Background: Leukocyte integrins are involved in cell adhesion, cell migration, and phagocytosis.
Results: CD11c/CD18, a leukocyte integrin expressed on monocytes, macrophages, and dendritic cells, is phosphorylated on Ser-1158 of the α-chain. Abrogation of phosphorylation results in impaired adhesion and phagocytosis.
Conclusion: Integrin inside-out activated adhesion is regulated by a single phosphorylation site on CD11c.
Significance: Regulation of integrin activity is essential for immune cells.
Keywords: Adhesion, Integrins, Leukocyte, Phagocytosis, Phosphorylation
Abstract
CD11c/CD18 (αXβ2, p150/95, or complement receptor 4, CR4) is a monocyte/macrophage-enriched integrin that has been reported to bind to a variety of ligands. These include cell surface proteins, extracellular matrix proteins, and soluble ligands. The regulation of ligand binding to CD11c/CD18 has remained poorly understood. Previous work has shown that both α-chain and β-chain phosphorylations of CD11a/CD18 and CD11b/CD18 are needed for activity, but no corresponding studies on CD11c/CD18 have been performed. In this study, we have identified the phosphorylation site of CD11c as Ser-1158 and show that it is pivotal for adherence and phagocytosis.
Introduction
The integrins are heterodimeric cell surface receptors, which convey signals across the plasma membrane in both directions (outside-in and inside-out) (1). CD11c/CD18 (αXβ2, p150.95, complement receptor 4, CR4) is one of the four members of the β2 leukocyte integrin (CD11/CD18) family. Other members include CD11a/CD18 (αLβ2, LFA-1), CD11b/CD18 (αMβ2, Mac-1), and CD11d/CD18 (αDβ2). The leukocyte CD11/CD18 integrins are involved in various immunological functions, including cell adhesion, migration, and phagocytosis (2–6).
CD11c/CD18 is expressed on macrophages, monocytes, granulocytes, subsets of T and B cells, and dendritic cells (6). It interacts with extracellular matrix molecules such as collagen I (7), soluble ligands such as iC3b (8, 9), heparin (10), and fibrinogen (11), and the cell surface immunoglobulin superfamily proteins intercellular adhesion molecules ICAM-1 (12), ICAM-2 (13), and ICAM-4 (14) and vascular adhesion molecule VCAM-1 (13). CD11c/CD18 has also been found to bind to denatured proteins (15), and especially negatively charged amino acid residues on decayed proteins (16).
The structure of the CD11c/CD18 extracellular portion has been solved (17), and it shows a relatively tight intersubunit interaction (8, 18). This feature may contribute to the fact that it is more resistant to activation than other leukocyte integrins. CD11c/CD18 has been considered as a marker for dendritic cells, but its function remained unclear for long. Recently, it has been shown to have an important role in antigen uptake and presentation, generating CD4 and CD8 T cell-mediated responses and providing a useful approach for antitumor vaccine development (19). Targeting CD11c has proven successful using either anti-CD11c Fab fragments (19) or peptides against CD11c/CD18 derived from ICAM-4 and ICAM-1-extracellular CD11c/CD18 binding domains (20). CD11c/CD18 has also been shown to have a role in the transendothelial migration of macrophages to atherosclerotic plaques in hypercholesterolemic mice (13, 21, 22). Another suggested role for CD11c/CD18 is in the removal of senescent red cells from the bloodstream through the interactions between red cell ICAM-4 and CD11c/CD18 on spleen macrophages (14).
Recent work has shown that integrin phosphorylations are of pivotal importance for the regulation of their activity (3). Phosphorylation of Thr-758 on the β2 chain of CD11a/CD18 results in recruitment of 14-3-3 proteins followed by binding of the adaptor protein Tiam1 and activation of the small G protein Rac-1 (3, 23, 24). These events result in reorganization of the cytoskeleton and increased adhesion. In addition, α-chain phosphorylation of Ser-1140 in CD11a/CD18 and Ser-1126 in CD11b/CD18 is needed for adhesion-related functions (23, 25).
The α-chain of CD11c/CD18 contains several potential phosphorylation sites (see Fig. 1B). It is known to be phosphorylated on serine, with traces of threonine phosphorylation (26, 27). We now report that Ser-1158 is the major phosphorylation site in CD11c and mutation of this residue abrogates CD11c/CD18-dependent functions. We also show that Thr-758 on the CD18 chain of CD11c/CD18 is necessary for its activity. In summary, our findings demonstrate that the CD11c/CD18 α-chain phosphorylation is needed for CD11c-mediated functions, but CD18 phosphorylation is responsible for the regulation of activity.
FIGURE 1.
