Abstract
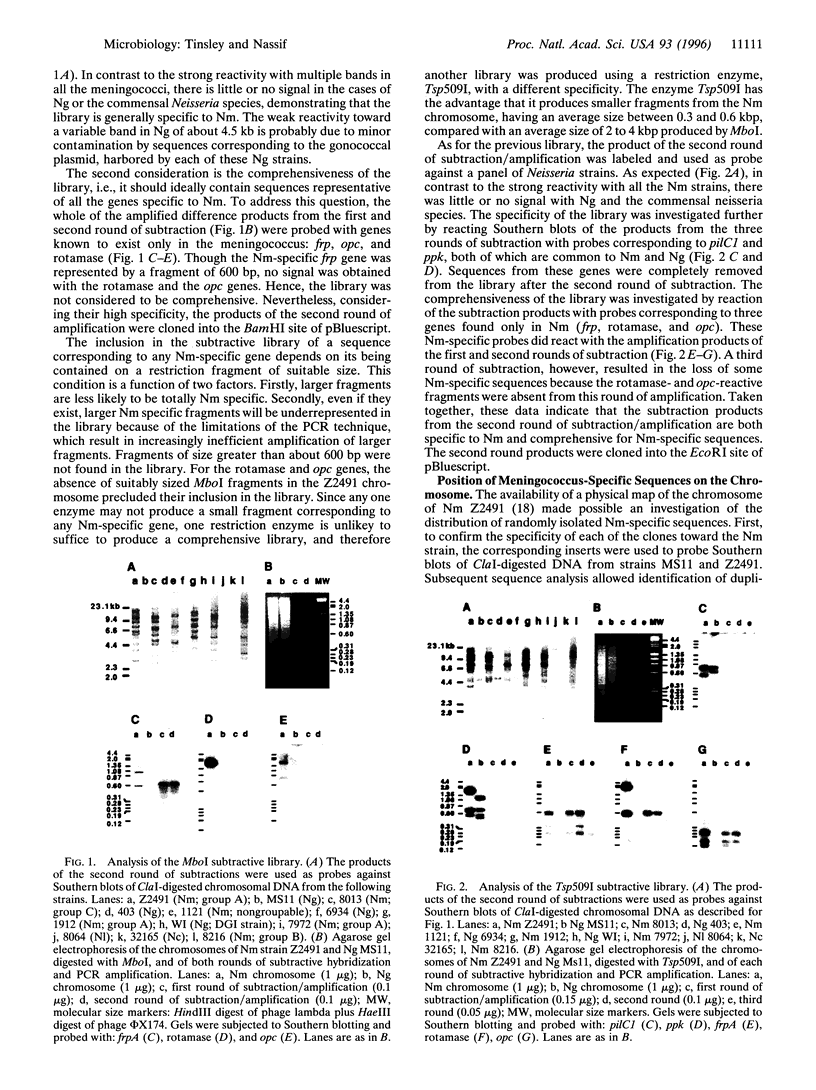
We have investigated genetic differences between the closely related pathogenic Neisseria species, Neisseria meningitidis and Neisseria gonorrhoeae, as a novel approach to the elucidation of the genetic basis for their different pathogenicities. N. meningitidis is a major cause of cerebrospinal meningitis, whereas N. gonorrhoeae is the agent of gonorrhoea. The technique of representational difference analysis was adapted to the search for genes present in the meningococcus but absent from the gonococcus. The libraries achieved are comprehensive and specific in that they contain sequences corresponding to the presently identified meningococcus-specific genes (capsule, frp, rotamase, and opc) but lack genes more or less homologous between the two species, e.g., ppk and pilC1. Of 35 randomly chosen clones specific to N. meningitidis, DNA sequence analysis has confirmed that the large majority have no homology with published neisserial sequences. Mapping of the cloned DNA fragments onto the chromosome of N. meningitidis strain Z2491 has revealed a nonrandom distribution of meningococcus-specific sequences. Most of the genetic differences between the meningococcus and gonococcus appear to be clustered in three distinct regions, one of which (region 1) contains the capsule-related genes. Region 3 was found only in strains of serogroup A, whereas region 2 is present in a variety of meningococci belonging to different serogroups. At a time when bacterial genomes are being sequenced, we believe that this technique is a powerful tool for a rapid and directed analysis of the genetic basis of inter- or intraspecific phenotypic variations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Neibert M., Crowe B. A., Strittmatter W., Kusecek B., Weyse E., Walsh M. J., Slawig B., Morelli G., Moll A. Purification and characterization of eight class 5 outer membrane protein variants from a clone of Neisseria meningitidis serogroup A. J Exp Med. 1988 Aug 1;168(2):507–525. doi: 10.1084/jem.168.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Wall R. A., Bopp M., Kusecek B., Morelli G., Saken E., Hassan-King M. Variation in class 5 protein expression by serogroup A meningococci during a meningitis epidemic. J Infect Dis. 1991 Aug;164(2):375–382. doi: 10.1093/infdis/164.2.375. [DOI] [PubMed] [Google Scholar]
- Ankenbauer R. G., Quan H. N. FptA, the Fe(III)-pyochelin receptor of Pseudomonas aeruginosa: a phenolate siderophore receptor homologous to hydroxamate siderophore receptors. J Bacteriol. 1994 Jan;176(2):307–319. doi: 10.1128/jb.176.2.307-319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen C. N., Sparling P. F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994 Dec;14(5):843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Wallace A. B., Cannon J. G. The physical map of the chromosome of a serogroup A strain of Neisseria meningitidis shows complex rearrangements relative to the chromosomes of the two mapped strains of the closely related species N. gonorrhoeae. J Bacteriol. 1995 Nov;177(22):6390–6400. doi: 10.1128/jb.177.22.6390-6400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch M., Müller A. Phospholipid substitution of capsular polysaccharides and mechanisms of capsule formation in Neisseria meningitidis. Mol Microbiol. 1993 May;8(3):483–493. doi: 10.1111/j.1365-2958.1993.tb01592.x. [DOI] [PubMed] [Google Scholar]
- Frosch M., Weisgerber C., Meyer T. F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibourdenche M., Popoff M. Y., Riou J. Y. Deoxyribonucleic acid relatedness among Neisseria gonorrhoeae, N. meningitidis, N. lactamica, N. cinerea and "Neisseria polysaccharea". Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):177–185. doi: 10.1016/s0769-2609(86)80106-5. [DOI] [PubMed] [Google Scholar]
