the study. In one TLD-cannulated rat, it proved difficult to collect the lymph continuously; therefore, we decided not to use this rat for further cannulation experiments. Thus, there were three rats in the TLD group. After confirming that the cannulated rats had regained full consciousness and stabilization, they were orally administered PK-d7 dissolved in a solution containing 10% ethanol, 0.1% SDS, and 0.1% Nonidet P-40 in water as a single dose of 10 μmol/kg body of weight. All the cannulated rats were kept in Ballman cages during collection of lymph, blood, and bile samples. Lymph and bile samples were continuously collected at 1-h intervals, and blood samples (200 μl) were collected hourly from the cannulated rats for 1 h before administering PK-d7 (one sample as a control) and for 0–6 h after administering PK-d7 (six samples). Volumes of lymph and bile were then measured. After collection of the samples, whole blood was withdrawn by heart puncture under light diethyl ether anesthesia, and plasma was obtained by centrifugation at 3,000 rpm for 10 min. The small intestine, liver, and heart were also excised, immersed and rinsed in ice-cold saline, and stored at −70 °C until analysis.
Measurements of PK-d7, MK-4-d7, Their Respective Epoxides, and PK-d7-derived MD in the Serum, Lymph, Bile, and Tissues of the Cannulated Rats Orally Administered PK-d7
PK-d7, MK-4-d7, and their respective epoxides in the intestine, liver, and heart of the cannulated rats orally administered PK-d7 were measured by APCI-LC-MS/MS as described above. For the serum, lymph, and bile assays, 20–80-μl aliquots were diluted to 500 μl with deionized water, and the measurements were performed using APCI-LC-MS/MS as described for the tissues. PK-d7-derived MD in the serum, lymph, and bile was measured by our novel method. In brief, 20 μl of serum, lymph, or bile was placed in a brown glass tube fitted with a Teflon-lined screw cap followed by the addition of 965 μl of buffered saline containing 1 mm DTT, 10 μl of rhUBIAD1 solution (described in detail later), and 5 μl of a solution containing GGPP-d5 (5 × 10−3 m) and incubation at 37 °C for 3 h. After incubation, the putative reaction product (MK-4-d12) was measured by APCI-LC-MS/MS under the same conditions as described above except for the following different parameters: precursor ion, m/z 457.2; collision energy, 33 V; product ion(s), m/z 194.2; and retention time, 19.5 min.
Preparation of rhUBIAD1 Solution
rhUBIAD1 was expressed in the endoplasmic reticulum membrane of Spodoptera frugiperda (Sf9) cells as described previously (2). In brief, cDNA for the UBIAD1 coding region was cloned into pIEx/Bac-1 (Merck) in Sf9 cells using Insect GeneJuiceTM (Merck). A recombinant baculovirus was generated using the BacMagic system (Merck) according to the manufacturer's protocol. Sf9 cells were grown at 28 °C in a tissue culture flask in Grace's insect medium (Invitrogen) supplemented with 10% FCS. To achieve the expression of UBIAD1, cell cultures (2 × 106 cells/ml) were infected at a multiplicity of infection of 10 followed by a 72-h expression period. Microsomes were prepared from the UBIAD1 baculovirus-infected Sf9 cells and suspended in a solution containing 0.5% N-cyclohexyl-3-aminopropanesulfonic acid, 0.2% phosphatidylcholine, and 20% glycerol in PBS (pH 7.4) at a protein concentration of 1 mg/ml.
HR-MS Analysis of PK-d7-derived MD in the Lymph of the Cannulated Rats Orally Administered PK-d7
Because MD is sensitive to light, all procedures were performed under dark conditions. Lymph was collected for 3–4 h from the TLD-cannulated rats after administration of PK-d7, and lipids were extracted from a 0.5-ml aliquot using 10 ml of acetone/ethanol (9:1) solution. The lipid extract was centrifuged at 3,000 rpm for 5 min at 4 °C, and the supernatant was evaporated under reduced pressure after addition of 50 μl of a 50% ethylene glycol solution for protection against vaporization. The resulting residue was then re-extracted using 8 ml of hexane/water (3:1). After centrifugation, the resulting residue was dissolved in 100 μl of methanol and used for HR-MS analysis. MS was performed on an Exactive Orbitrap mass spectrometer (Thermo Scientific, Hemel Hempstead, UK), which was operated in the positive ion mode. A heated electrospray source was used for analyses at the following settings: sheath gas, 40 arbitrary units; auxiliary gas, 10 arbitrary units; capillary temperature, 325 °C; capillary voltage, 35 V; and spray voltage, 5.0 kV. The instrument was operated in full scan mode from m/z 100 to 1,000 at 100,000 resolving power with a data acquisition rate of 10.0 Hz. The mass spectrometer was mass-calibrated just prior to starting the sequence of injections.
HR-MS and 1H NMR Analyses of MD-d3-derived MK-4 in the Reaction Mixture of MD-d3 and rhUBIAD1 in the Presence of GGPP
Because MK-4 is sensitive to light, all procedures were performed under dark conditions. MD-d3 (2 × 10−4 m) was incubated at 37 °C for 3 h with rhUBIAD1 (10 μg as protein weight of the microsomes prepared from UBIAD1 baculovirus-infected Sf9 cells) in the presence of GGPP (2 × 10−4 m) and DTT (1 mm) in 100 mm Tris-HCl buffer (pH 7.8). After incubation, the lipids were extracted using 10 ml of acetone/ethanol (9:1) solution. The extract was centrifuged at 2,500 rpm for 5 min at 4 °C, and the supernatant was evaporated under reduced pressure. This procedure was repeated 1,200 times. All residues were then combined and re-extracted using 8 ml of hexane/water (3:1). After centrifugation of the mixture, the hexane layer was loaded onto a Sep-Pak Vac Silica cartridge column, which was prewashed with 10 ml of hexane, and eluted with 5 ml of hexane/diethyl ether (97:3). The eluate was evaporated under reduced pressure, dissolved in 100 μl of methanol, and then purified using a Shimadzu HPLC system consisting of a C-R8A Chromatopac, SPD-20A UV spectrophotometric detector, and LC-20A liquid chromatograph (Shimadzu). Separations were performed using a reversed-phase C18 column (Capcell PAK C18 UG120, 5 μm, 4.6-mm inner diameter × 250 mm; Shiseido) with a mobile phase containing methanol/ethanol (95:5). This mobile phase was passed through the column at a flow rate of 1.0 ml/min. Samples with a peak corresponding to authentic MK-4 were collected, evaporated to dryness, and then redissolved in the mobile phase. After further purification of the combined samples using the same HPLC system, the samples were sufficiently pure for 1H NMR and HR-MS analyses. The residues were redissolved in 2 ml of hexane, evaporated under reduced pressure, and then dissolved in 60 μl of CDCl3 in a nanoprobe for 1H NMR spectrometry. The 500-MHz 1H NMR spectra of authentic MK-4-d3 and the product were measured on a Varian VNS-500 (proton, 500 MHz). After 1H NMR analysis, the sample was evaporated under reduced pressure, dissolved in 100 μl of methanol, and used for HR-MS analysis. MS was performed on an Exactive Orbitrap mass spectrometer (Thermo Scientific), which was operated in the positive ion mode.