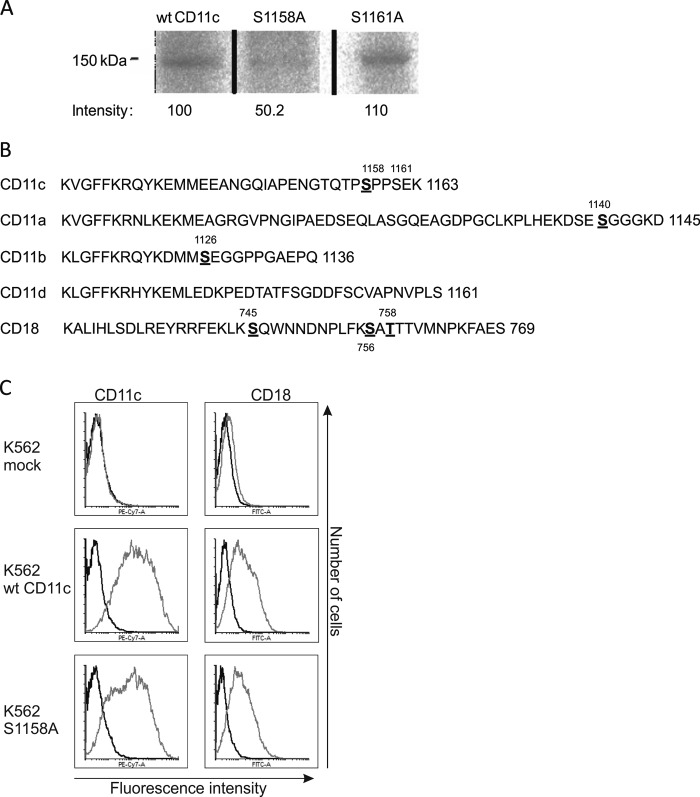
Ser-1158 is the major phosphorylation site in CD11c. A, in vivo phosphorylation of CD11c. COS-1 cells were transiently transfected with WT CD11c, S1158A CD11c, or S1161A CD11c together with WT CD18. Cells were metabolically labeled with radioactive phosphate, and CD11c/CD18 heterodimers were immunoprecipitated and analyzed using SDS-PAGE and autoradiography. B, sequence of the CD11c cytoplasmic domain as compared with CD11a, CD11b, CD11d, and CD18 cytoplasmic domains. The phosphorylation sites are shown in bold and numbered. C, flow cytometric analysis of K562 cells transfected with empty plasmid (mock), WT CD11c/CD18, or S1158A CD11c/CD18.
EXPERIMENTAL PROCEDURES
Monoclonal Antibodies
The activating anti-CD18 CBR LFA-1/2 and heterodimer-recognizing anti-CD18 IB4 were gifts from T. A. Springer (Harvard Medical School, Boston, MA) and M. A. Arnaout (Massachusetts General Hospital, Boston, MA), respectively. Anti-CD11c 3.9 was from N. Hogg (Cancer Research UK, London, UK). Anti-CD18 7E4 has been reported previously (28). Anti-Syk2 was from BD Biosciences, and anti-phospho-Syk (Tyr-525/526) from Cell Signaling (Danvers, MA).
Plasmid DNA
Human WT CD18 and T758A CD18 in πH3M plasmid have been described (23). The cDNA coding for full-length human CD11c in the pCDM8 vector was a gift from Y. van Kooyk (Vrije Universiteit Medical Center, Amsterdam, The Netherlands). CD11c mutants were created by using site-directed mutagenesis and sequenced. CD18 in pCDNA3.1 (+) was from Addgene (Cambridge, MA) (plasmid 8640, Ref. 29). Rap1V12-pMT2-HA (30) was a gift from J. L. Bos (Molecular Cancer Research, University Medical Center Utrecht, The Netherlands).
Cell Culture and Transfection
HEK293T and COS-1 cell lines were cultured in DMEM (Sigma-Aldrich) supplemented with 10% FBS (Thermo Fisher Scientific), 2 mm l-glutamine, and 100 units/ml penicillin-streptomycin (Lonza, Basel, Switzerland). Cells were transiently transfected with WT CD18 or T758A CD18 and WT or mutated CD11c using TurboFect (Thermo Fisher Scientific).
The K562 cell line, which lacks β2 integrins (Ref. 31, ATCC CCL-243), was cultured in Iscove's modified Dulbecco's medium (Lonza) supplemented as above. Stable transfectants were generated by transfecting the cells with WT CD11c or S1158A CD11c together with CD18-πH3M or with empty pCDM8 plasmid alone (mock cells), using Lipofectamine LTX reagent (Invitrogen) and further enriched with MACS magnetic beads coated with anti-CD11c or anti-CD18 mAbs (Miltenyi Biotech, Bergisch Gladbach, Germany). Stably transfected cells were cultured in the above mentioned medium supplemented with 0.5 mg/ml G418 (Calbiochem/Merck Millipore). When needed, stable K562 transfectants were co-transfected with Rap1V12-pMT2-HA using Lipofectamine LTX.
