Abstract
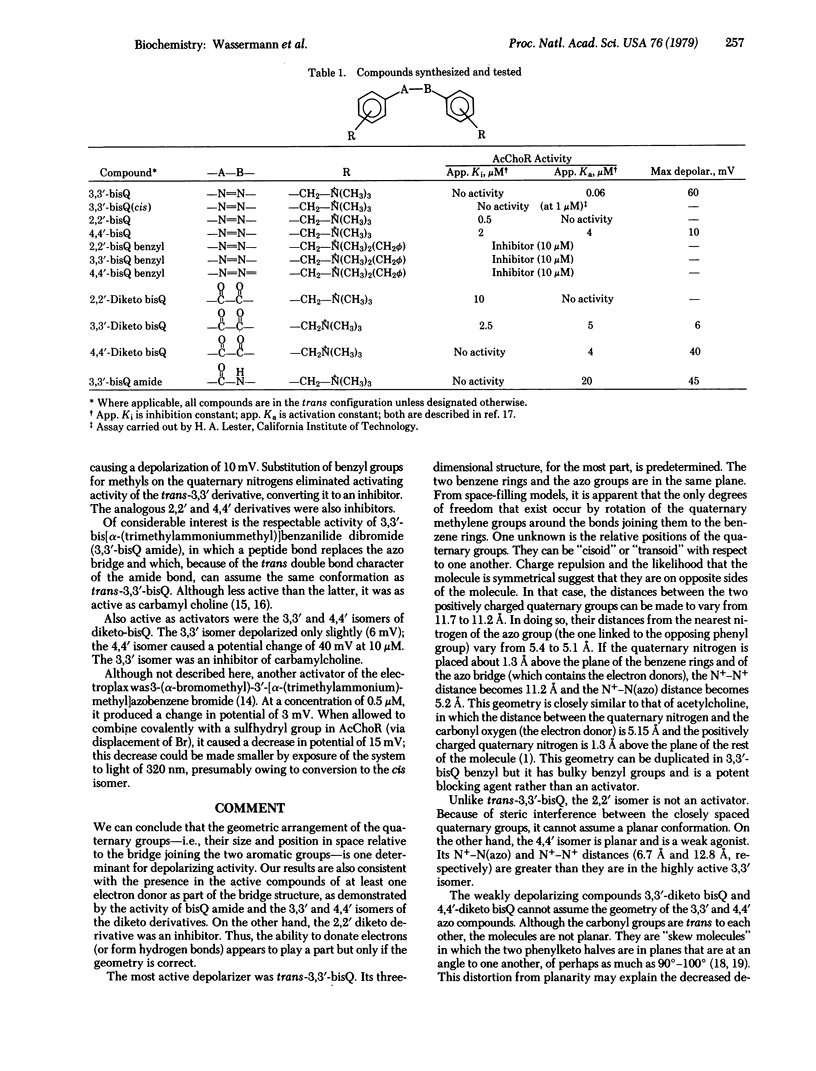
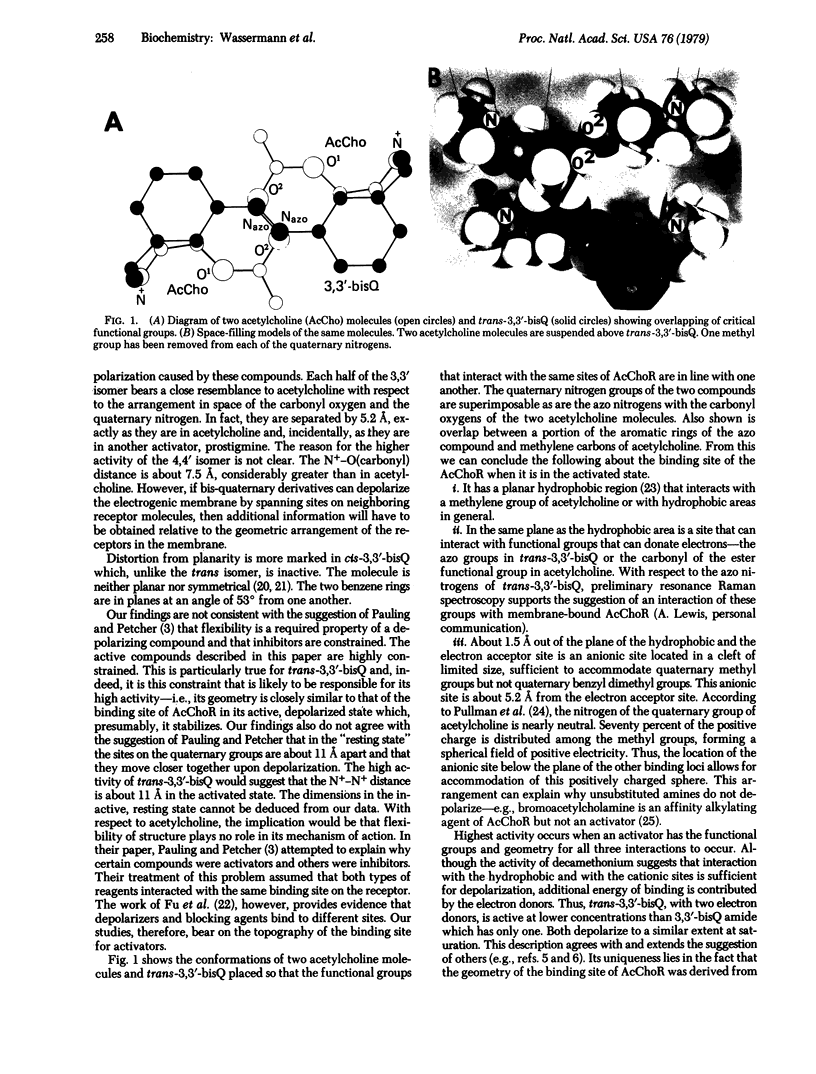
Conformational aspects of the acetylcholine receptor (AcChoR) of Electrophorus electricus have been examined by studies of its interaction with structurally related, constrained aromatic bis quaternary compounds. Among the compounds synthesized was 3,3′-bis[α-(trimethylammonium)-methyl]azobenzene dibromide (3,3′-bisQ). This compound is photochromic and can exist in a cis or trans isomeric form, both of which have now been isolated in pure form. Trans-3,3′-bisQ is the most potent activator known, producing a 60-mV depolarization at 0.2 μM and 50% activity at 0.06 μM. The cis isomer is less than 1% as active. Its high activity and constrained structure suggest that trans-3,3′-bisQ can be considered to be a “template” of the combining site of AcChoR, when the latter is in the activated state. The following conclusions can then be drawn concerning the AcChoR binding site. (i) Depolarization can occur by interaction with reagents that are essentially inflexible. (ii) The binding site has a planar hydrophobic region that interacts with methylene groups of acetylcholine and with hydrophobic areas in general. (iii) In the same plane as the hydrophobic area is a site that interacts with electron-donating functional groups including the carbonyl oxygen of acetylcholine and the azo nitrogens of trans-3,3′-bisQ. (iv) About 1.5 Å out of the plane of the hydrophobic and the electron acceptor site is an anionic site; when the AcChoR is in the activated state, this site is separated from the electron acceptor site by 5.2 Å and from another anionic site by 11 Å. (v) The anionic sites are located within a cleft of limited size, sufficient to accommodate quaternary methyl groups. (vi) Although depolarization can occur with reagents that possess only hydrophobic and cationic groups if their geometric arrangement is proper, the highest activity resides in compounds capable of all of the interactions cited above.