Measurements of Serum PK, MK-4, and MD of Healthy Adult Volunteers Who Orally Ingested PK Capsules
Blood samples were collected hourly from six healthy adult volunteers (four males and two females; 29–63 years old) for 1 h before a single oral ingestion of PK capsules (two capsules; 40 mg of PK) and for 0–6 h after ingestion of PK. Concentrations of PK, MK-4, and MD in serum were measured using APCI-LC-MS/MS as described above. Ethical approval was obtained from the Ethics Committee of the Kyoto Women's University, and all participants provided written informed consent.
Measurements of MK-4-d7 and Its Epoxide in Lipids Extracted from Rat Small Intestine Epithelial (IEC-6) Cells Treated with PK-d7
IEC-6 cells were obtained from Riken Cell Bank (Tsukuba, Japan) and cultured at a density of 5 × 106 cells/well in 6-well tissue culture plates in low glucose DMEM with 10% FCS at 37 °C in 5% CO2 in a humidified atmosphere for 3 days. Subsequently, the cells were incubated with PK-d7 (1 μm) at 37 °C for 24 h. The cells were collected, the lipids were extracted using 3 ml of hexane/diethyl ether (97:3), and the amount of MK-4-d7 and its epoxide was measured using APCI-LC-MS/MS as described above.
RESULTS
Time Course Changes in the Concentrations of PK-d7, MK-4-d7, and Their Epoxides in the Intestine, Liver, and Cerebrum of Mice Orally Administered PK-d7
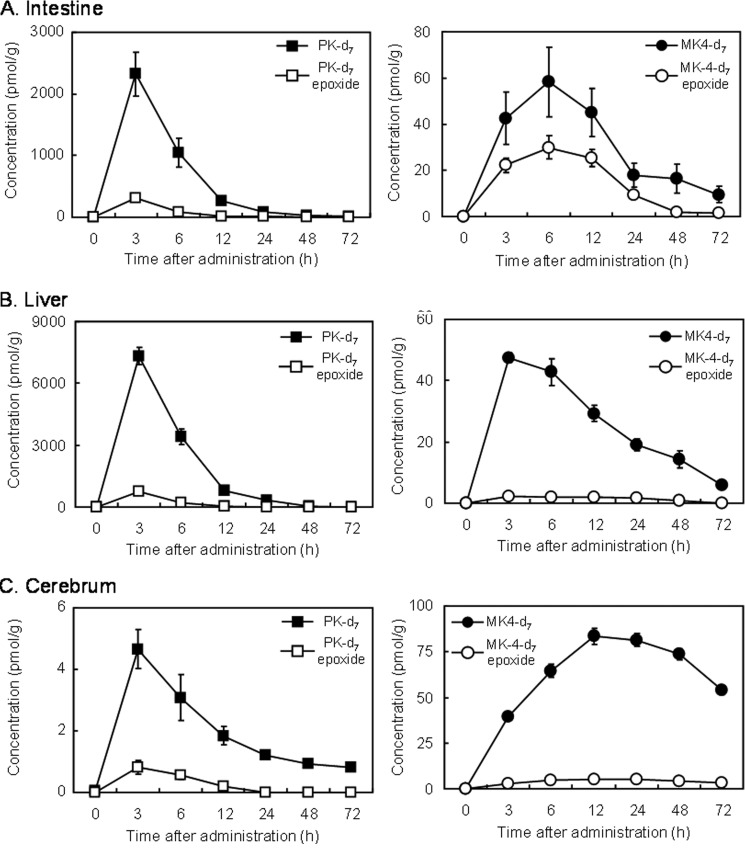
Following oral administration of PK-d7 as a single dose of 10 μmol/kg of body weight in mice, PK-d7 and its epoxide were found in all tissues examined and exhibited similar tissue time-distribution patterns, although their concentrations varied greatly among the tissues (Fig. 1). Tissue concentrations of PK-d7 and its epoxide reached a maximum at 3 h postadministration and rapidly decreased thereafter in the intestine and liver and gradually in the cerebrum. MK-4-d7 and its epoxide converted from oral PK-d7 were also found in the same tissues, and their concentrations reached a maximum at 3 h in the liver, 6 h in the intestine, and 12 h in the cerebrum and gradually decreased thereafter. Despite the marked difference in concentrations of PK-d7 and its epoxide among the tissues, concentrations of MK-4-d7 did not differ to the same extent among the tissues throughout the experimental period. Concentrations of MK-4-d7 in the cerebrum were considerably higher than those of PK-d7. This result suggested that tissue MK-4-d7 does not originate from tissue PK-d7 but rather from MD-d7 that is released from PK-d7 during intestinal absorption and then delivered to tissues. To investigate this possibility, we conducted cannulation experiments in rats. Prior to the initiation of these experiments, we examined whether gut microflora participated in the conversion of PK-d7 to MK-4-d7. Neither small nor large intestinal luminal contents showed conversion activity (data not shown).
FIGURE 1.