Flow Cytometry
Expression of integrins was studied with the following antibodies: CD11c-PC7 and CD18-FITC (Beckman Coulter, Brea, CA). Cells were run on LSR II flow cytometer (BD Biosciences).
32P Labeling, Immunoprecipitation, and SDS-PAGE
Metabolic 32P labeling, immunoprecipitation, and SDS-PAGE were done as described previously (23). Briefly, COS-1 cells were transiently transfected with WT CD11c, S1158A CD11c, or S1161A CD11c together with WT CD18 48 h before labeling. Cells were metabolically labeled with [32P]orthophosphate (GE Healthcare, Buckinghamshire, UK) in the presence of 1.5 μm okadaic acid, treated with 200 nm phorbol 12,13-dibutyrate, and lysed. CD11c/CD18 heterodimers were immunoprecipitated, subjected to SDS-PAGE, transferred onto polyvinylidene fluoride membranes, and exposed on a phosphorimaging plate. Plates were read by BAS-1500 (Fujifilm, Tokyo, Japan), and the intensity of the radiolabeled bands was quantified.
Cell Adhesion Assays
Cell adhesion assays were performed essentially as described previously (14). Mock-, WT CD11c/CD18-, or S1158A CD11c/CD18-transfected K562 cells were allowed to bind to iC3b (Calbiochem) coated on NUNC MaxiSorp 96-well plates (Thermo Fisher Scientific). 1–2 × 105cells were incubated in the coated wells without treatment or after incubation with the adhesion inhibitory antibodies 7E4 (anti-CD18) or 3.9 (anti-CD11c) or the activating antibody CBR LFA-1/2. In some experiments, K562 cells co-transfected with constitutively active Rap1 (Rap1 V12-HA) were used. The wells were washed by floating upside down, and the bound cells were lysed and detected with phosphatase substrate (Sigma-Aldrich). Adhesion is reported as the percentage of bound cells out of those added. HEK293T cells, transiently transfected with CD11c (WT or mutants) together with CD18 (WT or T758A CD18), were allowed to bind to iC3b or denatured BSA (heated for 5 min at 95 °C) coated on plastic as above.
Phagocytosis of iC3b-coated Microspheres
Fluoresbrite carboxy YG 2.0 micron microspheres (Polysciences Inc., Warrington, PA) were coated with iC3b according to the manufacturer's instructions. Control beads were left uncoated. For phagocytosis assays, K562 cells were induced with 10 ng/ml phorbol 12-myristate 13-acetate (Sigma-Aldrich) for 24 h before phagocytosis assay. Cells were washed with Krebs-Ringer PBS (PBS, 1 mm Ca2+, 1,5 mm Mg2+, 5.5 mm d-glucose) and incubated with the iC3b-coated beads for 2 h. Cytochalasin D (20 μg/ml) was used to prevent phagocytosis. After incubation, the cells were washed with PBS and subjected to flow cytometry. Trypan blue was used to quench the fluorescence of the beads bound to the cell surface. Cells were gated, and mean fluorescence intensities on the green channel were compared relative to the expression level of CD11c.
Detection of Phosphorylated Syk
K562 cells expressing WT CD11c/CD18 or S1158A CD11c/CD18 were activated with CBR LFA-1/2 or control-IgG for 30 min. Cells were lysed in 10 mm Tris, pH 8,0, 150 mm NaCl, 1% Nonidet P-40 containing protease and phosphatase inhibitor (Pierce). Lysates were treated with anti-Syk or control antibody and protein G-Sepharose (Invitrogen). Immunoprecipitates were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Phosphorylated Syk was detected with phospho-Syk (Tyr-525/526) antibody, after which the blots were stripped and total Syk was detected with the anti-Syk antibody.
RESULTS AND DISCUSSION
CD11c/CD18 Is Phosphorylated on Ser-1158 of the CD11c Chain
Previous work has shown that α-chain phosphorylation is important for CD11a/CD18 and CD11b/CD18 activities (23, 25). CD11c is known to be phosphorylated on serine (26), but the phosphorylation site has remained unknown. To determine the site(s), we substituted the possible phosphorylatable serines (Ser-1158 and Ser-1161) with alanine and transiently transfected COS-1 cells with WT or mutated CD11c/CD18. The cells were then radioactively labeled, the integrins were immunoprecipitated, and the phosphorylated protein was detected by autoradiography. The S1158A CD11c mutant showed weak radioactive labeling, whereas in the S1161A CD11c transfectants, the labeling was at the level of WT CD11c (Fig. 1A). The levels of expression were similar as shown by flow cytometry (not shown). This indicates that Ser-1158 is the major phosphorylation site on CD11c.