- Gustafson C. E., Chu S., Trust T. J. Mutagenesis of the paracrystalline surface protein array of Aeromonas salmonicida by endogenous insertion elements. J Mol Biol. 1994 Apr 8;237(4):452–463. doi: 10.1006/jmbi.1994.1247. [DOI] [PubMed] [Google Scholar]
- Knight A. I., Ni H., Cartwright K. A., McFadden J. J. Identification and characterization of a novel insertion sequence, IS1106, downstream of the porA gene in B15 Neisseria meningitidis. Mol Microbiol. 1992 Jun;6(11):1565–1573. doi: 10.1111/j.1365-2958.1992.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N., Lisitsyn N., Wigler M. Cloning the differences between two complex genomes. Science. 1993 Feb 12;259(5097):946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- Mahairas G. G., Sabo P. J., Hickey M. J., Singh D. C., Stover C. K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996 Mar;178(5):1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. D., Sparling P. F. Isolation and identification of a glutathione peroxidase homolog gene, gpxA, present in Neisseria meningitidis but absent in Neisseria gonorrhoeae. Infect Immun. 1995 Apr;63(4):1603–1607. doi: 10.1128/iai.63.4.1603-1607.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X., Beretti J. L., Lowy J., Stenberg P., O'Gaora P., Pfeifer J., Normark S., So M. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3769–3773. doi: 10.1073/pnas.91.9.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olyhoek A. J., Sarkari J., Bopp M., Morelli G., Achtman M. Cloning and expression in Escherichia coli of opc, the gene for an unusual class 5 outer membrane protein from Neisseria meningitidis (meningococci/surface antigen). Microb Pathog. 1991 Oct;11(4):249–257. doi: 10.1016/0882-4010(91)90029-a. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson B. A., Gotschlich E. C. Neisseria meningitidis encodes an FK506-inhibitable rotamase. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1164–1168. doi: 10.1073/pnas.89.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Johnson W. M. Identification of epidemiologic markers for Neisseria meningitidis using difference analysis. Gene. 1995 Dec 1;166(1):105–110. doi: 10.1016/0378-1119(95)00568-3. [DOI] [PubMed] [Google Scholar]
- Straus D., Ausubel F. M. Genomic subtraction for cloning DNA corresponding to deletion mutations. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1889–1893. doi: 10.1073/pnas.87.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Zeligs B., Siam M. A., Parrott C. Studies on gonococcus infection. V. Observations on in vitro interactions of gonococci and human neutrophils. Infect Immun. 1974 Sep;10(3):633–644. doi: 10.1128/iai.10.3.633-644.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. A., Wang L. L., Sparling P. F. Cloning and nucleotide sequence of frpC, a second gene from Neisseria meningitidis encoding a protein similar to RTX cytotoxins. Mol Microbiol. 1993 Jul;9(1):85–96. doi: 10.1111/j.1365-2958.1993.tb01671.x. [DOI] [PubMed] [Google Scholar]
- Thompson S. A., Wang L. L., West A., Sparling P. F. Neisseria meningitidis produces iron-regulated proteins related to the RTX family of exoproteins. J Bacteriol. 1993 Feb;175(3):811–818. doi: 10.1128/jb.175.3.811-818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley C. R., Gotschlich E. C. Cloning and characterization of the meningococcal polyphosphate kinase gene: production of polyphosphate synthesis mutants. Infect Immun. 1995 May;63(5):1624–1630. doi: 10.1128/iai.63.5.1624-1630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]