Time course changes in the concentrations of PK-d7, MK-4-d7, and their respective epoxides in the intestine (A), liver (B), and cerebrum (C) of mice orally administered PK-d7. Female mice aged 10 weeks were orally administered PK-d7 as a single dose of 10 μmol/kg of body weight after a 12-h fast. At 0, 3, 6, 12, 24, 48, and 72 h postadministration, the mice were sacrificed, and the intestine, liver, and cerebrum were collected to measure PK-d7, MK-4-d7, and their respective epoxides by APCI-LC-MS/MS as described under “Experimental Procedures.” Results represent the means for 6 mice (values shown as broken lines) and standard errors (vertical bars).
Time Course Changes in the Concentrations of PK-d7, MK-4-d7, and MD-d7 in the Bile, Lymph, and Serum Collected from the Cannulated Rats Orally Administered PK-d7
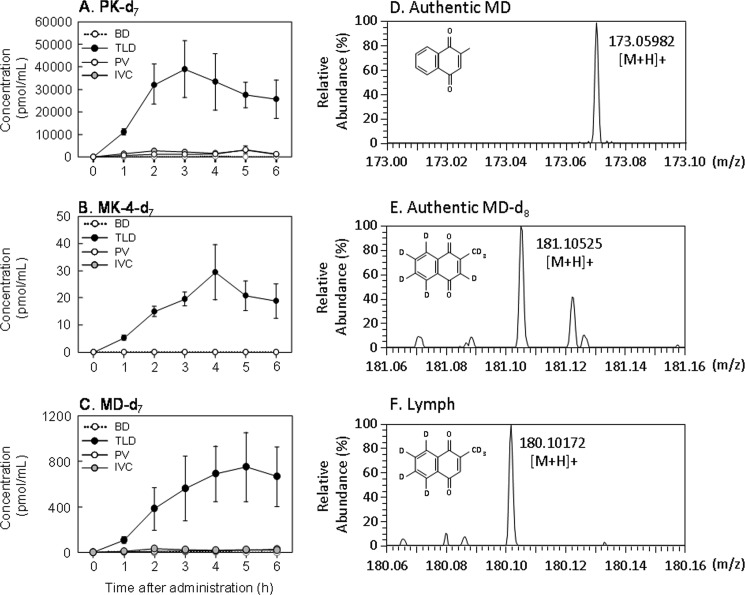
Following oral administration of PK-d7 as a single dose of 10 μmol/kg of body weight in cannulated rats, PK-d7 and MK-4-d7 were predominantly observed in the lymph collected from the TLD-cannulated rats throughout the experimental period. However, concentrations of MK-4-d7 were ∼1,000-fold lower than those of PK-d7 (Fig. 2). Concentrations of both PK-d7 and MK-4-d7 increased 1 h postadministration, reached a maximum at 3 h for PK-d7 and 4 h for MK-4-d7, and decreased gradually thereafter. For up to 6 h postadministration, the lymph time-concentration profiles of PK-d7 and MK-4-d7 were almost identical to the tissue time-distribution profiles of these K vitamers in the mice (Fig. 1). Concentrations of PK-d7 and MK-4-d7 in the serum of the PV and IVC of the PV- and IVC-cannulated rats, respectively, were markedly lower than those in the lymph, whereas neither PK-d7 nor MK-4-d7 was detected in bile from the BD-cannulated rats throughout the experimental period. Similarly, in the TLD-cannulated rats, MD-d7 was predominantly found in the lymph at concentrations ∼50-fold lower than those of PK-d7 and ∼25-fold higher than those of MK-4-d7. These results indicate that a large portion of the PK absorbed from the intestine is delivered in its original form through the mesenteric lymphatic system to the blood circulation, but ∼2 and 0.1% of the absorbed PK is converted to MD-d7 and MK-4-d7 in the intestine, respectively. MK-4-d7 then enters the blood circulation through the mesenteric lymphatic system.
FIGURE 2.
Time course changes in the concentrations of PK-d7 (A), MK-4-d7 (B), and MD-d7 (C) in the bile, lymph, and serum collected from cannulated rats orally administered PK-d7 as a single dose of 10 μmol/kg of body weight are shown. PK-d7 and MK-4-d7 in the samples were measured by APCI-LC-MS/MS as described under “Experimental Procedures.” MD-d7 was measured by the in vitro bioassay using rhUBIAD1 with GGPP-d5 as described under “Experimental Procedures.” Except for the TLD experiment, which included three rats, the results represent the means for four rats (values shown as broken lines) and the standard errors (vertical bars). The abbreviations BD, TLD, PV, and IVC represent bile from bile duct-, lymph from thoracic lymph duct-, serum from portal vein-, and serum from inferior vena cava-cannulated rats, respectively. HR-MS analysis of authentic MD (D), MD-d8 (E), and PK-d7-derived MD (F) in the lymph collected as described above is shown. A lipid extract of lymph was dissolved in 100 μl of methanol, and an aliquot of the solution was used for HR-MS analysis. MS was performed on an Exactive Orbitrap mass spectrometer (Thermo Scientific), which was operated in the positive ion mode as described under “Experimental Procedures.”
HR-MS Analysis of PK-d7-derived MD
To demonstrate the occurrence of MD-d7 as a side chain-cleaved form of PK-d7 in the lymph collected from the TLD-cannulated rats orally administered PK-d7, we extracted lipid from the lymph and subjected it to HR-MS analysis as described under “Experimental Procedures.” This confirmed that the peak corresponded to MD-d7 [M + H, m/z 180.10172, C11H22H7O2] (Fig. 2).