S1158A Mutation of CD11c Chain Decreases the Binding to iC3b
To study functional roles of CD11c phosphorylation, we generated a K562 leukemia cell line stably expressing WT CD11c/CD18 or the phosphorylation mutant S1158A CD11c/CD18. The mutation of Ser-1158 to alanine did not alter the surface expression of CD11c/CD18 on K562 cells (Fig. 1C), nor on transiently transfected COS-1 or HEK293T cells (not shown).
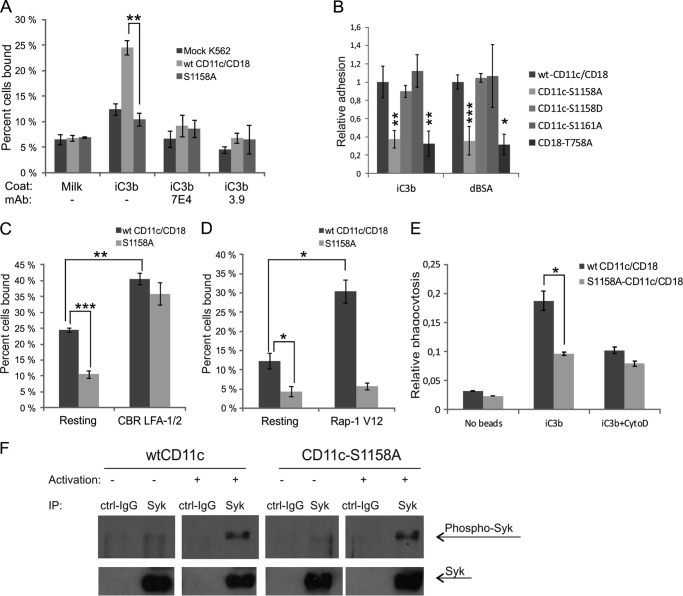
K562 transfectants were allowed to adhere to CD11c/CD18 ligand iC3b coated on plastic. Mutation of the α-chain phosphorylation site to alanine abrogated the binding (Fig. 2A). The binding was CD11c/CD18-dependent as it was inhibited by the adhesion inhibiting antibodies, 3.9 against CD11c and 7E4 against CD18. Interestingly, the iC3b binding to CD11b/CD18, a close homologue of CD11c/CD18, is not phosphorylation-dependent, whereas the binding of CD11b/CD18 to ICAM-1 and ICAM-2 is dependent on α-chain phosphorylation on Ser-1126 (25). However, binding of CD11a/CD18 to all ligands tested requires phosphorylatable Ser-1140 (23). The binding of the CD11c/CD18 extracellular part to iC3b has been studied in detail with negative stain EM, and the binding sites on the integrin I domain are well characterized (9). Also the binding site of iC3b on CD11b/CD18 has been localized to the I domain and more specifically to a 14-mer peptide taking part in divalent cation chelation (32).
FIGURE 2.
Phosphorylation on Ser-1158 regulates CD11c/CD18 inside-out activation, phagocytosis, and adhesion to ligands. A, mock-, WT CD11c/CD18-, or S1158A CD11c/CD18-transfected K562 cells were allowed to bind to iC3b coated on plastic. Cells were incubated in the ligand-coated wells with or without incubation with the adhesion inhibitory antibodies 7E4 (anti-CD18) or 3.9 (anti-CD11c). Adhesion is reported as the percentage of bound cells out of those added in total. B, HEK293T cells transiently transfected with WT CD11c/CD18, S1158A CD11c/CD18, S1158D CD11c/CD18, S1161A CD11c/CD18, or T758A CD11c/CD18 were allowed to bind to iC3b or denatured BSA (dBSA)-coated on plastic. Cell binding is reported relative to WT CD11c/CD18 transfectant binding. Asterisks indicate p values as compared with WT CD11c/CD18 binding. C and D, K562 transfectants were allowed to bind to iC3b. Before adhesion, cells were stimulated with the activating antibody CBR LFA-1/2 or co-transfected with constitutively active Rap1 (Rap1 V12-HA). E, K562 cells were pretreated with phorbol 12-myristate 13-acetate for 24 h and incubated with iC3b-coated microspheres with or without cytochalasin D (CytoD). Phagocytosis of fluorescent microspheres was detected with flow cytometry after quenching the extracellular microsphere fluorescence. The results are reported relative to CD11c expression level. F, K562 were activated with control IgG or the activating antibody CBR LFA-1/2 for 30 min, lysed, and subjected to immunoprecipitation (IP) with anti-Syk antibody. Western blots were detected with anti-phospho-Syk (Tyr-525/526), stripped, and reprobed with anti-Syk to show the amount of total Syk protein. ***, p < 0.001 **, p < 0.01 *, p < 0.05.