Keywords: activators, inhibitors, dimensions of binding sites, binding site template, ligand flexibility
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels E., Wassermann N. H., Erlanger B. F. Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1820–1823. doi: 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers W. H., Reich E. Structure and activity of acetylcholine. Nature. 1970 Dec 5;228(5275):917–922. doi: 10.1038/228917a0. [DOI] [PubMed] [Google Scholar]
- Bieth J., Vratsanos S. M., Wassermann N. H., Cooper A. G., Erlanger B. F. Photoregulation of biological activity by photochromic reagents. Inactivators of acetylcholinesterase. Biochemistry. 1973 Jul 31;12(16):3023–3027. doi: 10.1021/bi00740a012. [DOI] [PubMed] [Google Scholar]
- Chothia C., Pauling P. The conformation of cholinergic molecules at nicotinic nerve receptors. Proc Natl Acad Sci U S A. 1970 Mar;65(3):477–482. doi: 10.1073/pnas.65.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T. Purification and molecular properties of the acetylcholine receptor from Torpedo electroplax. Arch Biochem Biophys. 1973 Nov;159(1):362–373. doi: 10.1016/0003-9861(73)90462-1. [DOI] [PubMed] [Google Scholar]
- Fu J. L., Donner D. B., Hess G. P. Half-of-the-sites reactivity of the membrane-bound Electrophorus electricus acetylcholine receptor. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1072–1080. doi: 10.1016/0006-291x(74)90422-7. [DOI] [PubMed] [Google Scholar]
- HIGMAN H. B., PODLESKI T. R., BARTELS E. APPARENT DISSOCIATION CONSTANTS BETWEEN CARBAMYLCHOLINE, DELTA-TUBOCURARINE AND THE RECEPTOR. Biochim Biophys Acta. 1963 Sep 24;75:187–193. doi: 10.1016/0006-3002(63)90597-3. [DOI] [PubMed] [Google Scholar]
- Herdklotz J. K., Sass R. L. The crystal structure of acetylcholine chloride: a new conformation for acetylcholine. Biochem Biophys Res Commun. 1970 Aug 11;40(3):583–588. doi: 10.1016/0006-291x(70)90942-3. [DOI] [PubMed] [Google Scholar]
- Karlin A., Cowburn D. The affinity-labeling of partially purified acetylcholine receptor from electric tissue of Electrophorus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3636–3640. doi: 10.1073/pnas.70.12.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. The acetylcholine receptor: progress report. Life Sci. 1974 Apr 16;14(8):1385–1415. doi: 10.1016/0024-3205(74)90150-7. [DOI] [PubMed] [Google Scholar]
- Karlsson E., Heilbronn E., Widlund L. Isolation of the nicotinic acetylcholine receptor by biospecific chromatography on insolubilized Naja naja neurotoxin. FEBS Lett. 1972 Nov 15;28(1):107–111. doi: 10.1016/0014-5793(72)80688-4. [DOI] [PubMed] [Google Scholar]
- Klett R. P., Fulpius B. W., Cooper D., Smith M., Reich E., Possani L. D. The acetylcholine receptor. I. Purification and characterization of a macromolecule isolated from Electrophorus electricus. J Biol Chem. 1973 Oct 10;248(19):6841–6853. [PubMed] [Google Scholar]
- Mautner H. G., Bartels E., Webb G. D. Sulfur and selenium isologs related to acetycholine. and choline IV. Activity in the electroplax preparation. Biochem Pharmacol. 1966 Feb;15(2):187–193. doi: 10.1016/0006-2952(66)90059-1. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Changeux J. P. Comparison between the affinities for reversible cholinergic ligands of a purified and membrane bound state of the acetylcholine-receptor protein from Electrophorus electricus. FEBS Lett. 1973 May 15;32(1):143–148. doi: 10.1016/0014-5793(73)80758-6. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Olsen R., Menez A., Morgat J. L., Fromageot P., Ronseray A. M., Boquet P., Changeux J. P. Quelques propriétés physique de la protéine réceptrice de l'acétylcholine étudiées à l'aide d'une neurotoxine radioactive. C R Acad Sci Hebd Seances Acad Sci D. 1971 Aug 2;273(5):595–598. [PubMed] [Google Scholar]
- Pauling P., Petcher T. J. Neuromuscular blocking agents: structure and activity. Chem Biol Interact. 1973 Jun;6(6):351–365. doi: 10.1016/0009-2797(73)90056-2. [DOI] [PubMed] [Google Scholar]
- Pullman B., Courrière P., Coubeils J. L. Quantum mechanical study of the conformational and electronic properties of acetylcholine and its agonists muscarine and nicotine. Mol Pharmacol. 1971 Jul;7(4):397–405. [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. Use of affinity chromatography for acetylcholine receptor purification. Biochem Biophys Res Commun. 1972 Oct 17;49(2):572–578. doi: 10.1016/0006-291x(72)90449-4. [DOI] [PubMed] [Google Scholar]