Comparison of the Concentrations of PK-d7, MK-4-d7, and Their Epoxides in the Serum and Tissues of the Cannulated Rats Orally Administered PK-d7
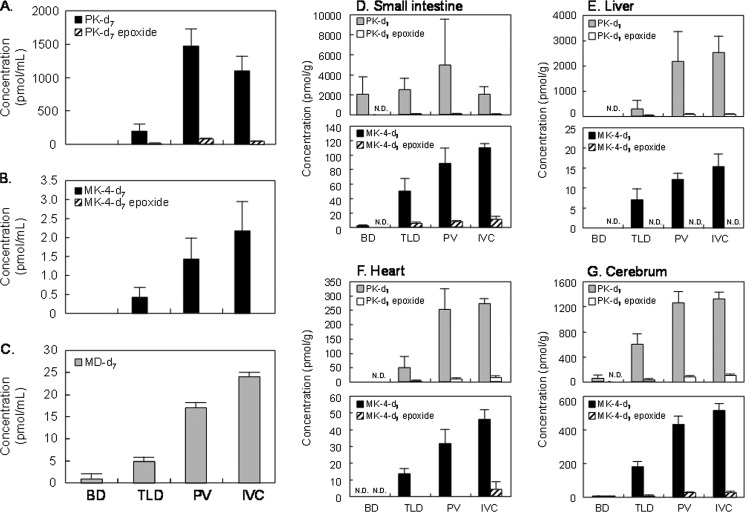
To examine the tissue distribution of PK-d7, MK-4-d7, and their epoxides after oral administration of PK-d7 as a single dose of 10 μmol/kg body of weight, the cannulated rats were sacrificed by heart puncture immediately after final sample collection (6 h after administration of PK-d7), and the blood, intestine, liver, heart, and cerebrum were collected to measure the concentrations of PK-d7, MK-4-d7, and their epoxides by APCI-LC-MS/MS. The serum concentrations of PK-d7, MK-4-d7, and their respective epoxides in the BD- and TLD-cannulated rats were markedly lower and undetectable, respectively, compared with those in the PV- and IVC-cannulated rats (Fig. 3). Similarly, the concentrations of PK-d7, MK-4-d7, and their epoxides in the liver, heart, and cerebrum of the TLD- and BD-cannulated rats were markedly lower than those in the PV- and IVC-cannulated rats (Fig. 3). Because the mesenteric lymphatic system is considered to be a major transport pathway of PK from the intestine to the blood, it is possible that cannulation of the TLD led to low concentrations of PK-d7 and MK-4-d7 in both serum and tissues of the TLD-cannulated rats. Despite almost equal concentrations of PK-d7 in the intestines of all cannulated rats, concentrations of MK-4-d7 and its epoxide in the liver, heart, and cerebrum of the BD-cannulated rats were extremely low or undetectable compared with those in the other three types of cannulated rats. Because dietary PK is known to be absorbed from the intestine in micelle form with bile salts, it is possible that cannulation of the BD caused the low uptake of PK-d7 into chylomicrons by enterocytes of the intestine, resulting in low or undetectable concentrations of PK-d7, MK-4-d7, and their epoxide in the serum and tissues of the BD-cannulated rats.
FIGURE 3.
Concentrations of PK-d7 (A), MK-4-d7 (B), their respective epoxides, and MD-d7 (C) in serum collected by heart puncture 6 h postadministration from cannulated rats orally administered PK-d7 as a single dose of 10 μmol/kg body of weight are shown. PK-d7, MK-4-d7, and their epoxides in the samples were measured by APCI-LC-MS/MS as described under “Experimental Procedures.” MD-d7 was measured by in vitro bioassay using rhUBIAD1 with GGPP-d5 as described under “Experimental Procedures.” Concentrations of PK-d7, MK-4-d7, and their respective epoxides in small intestine (D), liver (E), heart (F), and cerebrum (G) collected 6 h postadministration from cannulated rats orally administered PK-d7 as a single dose of 10 