Decrease of Adhesion Is Specific for the S1158A Mutation
To find out whether the inhibition of adhesion is due to general alterations in the cytoplasmic moiety of CD11c or CD18, and whether phosphorylation affects binding to iC3b alone or also to other ligands, we used HEK293T cells due to their ease of transfection. HEK293T cells were transfected with different cytoplasmic mutants of the CD11c and CD18 chains, and their adhesion to iC3b and denatured BSA was measured. In these cells, the S1158A mutation of the CD11c chain had a similar effect as in K562 cells, whereas the mutation of Ser-1161 did not affect the binding to iC3b. To mimic phosphorylation, we also used an S1158D mutated integrin, which bound to iC3b like WT CD11c/CD18. Phosphorylation of Thr-758 on CD18 chain has been shown to be important for the binding of CD11a/CD18 to its ligands (23). As shown in Fig. 2B, the mutation of Thr-758 to alanine disrupted the adhesion of CD11c/CD18-transfected cells to the same level as the S1158A mutation of CD11c. Similar levels of CD11c/CD18 expression were ascertained using flow cytometry (not shown). We conclude that the alteration of adhesion to iC3b and denatured BSA is due to lack of phosphorylation on specific α- and β-chain sites.
The adhesion efficiency of WT CD11c/CD18 K562 and HEK293T transfectants is relatively high, taking into account that CD11c/CD18 is usually considered to be in an inactive conformation (18, 33). We therefore studied the activity of the integrin using mAb KIM127 that recognizes the extended conformation considered to be needed for ligand binding (34). Our finding was supported by relatively high expression of KIM127 epitope on K562 cells even without activation of the cells, indicating a basal activation of CD11c/CD18 in transfected K562 cells (not shown).
The S1158A Mutation Does Not Affect Outside-in Activation
We further studied the effect of CD11c phosphorylation on its ligand binding properties by activating the K562 cells with the CBR LFA-1/2 antibody that binds to the extracellular domain of CD11c/CD18, induces the extended-open conformation, and enhances the binding to iC3b (35). The S1158A-containing cells were able to bind iC3b as strongly as WT CD11c cells upon activation with CBR LFA-1/2 (Fig. 2C). Furthermore, we investigated the effect of extracellular activation to induce downstream signaling in K562 cells transfected with WT or S1158A CD11c/CD18. For that purpose, we studied the phosphorylation of Syk, a nonreceptor tyrosine kinase that has been shown to be important for spreading of polymorphonuclear cells (36) (Fig. 2F). Cells were activated with CBR LFA-1/2 and lysed, and Syk was immunoprecipitated from the lysates. Activation of Syk was detected with anti-phospho-Syk (Tyr-525/526) antibody. Phosphorylated Syk was detected in a similar amount in both cell lines after activation. The results showed that the outside-in signaling was not changed. Thus the α-chain phosphorylation is not needed for outside-in signaling.
The S1158A Mutation Inhibits Inside-out Activation
To study the inside-out activation of CD11c/CD18, we used a constitutively active Rap1 construct. Rap1 is a small GTPase that has been shown to be involved in the affinity regulation of CD11a/CD18 and CD11b/CD18 (37). In the Jurkat T-cell line, Rap1 was not able to activate the cells expressing the phosphorylation mutant S1140A CD11a/CD18, whereas the WT CD11a/CD18 was activated to bind ICAM-1 (23). The effect of Rap1 on CD11b/CD18 function and activation has been studied in detail, and in COS-7 cells, the inside-out activation produced by Rap1 is shown to occur through the CD18 chain (38). To find out how the phosphorylation of CD11c affects Rap1-mediated activation, we co-transfected the WT CD11c/CD18- or S1158A CD11c/CD18-expressing K562 cells with constitutively active Rap1 (Rap1V12). The S1158A CD11c/CD18 cells could not be activated with Rap1V12, whereas the WT CD11c/CD18 cells showed high binding to iC3b (Fig. 2D). Equal Rap1V12-HA expression was determined by ELISA. Our results demonstrate that Rap1 can activate CD11c/CD18 and that the phosphorylation on Ser-1158 plays a role in inside-out activation through Rap1.