μmol/kg of body weight are shown. PK-d7, MK-4-d7, and their respective epoxides in tissues were measured by APCI-LC-MS/MS as described under “Experimental Procedures.” Except for the TLD experiment, which included three rats, the results represent the means for four rats (values are shown as columns) and the standard errors (vertical bars). The abbreviations BD, TLD, PV, and IVC represent bile duct-, thoracic lymph duct-, portal vein-, and inferior vena cava-cannulated rats, respectively.
Time Course Changes in the Serum Concentrations of PK, MK-4, and MD in Healthy Adult Volunteers Orally Administered PK Capsules
Following oral ingestion of 40 mg of PK, which is similar to the therapeutic dose (45 mg of MK-4) for the treatment of osteoporosis in Japan, concentrations of both PK and MD increased at 1 h postadministration, reached a maximum at 3 h for PK and MD, and decreased gradually thereafter (Fig. 4). For up to 6 h postadministration, serum time-concentration profiles of PK, MK-4, and MD were almost identical to the lymph time-concentration profiles of these K vitamers in the cannulated rats (Fig. 2).
FIGURE 4.
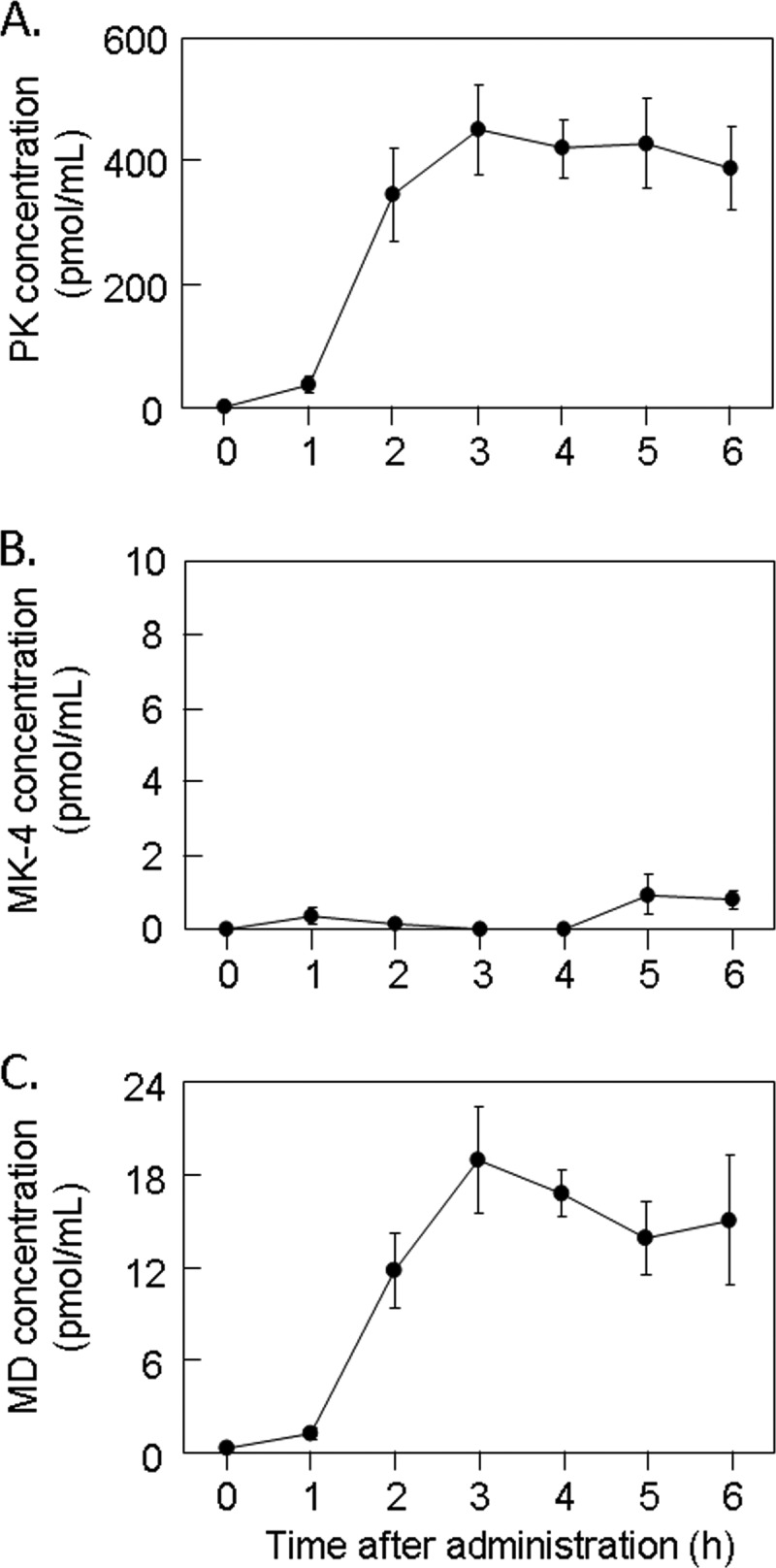
Time course changes in concentrations of PK (A), MK-4 (B), and MD (C) in serum collected from healthy adult volunteers orally administered PK capsules as a single dose of 40 mg. PK and MK-4 in the samples were measured by APCI-LC-MS/MS as described under “Experimental Procedures.” MD was measured by in vitro bioassay using rhUBIAD1 with GGPP-d5 as described under “Experimental Procedures.” Error bars represent S.E.
Conversion of PK-d7 to MK-4-d7 in IEC-6 Cells
Based on the above findings, it was expected that the phytyl side chain of PK-d7 was cleaved to some extent by an unknown enzyme(s) in the intestine before delivery to tissues and conversion to MK-4-d7. To confirm the occurrence of this conversion in the intestine, we examined the conversion of PK-d7 to MK-4-d7 in the rat intestine epithelial cell line IEC-6. As shown in Fig. 5, IEC-6 cells were able to convert PK-d7 to MK-4-d7.
FIGURE 5.
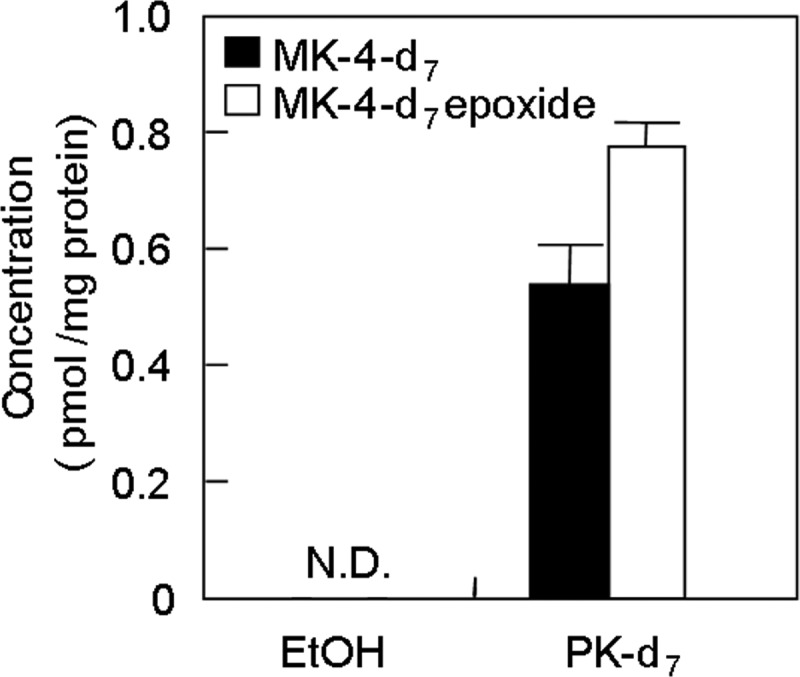
Conversion of PK-d7 to MK-4-d7 in the rat small intestine epithelial cell line IEC-6. IEC-6 cells were cultured for 3 days at a density of 5 × 106 cells/well in 6-well tissue culture plates in low glucose DMEM containing 10% FCS at 37 °C in 5% CO2 in a humidified atmosphere. The cells were then incubated with PK-d7 (1 μm) at 37 °C for 24 h. The cells were collected, and the lipids were extracted using 3 ml of hexane/diethyl ether (97:3). MK-4-d7 and its epoxide were measured by APCI-LC-MS/MS as described under “Experimental Procedures.” N.D., not detected. Error bars represent S.E.