Phosphorylation of Ser-1158 Is Important in Phagocytosis
To study whether the phosphorylation is important for other functions, we studied the effect of the phosphorylatable Ser-1158 on phagocytosis. The K562 cell line is an erythroid precursor line that can be induced to monocyte/macrophage differentiation by phorbol esters (31, 39). For phagocytosis assays, K562 cells were allowed to differentiate toward macrophages and incubated with iC3b-coated or control fluorescent microspheres. Expression of WT CD11c/CD18 on K562 significantly enhanced phagocytosis, whereas expression of S1158A CD11c/CD18 did not change the phagocytosis level from that of nontransfected cells (Fig. 2E).
The phosphorylation of the CD18 chain on T758 is a major event in the activation of β2 integrins, and it has been shown to release the binding of talin to CD18 and further enable the binding of 14-3-3 to the cytoplasmic tail of CD18. It has been suggested that this binding occurs at later time points after stimulation and that 14-3-3 outcompetes talin head domain binding in the activation cascade (40). Further events in T cell adhesion have also been studied, and it has been shown that the 14-3-3 dimer bound to the T758-phosphorylated CD18 intracellular tail is able to activate Rac1 and Cdc-42 through Tiam1, a Rac1-activating guanine nucleotide exchange factor. This leads to actin cytoskeleton rearrangements, cell adhesion, and migration (24).
The importance of integrin β chain phosphorylations in these activation events leading to integrin activation, actin cytoskeleton reorganization, cell adhesion, migration, and phagocytosis is well established, but far less is known about α-chain cytoplasmic events involved in integrin activity and functions (3, 41). It has previously been shown that in neutrophils, monocytes, and mononuclear cells, CD11c is constitutively phosphorylated and the CD18 becomes phosphorylated upon activation (26, 27).
Although the extracellular domain structures of CD11c/CD18 in different activation states and binding sites of iC3b have been solved (9, 17), no studies on CD11c/CD18 intracellular domain signaling and interactions have been reported. However, the structure of the cytosolic tail of CD11c/CD18 has been solved with NMR, and interesting similarities but also differences with other α-chain structures were observed (42). Together with our present results, these findings may help to clarify the regulation of the CD11c/CD18 integrin. Furthermore, most studies on β2 integrin cytoplasmic regulation have been made in T cells and transfected cell lines (23–25, 37, 40) but not in cells of myelomonocytic or dendritic lineages, where CD11c/CD18 is highly expressed. In addition, most of the recent studies on activation of CD11c/CD18 have been made with purified proteins and not in a cellular context.
In summary, our findings demonstrate the importance of CD11c/CD18 α-chain phosphorylation for integrin activity and adhesion. In general, it may be that the role of the CD11c/CD18 is also relevant outside the circulation as it is enriched in macrophages and dendritic cells and binds to several extracellular matrix ligands. All the suggested functions involve adhesion, and understanding the regulation of CD11c/CD18 is a prerequisite for further elucidation of this complex integrin.
Acknowledgments
We thank T. A. Springer, M. A. Arnaout, and N. Hogg for antibodies, Y. van Kooyk and J. L. Bos for plasmids, L. Kuoppasalmi for expert technical support, and Y. Heinilä, L. Sokura, H. Sarkimaa, and K. Fellman for secretarial assistance. We thank M. Grönholm for valuable comments on the manuscript.
This work was supported in part by grants from the Academy of Finland, the Sigrid Jusélius Foundation, the Finska Läkaresällskapet, the Liv och Hälsa Foundation, the Magnus Ehrnrooth Foundation, and the Wilhelm and Else Stockmann Foundation.
- Syk
- spleen tyrosine kinase.