HR-MS and 1H NMR Analyses of the Product Generated from the Incubation of MD-d3 with rhUBIAD1 in the Presence of GGPP
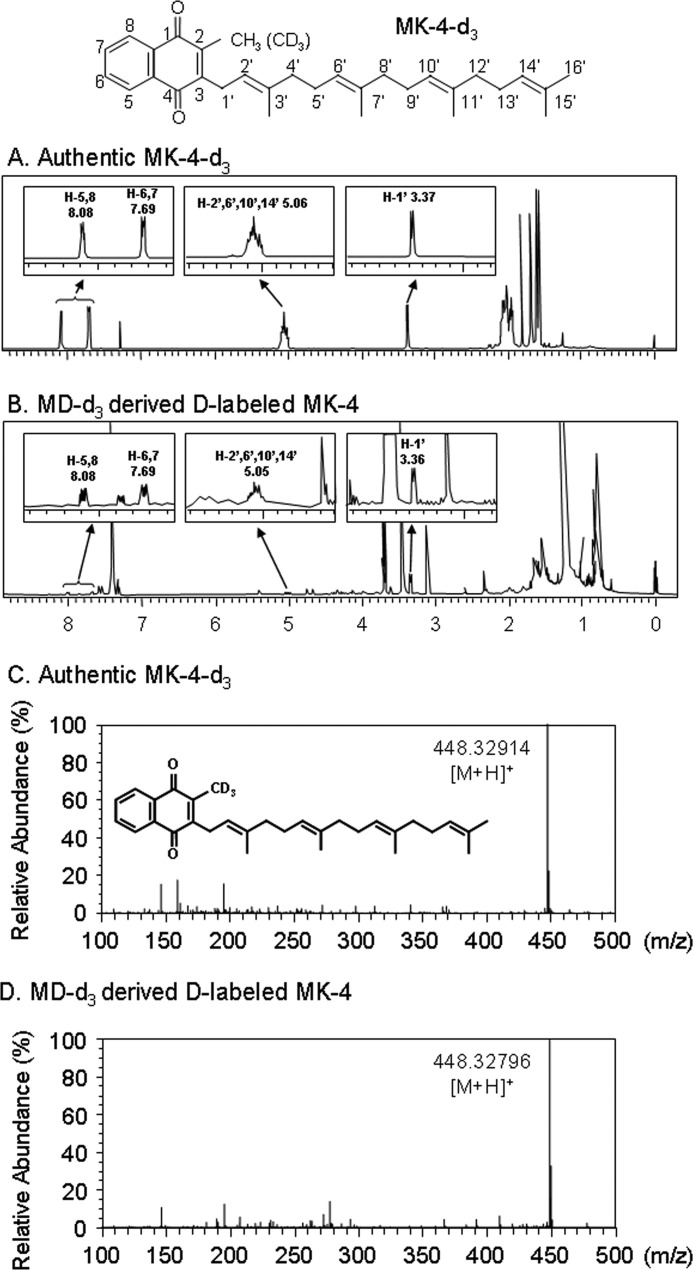
Next, we examined the mechanism underlying the conversion of MD to MK-4 catalyzed by rhUBIAD1. rhUBIAD1 was incubated with 10 μg of MD-d3 in the presence of 1 mm GGPP at 37 °C for 3 h. After purification by HPLC, as described under “Experimental Procedures,” we obtained ∼4.0 μg of the product MD-d3-derived MK-4 and subjected it to HR-MS analysis. We first analyzed the product by 1H NMR spectrometry. The 1H NMR spectra of authentic MK-4-d3 and the product are shown in Fig. 6. The chemical shifts derived from the 2-methyl-1,4-naphthoquinone ring and geranylgeranyl side chain of the product exactly coincided with those of authentic MK-4-d3. We further confirmed that the peak corresponded to MK-4-d3 [M + H, m/z 448.32796, C31H342H3O2] but not MK-4-d2 (Fig. 6). Based on the combined results of HR-MS and 1H NMR analyses, it is evident that hydroquinone, but not quinone of MD, was the substrate for the generation of MK-4 by rhUBIAD1. These findings suggest that PK is cleaved by an unknown enzyme to release MD in the intestine. Thereafter, MD enters the blood circulation through the thoracic lymphatic system and is delivered to tissues, reduced by redox enzyme(s), and converted to MK-4 by UBIAD1 with GGPP.
FIGURE 6.
1H NMR analysis and HR-MS analysis of menadione labeled with deuterium at the 2-methyl group (MD-d3)-derived MK-4 generated by the incubation of MD-d3 with rhUBIAD1 in the presence of GGPP. MD-d3 (2 × 10−4 m) was incubated at 37 °C for 3 h with rhUBIAD1 (10 μg as protein weight of the microsomes prepared from UBIAD1 baculovirus-infected Sf9 cells) in the presence of GGPP (2 × 10−4 m) and DTT (1 mm) in 100 mm Tris-HCl buffer (pH 7.8). After incubation, the lipids were extracted with 10 ml of acetone/ethanol (9:1) solution. The extract was centrifuged at 2,500 rpm for 5 min at 4 °C, and the supernatant (2.5 ml) was evaporated under reduced pressure. This procedure was repeated 1,200 times. All the residues were then redissolved in 2 ml of hexane, evaporated under reduced pressure, and then dissolved in 60 μl of CDCl3 to yield the resulting samples. An aliquot of the solution was used for 1H NMR analysis. A and B, 1H NMR spectra of authentic MK-4-d3 and MD-d3-derived MK-4, respectively. The number and letter H in each spectrum refer to the chemical shift (ppm) and the respective position of the proton in the 2-methyl-1,4-naphthoquinone ring or the side chain of MK-4-d3. After 1H NMR analysis of MD-d3-derived MK-4, the sample solution was evaporated under reduced pressure, dissolved in 100 μl of methanol, and used for HR-MS analysis. C and D, HR-MS spectra of authentic MK-4-d3 and MD-d3-derived MK-4, respectively. MS was performed on an Exactive Orbitrap mass spectrometer (Thermo Scientific) operating in the positive ion mode as described under “Experimental Procedures.”
DISCUSSION
The present study provides unequivocal evidence that MD-d7 derived from oral PK-d7 is present in the lymph and blood of rats in its original form and contributes as a circulating precursor to tissue MK-4-d7 synthesis. To our knowledge, this is the first study to demonstrate directly the occurrence of MD-d7 released from PK-d7 during the course of intestinal absorption on the basis of structural assignment by HR-MS analysis together with the measurements by our novel in vitro assay using rhUBIAD1. Conversion of PK-d7 into MK-4-d7 by gut microflora did not occur (data not shown), suggesting that the conversion occurred in the enterocytes of the intestine. This unique activity was further examined in animal models using four site-specific cannulation techniques. Except for BD cannulation in which the secretion of bile salts into the intestine is completely blocked, resulting in severe reduction of PK uptake into chylomicrons, irrespective of the difference in cannulation sites, MD-d7 was consistently found in the lymph and serum of the cannulated rats (Figs. 2 and 3). Therefore, it appears that MD is an intermediate generated in the course of the intestinal absorption of dietary PK (4, 5).
Concentrations of PK-d7 in the lymph of the TLD-cannulated rats were ∼50-fold higher than those of MD-d7 and ∼1,000-fold higher than those of MK-4-d7 for the duration of cannulation experiments. Furthermore, the time to reach the maximum concentration of PK-d7 in the lymph was 3 h postadministration, whereas it was 4 and 5 h postadministration, respectively, for MK-4-d7 and MD-d7 (Fig. 2). Based on these observations, it is possible that most of the oral PK-d7 was absorbed from the intestine in its original form and entered the blood circulation through the mesenteric lymphatic system, small or trace amounts of PK-d7 were converted to MD-d7 and MK-4-d7 in the intestine, and MD-d7 and MK-4-d7 entered the mesenteric lymphatic system with a delay of 1–2 h compared with PK-d7 (Fig. 2).
There was a considerable difference in the tissue distribution profiles of PK-d7, MK-4-d7, and their epoxides 6 h postadministration. In IVC-cannulated rats, the respective concentrations of PK-d7 and MK-4-d7 were ∼2,600 and 16 pmol/g (concentration ratio of PK-d7 versus MK-4-d7, 16.3) in the liver, ∼270 and 46 pmol/g (5.9) in the heart, and ∼1,300 and 650 pmol/g (2.0) in the cerebrum. This indicated that PK is stored mainly in the liver and that MK-4 accumulates to high concentrations in the cerebrum of rats. In mice, the respective tissue concentrations of PK-d7 and MK-4-d7 6 h postadministration were ∼1,000 and 60 pmol/g (concentration ratio of PK-d7 versus MK-4-d7, 16.7) in the intestine, ∼3,500 and 42 pmol/g (83.3) in the liver, and ∼3 and 65 pmol/g (0.02) in the cerebrum (Fig. 1). These findings and our previous report indicate that the cerebrum actively accumulates MK-4 in rats and mice with this activity being higher in mice than in rats (1). The reason why MK-4 accumulates to high concentrations in the cerebrum of rats and mice is not known, but one explanation is that because MD is more easily transferred into the brain through the blood-brain barrier than PK MD released from PK in the intestine is delivered to the cerebrum where it is converted to MK-4 by UBIAD1.
Thijssen et al. (7) reported a human study that showed that urinary MD excretion increased after oral intake of the K vitamers. This effect was apparent within 1–2 h and peaked at ∼3 h after intake. The amounts of MD excreted in 24 h after vitamin K intake ranged from 1 to 5% of the administered dose, indicating that ∼5–20% of the ingested vitamin K was catabolized to MD. They further reported that MD excretion was not enhanced by PK administered subcutaneously. These observations in humans and the results of our animal study are in good agreement in terms of the dependence on the oral route, rapid appearance of MD in the urine and lymph, and conversion efficiency of PK-d7 to MD-d7. We did not measure the amounts of MD-d7 excreted in the urine of the cannulated rats and therefore do not know how much MD-d7 was generated from oral PK-d7 in each rat. However, it appears that a significant fraction of MK-4 in tissues results from the uptake and prenylation of circulating MD.
There are conflicting reports concerning the tissue sites of MD release. Using HPLC analysis, Thijssen et al. (7) found that various cell lines were capable of converting MD into MK-4, but none of these cell lines was able to convert PK into MK-4 presumably because of a lack of MD-releasing activity. The current study clearly demonstrated that the release of MD-d7 from PK-d7 and the conversion of MD-d7 to MK-4-d7 occurred in the intestine of rats (Figs. 2 and 3) and suggested that the rat intestine epithelial cell line IEC-6 cleaved the side chain of PK-d7 to release MD and converted it to MK-4-d7 (Fig. 5). The reason for the inconsistency between our results and those of others is unclear, although one explanation may be the difference in the methods used to detect the conversion of PK to MK-4.
We tried to elucidate the comprehensive mechanism underlying this conversion reaction. Organic chemistry reasoning suggests that two final products, namely MK-4-d2 and MK-4-d3, are generated from MD-d3 by the two pathways shown in Fig. 7. To address this question, MD-d3 was incubated with rhUBIAD1 and GGPP, and the purified MK-4 fraction was analyzed by 1H NMR and HR-MS. The results showed that the final product, MK-4-d3, was generated by the hydroquinone form of MD-d3 by the second pathway. To our knowledge, this is the first evidence demonstrating the mechanism of MD to MK-4 conversion catalyzed by UBIAD1.
FIGURE 7.
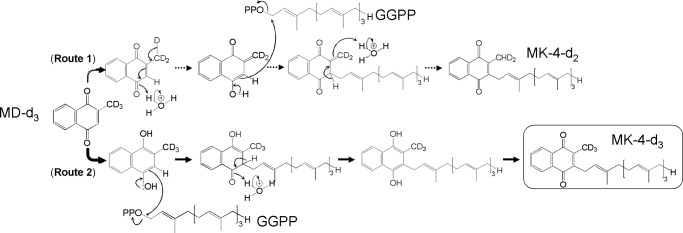
Pathways for the conversion of MD-d3 into MK-4-d3 by UBIAD1. The present study demonstrated that UBIAD1 converted MD-d3 to MK-4-d3 but not MK-4-d2 (Route 2), indicating that the hydroquinone, but not the quinone form of MD, is an intermediate in the course of conversion of PK to MK-4.
There are several limitations to this study. First, we were unable to detect MD-d7 in the liver, heart, and cerebrum of cannulated rats orally administered PK-d7. MD-d7 is not easily detected by APCI-LC-MS/MS because of the difficulties in the detection of either the precursor ion or product ion derived from MD-d7. Several studies have shown that MD is present in the form of conjugates, namely glucuronides or sulfates of menadiol in urine. MD was successfully measured by HPLC using a reversed-phase column with fluorescence detection after oxidation of the conjugates (16, 17). However, the sensitivity and accuracy of this method were not sufficient to detect MD present in the serum and tissues at low concentrations. Therefore, we measured MD-d7 in the tissues of the cannulated rats by our novel in vitro assay using rhUBIAD1 but failed to detect it in all tissues examined. This result suggested that MD-d7 is quite unstable and/or rapidly converted to MK-4-d7 in tissues.