REFERENCES
- 1. Hynes R. O. (2002) Integrins: Bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 2. Luo B. H., Carman C. V., Springer T. A. (2007) Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gahmberg C. G., Fagerholm S. C., Nurmi S. M., Chavakis T., Marchesan S., Grönholm M. (2009) Regulation of integrin activity and signalling. Biochim. Biophys. Acta 1790, 431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye F., Kim C., Ginsberg M. H. (2012) Reconstruction of integrin activation. Blood 119, 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gahmberg C. G., Tolvanen M., Kotovuori P. (1997) Leukocyte adhesion–structure and function of human leukocyte β2-integrins and their cellular ligands. Eur. J. Biochem. 245, 215–232 [DOI] [PubMed] [Google Scholar]
- 6. Tan S.-M. (2012) The leucocyte β2 (CD18) integrins: the structure, functional regulation and signaling properties. Biosci. Rep. 32, 241–269 [DOI] [PubMed] [Google Scholar]
- 7. Garnotel R., Rittié L., Poitevin S., Monboisse J. C., Nguyen P., Potron G., Maquart F. X., Randoux A., Gillery P. (2000) Human blood monocytes interact with type I collagen through αXβ2 integrin (CD11c-CD18, gp150–95). J. Immunol. 164, 5928–5934 [DOI] [PubMed] [Google Scholar]
- 8. Bilsland C. A., Diamond M. S., Springer T. A. (1994) The leukocyte integrin p150,95 (CD11c/CD18) as a receptor for iC3b. Activation by a heterologous β subunit and localization of a ligand recognition site to the I domain. J. Immunol. 152, 4582–4589 [PubMed] [Google Scholar]
- 9. Chen X., Yu Y., Mi L. Z., Walz T., Springer T. A. (2012) Molecular basis for complement recognition by integrin αXβ2. Proc. Natl. Acad. Sci. U.S.A. 109, 4586–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vorup-Jensen T., Chi L., Gjelstrup L. C., Jensen U. B., Jewett C. A., Xie C., Shimaoka M., Linhardt R. J., Springer T. A. (2007) Binding between the integrin αXβ2 (CD11c/CD18) and heparin. J. Biol. Chem. 282, 30869–30877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loike J. D., Sodeik B., Cao L., Leucona S., Weitz J. I., Detmers P. A., Wright S. D., Silverstein S. C. (1991) CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the Aα chain of fibrinogen. Proc. Natl. Acad. Sci. U.S.A. 88, 1044–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blackford J., Reid H. W., Pappin D. J., Bowers F. S., Wilkinson J. M. (1996) A monoclonal antibody, 3/22, to rabbit CD11c which induces homotypic T cell aggregation: Evidence that ICAM-1 is a ligand for CD11c/CD18. Eur. J. Immunol. 26, 525–531 [DOI] [PubMed] [Google Scholar]
- 13. Sadhu C., Ting H. J., Lipsky B., Hensley K., Garcia-Martinez L. F., Simon S. I., Staunton D. E. (2007) CD11c/CD18: Novel ligands and a role in delayed-type hypersensitivity. J. Leukoc. Biol. 81, 1395–1403 [DOI] [PubMed] [Google Scholar]
- 14. Ihanus E., Uotila L. M., Toivanen A., Varis M., Gahmberg C. G. (2007) Red-cell ICAM-4 is a ligand for the monocyte/macrophage integrin CD11c/CD18: Characterization of the binding sites on ICAM-4. Blood 109, 802–810 [DOI] [PubMed] [Google Scholar]
- 15. Davis G. E. (1992) The Mac-1 and p150,95 β2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Exp. Cell Res. 200, 242–252 [DOI] [PubMed] [Google Scholar]
- 16. Vorup-Jensen T., Carman C. V., Shimaoka M., Schuck P., Svitel J., Springer T. A. (2005) Exposure of acidic residues as a danger signal for recognition of fibrinogen and other macromolecules by integrin αXβ2. Proc. Natl. Acad. Sci. U.S.A. 102, 1614–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xie C., Zhu J., Chen X., Mi L., Nishida N., Springer T. A. (2010) Structure of an integrin with an αI domain, complement receptor type 4. EMBO J. 29, 666–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zang Q., Springer T. A. (2001) Amino acid residues in the PSI domain and cysteine-rich repeats of the integrin β2 subunit that restrain activation of the integrin αXβ2. J. Biol. Chem. 276, 6922–6929 [DOI] [PubMed] [Google Scholar]
- 19. Castro F. V. V., Tutt A. L., White A. L., Teeling J. L., James S., French R. R., Glennie M. J. (2008) CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses. Eur. J. Immunol. 38, 2263–2273 [DOI] [PubMed] [Google Scholar]
- 20. Faham A., Altin J. G. (2011) Ag-bearing liposomes engrafted with peptides that interact with CD11c/CD18 induce potent ag-specific and antitumor immunity. Int. J. Cancer 129, 1391–1403 [DOI] [PubMed] [Google Scholar]