Second, we were unable to identify the enzyme(s) involved in the cleavage of the phytyl side chain of PK in the intestine. Studies in search of the enzyme(s) are currently being conducted in our laboratory.
In summary, the present study shows for the first time that MK-4-d7 present in tissues of rats originates from oral PK-d7 by a mechanism that involves the release of MD-d7 from PK-d7 in the intestine followed by delivery of MD-d7 through the mesenteric lymphatic system and blood circulation to tissues. MD-d7 is then reduced by an unknown redox enzyme in the tissues and converted into MK-4-d7 by the prenylating enzyme UBIAD1 (Fig. 8). The present results may help to understand the metabolism of PK in tissues of animals and contribute to the development of new drugs for the treatment of diseases related to vitamin K nutrition.
FIGURE 8.
The side chain of PK is cleaved to release MD during intestinal absorption followed by delivery of MD through a mesenteric lymphatic system and blood circulation to local tissues. After MD is reduced to the hydroquinone form by redox enzyme(s), it is converted to MK-4 by UBIAD1 (working hypothesis).
Acknowledgment
We thank Enago for the English language review.
This work was supported in part by Grant-in-aid for Scientific Research (B) 23390022 (to T. O.), Grant-in-aid for Scientific Research (Young Scientists-B) 23790110 (to K. N.) from the Japan Society for the Promotion of Science (JSPS), and Grant-in-aid for JSPS Fellows 24-7941 (to Y. H.).
- PK
- vitamin K1 (phylloquinone)
- MK-4
- vitamin K2 (menaquinone-4)
- UBIAD1
- UbiA prenyltransferase domain-containing 1
- MD
- vitamin K3 (menadione)
- IVC
- inferior vena cava
- PV
- portal vein
- TLD
- thoracic lymph duct
- BD
- bile duct
- rhUBIAD1
- recombinant human UBIAD1
- GGPP
- geranylgeranyl pyrophosphate
- APCI
- atmospheric pressure chemical ionization
- HR
- high resolution.
REFERENCES
- 1. Okano T., Shimomura Y., Yamane M., Suhara Y., Kamao M., Sugiura M., Nakagawa K. (2008) Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 283, 11270–11279 [DOI] [PubMed] [Google Scholar]
- 2. Nakagawa K., Hirota Y., Sawada N., Yuge N., Watanabe M., Uchino Y., Okuda N., Shimomura Y., Suhara Y., Okano T. (2010) Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 468, 117–121 [DOI] [PubMed] [Google Scholar]
- 3. Taggart W. V., Matschiner J. T. (1969) Metabolism of menadione-6,7-3H in the rat. Biochemistry 8, 1141–1146 [DOI] [PubMed] [Google Scholar]
- 4. Ronden J. E., Drittij-Reijnders M. J., Vermeer C., Thijssen H. H. (1998) Intestinal flora is not an intermediate in the phylloquinone-menaquinone-4 conversion in the rat. Biochim. Biophys. Acta 1379, 69–75 [DOI] [PubMed] [Google Scholar]
- 5. Davidson R. T., Foley A. L., Engelke J. A., Suttie J. W. (1998) Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J. Nutr. 128, 220–223 [DOI] [PubMed] [Google Scholar]
- 6. Shearer M. J., Fu X., Booth S. L. (2012) Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv. Nutr. 3, 182–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thijssen H. H., Vervoort L. M., Schurgers L. J., Shearer M. J. (2006) Menadione is a metabolite of oral vitamin K. Br. J. Nutr. 95, 260–266 [DOI] [PubMed] [Google Scholar]
- 8. Al Rajabi A., Booth S. L., Peterson J. W., Choi S. W., Suttie J. W., Shea M. K., Miao B., Grusak M. A., Fu X. (2012) Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J. Nutr. 142, 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suhara Y., Murakami A., Nakagawa K., Mizuguchi Y., Okano T. (2006) Comparative uptake, metabolism, and utilization of menaquinone-4 and phylloquinone in human cultured cell lines. Bioorg. Med. Chem. 14, 6601–6607 [DOI] [PubMed] [Google Scholar]
- 10. Suhara Y., Wada A., Okano T. (2009) Elucidation of the mechanism producing menaquinone-4 in osteoblastic cells. Bioorg. Med. Chem. Lett. 19, 1054–1057 [DOI] [PubMed] [Google Scholar]
- 11. Suhara Y., Wada A., Tachibana Y., Watanabe M., Nakamura K., Nakagawa K., Okano T. (2010) Structure-activity relationships in the conversion of vitamin K analogues into menaquinone-4. Substrates essential to the synthesis of menaquinone-4 in cultured human cell lines. Bioorg. Med. Chem. 18, 3116–3124 [DOI] [PubMed] [Google Scholar]
- 12. Yang J., Maarek J. M., Holschneider D. P. (2005) In vivo quantitative assessment of catheter patency in rats. Lab. Anim. 39, 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strubbe J. H., Bruggink J. E., Steffens A. B. (1999) Hepatic portal vein cannulation for infusion and blood sampling in freely moving rats. Physiol. Behav. 65, 885–887 [DOI] [PubMed] [Google Scholar]
- 14. Ionac M. (2003) One technique, two approaches, and results: thoracic duct cannulation in small laboratory animals. Microsurgery 23, 239–245 [DOI] [PubMed] [Google Scholar]
- 15. Medinsky M. A., Dent J. G. (1983) Biliary excretion and enterohepatic circulation of 2,4-dinitrotoluene metabolites in Fischer-344 rats. Toxicol. Appl. Pharmacol. 68, 359–366 [DOI] [PubMed] [Google Scholar]
- 16. Speek A. J., Schrijver J., Schreurs W. H. (1984) Fluorimetric determination of menadione sodium bisulphite (vitamin K3) in animal feed and premixes by high-performance liquid chromatography with post-column derivatization. J. Chromatogr. 301, 441–447 [DOI] [PubMed] [Google Scholar]
- 17. Al Rajabi A., Peterson J., Choi S. W., Suttie J., Barakat S., Booth S. L. (2010) Measurement of menadione in urine by HPLC. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 878, 2457–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]