- 21. Wu H., Gower R. M., Wang H., Perrard X.-Y., Ma R., Bullard D. C., Burns A. R., Paul A., Smith C. W., Simon S. I., Ballantyne C. M. (2009) Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation 119, 2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gower R. M., Wu H., Foster G. A., Devaraj S., Jialal I., Ballantyne C. M., Knowlton A. A., Simon S. I. (2011) CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to VCAM-1. Arterioscler. Thromb. Vasc. Biol. 31, 160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fagerholm S. C., Hilden T. J., Nurmi S. M., Gahmberg C. G. (2005) Specific integrin α and β chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. J. Cell Biol. 171, 705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grönholm M., Jahan F., Marchesan S., Karvonen U., Aatonen M., Narumanchi S., Gahmberg C. G. (2011) TCR-induced activation of LFA-1 involves signaling through Tiam1. J. Immunol. 187, 3613–3619 [DOI] [PubMed] [Google Scholar]
- 25. Fagerholm S. C., Varis M., Stefanidakis M., Hilden T. J., Gahmberg C. G. (2006) α-Chain phosphorylation of the human leukocyte CD11b/CD18 (Mac-1) integrin is pivotal for integrin activation to bind ICAMs and leukocyte extravasation. Blood 108, 3379–3386 [DOI] [PubMed] [Google Scholar]
- 26. Chatila T. A., Geha R. S., Arnaout M. A. (1989) Constitutive and stimulus-induced phosphorylation of CD11/CD18 leukocyte adhesion molecules. J. Cell Biol. 109, 3435–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buyon J. P., Slade S. G., Reibman J., Abramson S. B., Philips M. R., Weissmann G., Winchester R. (1990) Constitutive and induced phosphorylation of the α- and β-chains of the CD11/CD18 leukocyte integrin family. J. Immunol. 144, 191–197 [PubMed] [Google Scholar]
- 28. Nortamo P., Patarroyo M., Kantor C., Suopanki J., Gahmberg C. G. (1988) Immunological mapping of the human leucocyte adhesion glycoprotein gp90 (CD18) by monoclonal antibodies. Scand. J. Immunol. 28, 537–546 [DOI] [PubMed] [Google Scholar]
- 29. Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. (1987) Cloning of the β subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell 48, 681–690 [DOI] [PubMed] [Google Scholar]
- 30. Zwartkruis F. J., Wolthuis R. M., Nabben N. M., Franke B., Bos J. L. (1998) Extracellular signal-regulated activation of Rap1 fails to interfere in Ras effector signalling. EMBO J. 17, 5905–5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersson L. C., Jokinen M., Gahmberg C. G. (1979) Induction of erythroid differentiation in the human leukaemia cell line K562. Nature 278, 364–365 [DOI] [PubMed] [Google Scholar]
- 32. Ueda T., Rieu P., Brayer J., Arnaout M. A. (1994) Identification of the complement iC3b binding site in the β2 integrin CR3 (CD11b/CD18). Proc. Natl. Acad. Sci. U.S.A. 91, 10680–10684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen X., Xie C., Nishida N., Li Z., Walz T., Springer T. A. (2010) Requirement of open headpiece conformation for activation of leukocyte integrin αXβ2. Proc. Natl. Acad. Sci. U.S.A. 107, 14727–14732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu C., Ferzly M., Takagi J., Springer T. A. (2001) Epitope mapping of antibodies to the C-terminal region of the integrin β2 subunit reveals regions that become exposed upon receptor activation. J. Immunol. 166, 5629–5637 [DOI] [PubMed] [Google Scholar]
- 35. Petruzzelli L., Maduzia L., Springer T. A. (1995) Activation of lymphocyte function-associated molecule-1 (CD11a/CD18) and mac-1 (CD11b/CD18) mimicked by an antibody directed against CD18. J. Immunol. 155, 854–866 [PubMed] [Google Scholar]
- 36. Mócsai A., Abram C. L., Jakus Z., Hu Y., Lanier L. L., Lowell C. A. (2006) Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat. Immunol. 7, 1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bos J. L. (2005) Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17, 123–128 [DOI] [PubMed] [Google Scholar]
- 38. Lim J., Dupuy A. G., Critchley D. R., Caron E. (2010) Rap1 Controls activation of the aMb2 integrin in a talin-dependent manner. J. Cell. Biochem. 111, 999–1009 [DOI] [PubMed] [Google Scholar]
- 39. Sutherland J. A., Turner A. R., Mannoni P., McGann L. E., Turc J. M. (1986) Differentiation of K562 leukemia cells along erythroid, macrophage, and megakaryocyte lineages. J. Biol. Response Mod. 5, 250–262 [PubMed] [Google Scholar]
- 40. Takala H., Nurminen E., Nurmi S. M., Aatonen M., Strandin T., Takatalo M., Kiema T., Gahmberg C. G., Ylänne J., Fagerholm S. C. (2008) β2 integrin phosphorylation on Thr758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood 112, 1853–1862 [DOI] [PubMed] [Google Scholar]
- 41. Kinashi T. (2005) Intracellular signaling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 5, 546–559 [DOI] [PubMed] [Google Scholar]
- 42. Chua G.-L., Tang X.-Y., Patra A. T., Tan S.-M., Bhattacharjya S. (2012) Structure and binding interface of the cytosolic tails of αXβ2 integrin. PLoS ONE 7, e41924. [DOI] [PMC free article] [PubMed] [Google Scholar]